Abstract
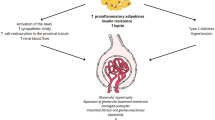
Obesity epidemic is in rise in almost every industrialized country and continues to be a growing problem worldwide. In fact, obesity per se has been recognized as a chronic disease. Consequently, there has been a cascade of metabolic changes initiated by the markedly risen prevalence that contributes to the increased incidence of diabetes, hypertension, and cardiovascular disease. Moreover, obesity is also associated with an increased risk of chronic kidney disease (CKD). The majority of the studies indicate a direct relationship between body mass index (BMI) and CKD risk. Moreover, current evidence emphasized the fact that central obesity measurements, such as waist circumference, could be a better predictor of CKD progression and mortality than BMI. The detrimental effects of obesity on kidney outcome have been recognized in nondialysis-dependent (NDD)-CKD patients. However, survival in overweight or obese CKD patients undergoing maintenance hemodialysis is paradoxically opposed compared with the general population. This “reverse epidemiology,” however, is valid mainly for the inflammated end-stage renal disease (ESRD) patients. In fact, renal transplant recipients with higher BMI have inferior patient and graft survival compared to patients with lower BMI. This review also provides perspectives concerning the mechanisms associated with obesity, such as the renin–angiotensin–aldosterone system (RAAS) activation, and the role of leptin, adiponectin, fetuin-A, and adipose tissue, as factors that contribute to the development of CKD. Prevention strategies for CKD patients are also discussed and should be considered by clinicians.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The markedly high prevalence of obesity contributes to the increased incidence of chronic diseases, such as diabetes, hypertension, sleep apnea, and heart disease [1–3]. Actually, obesity per se has been recognized as a chronic disease since 1985 [4], and the effects have also been recognized in patients with diagnosed kidney disease [5–10], as well as among the general population [11–19]. Moreover, obesity is a well-known risk factor for CKD [11, 14, 15, 20], independent of diabetes and hypertension [21, 22]. This review discusses the effects of obesity on kidney and mortality outcomes in the general population, in non-dialysis-dependent (NDD)-CKD patients, in CKD patients undergoing maintenance hemodialysis, and in renal transplant recipients. Furthermore, it provides additional perspectives regarding the mechanisms of obesity as a factor that contributes to the occurrence of CKD. Finally, prevention strategies for kidney disease or obesity-related CKD patients, are also discussed and should be considered by clinicians.
Epidemiology of obesity and CKD
Over the past 20 years, the prevalence of obesity among adults aged 20–74 years has doubled, from 15 to 30% [23], and this increase occurred in both men and women, of all age-groups [23]. More recent data have shown that obesity afflicts 33% of the US population [24]. It is noteworthy that men were not more susceptible to these risk factors contrary to women [25].
Among adults, the body mass index (BMI) defines overweight and obesity as 25 kg/m2 or greater and 30 kg/m2 or greater, respectively. Among children and adolescents, overweight is defined as a BMI for age at or above the 95th percentile of a specified reference population. In 2003–2004, 32.9% of adults 20–74 years old were obese and more than 17% of teenagers (aged 12–19) were overweight [21]. Of note, children’s obesity rate is lower; however, the increase in the prevalence of overweight is generally similar to the rise in obesity among adults [26].
Obesity initiates a cascade of metabolic and endocrine changes that contributes to an increased risk of diabetes, hypertension, and cardiovascular disease [1–3]. For example, it is suggested that 65–75% of the risk of hypertension is attributed to excess body weight [27, 28]. On the other hand, diabetes mellitus, which occurs with greater frequency in obese individuals [29], is the most common cause of CKD and end-stage renal disease (ESRD).
The specific risk level associated with a given obesity level may vary depending on gender, race, and socioeconomic condition [30]. Furthermore, race/ethnic differences in lifestyle and economic status may account for some of the race disparity in obesity-related diseases and outcomes [31]. On the other hand, socioeconomic status seems to play a strong role in the risk of obesity [32–34]. There is no evidence that obese or overweight patients receive inferior quality of care, compared with normal-weight patients [35]. Most of the excessive obesity trends have been noticed in communities with the highest rates of poverty and the lowest levels of education [36].
Obesity epidemic is in concert with the crescendo of adults with ESRD, a population that is expected to get doubled over the next decade [37]. CKD is a major worldwide public health problem [38], with an estimated prevalence of about 14.8% of the general population [39]. Both the incidence and prevalence of kidney failure treated by dialysis continue to increase [40] and during the past three decades they have been raising progressively [41].
Obesity and the risk for CKD
Obesity as a risk factor for CKD in epidemiological studies
Obesity epidemic has been paralleled by the increase in the incidence of CKD. In fact, there is a rapidly increasing prevalence of overweight and obese patients with CKD [23]. Obesity is not only a well-recognized risk factor for hypertension [42, 43] and diabetes [44], the most common etiologies of ESRD, [45] and indirectly linked to the development of CKD, but it predisposes directly to kidney disease, independently of diabetes and hypertension [17, 21, 22]. There is mounting evidence suggesting that increased BMI increases the risk of developing kidney impairment in the general population (Table 1). Noteworthily, long-term observational studies have shown a correlation between BMI and the incidence ESRD [11–19]. Hsu et al. assessed the impact of BMI on the risk of ESRD in a cohort of 330,252 persons from a large integrated health care delivery system in California, between 1964 and 1985. There was a strong dose–response relationship between the baseline BMI and the risk of ESRD. In particular, it has been demonstrated that the relative risk (RR) of ESRD was 3.57 [(95% coefficient interval (CI) 3.05,4.18)] compared to patients with an ideal BMI (18.5–24.9 kg/m), for those with stage 1 obesity (BMI 30–34.9 kg/m2), 6.12 (95% CI 4.97,7.54) for those with stage 2 obesity (BMI 35–39.9 kg/m2), and 7.07 (95% CI 5.37, 9.31) for those with morbid obesity (BMI ≥ 40 kg/m2) [14]. In another study, Iseki et al., using data from a community-based screening of 100,753 subjects, provided evidence that higher BMI at baseline increased the risk of ESRD after 17 years of follow-up only in men, but not in women [13]. Furthermore, a recent meta-analysis showed that overweight persons have a 40% higher risk of CKD and that obese persons have an 83% higher risk [46].
Obesity in individuals with normal renal function and in patients with mild and moderate CKD
The initial clinical evidence of kidney involvement in obesity is the presence of proteinuria, which usually precedes the glomerular filtration rate (GFR) decline by several years [20, 47]. In overweight and obese patients, the risk of incident dipstick proteinuria is 43–56% higher than in individuals with a BMI below 25 kg/m2 [6, 48]. Proteinuria is less likely to be nephrotic [49] than in idiopathic focal and segmental glomerulosclerosis. In the Look AHEAD (Action for Health in Diabetes) Study, among 4,985 participants, the highest quartiles of BMI, [odds ratio (OR) 1.72 (95% CI 1.40–2.11)], and waist circumference (WC), [OR 1.75 (95% CI 1.42–2.15)], were significantly associated with albuminuria, compared to the lowest quartiles, after adjustment for covariates [50]. Results from the Framingham study cohort with more than 18.5 years of follow-up, 36% of whom were overweight and 12% obese, showed that 14.4% developed proteinuria and 7.9% stage 3 CKD. This increase in risk of stage 3 CKD was no longer significant after adjustment for cardiovascular risk factors. Thus, the relationship between obesity and stage 3 CKD may be mediated through cardiovascular disease risk factors [11].
Proteinuria is thought to be the result of glomerular hypertrophy and hyperfiltration due to hemodynamic changes. The question is whether interventions that reduce abdominal obesity could prevent the development of albuminuria. A recent meta-analysis showed that weight loss is associated with decreased proteinuria [51]; however there are no data to evaluate the effect of weight loss on CKD progression.
The effects of obesity on renal outcomes have been studied in patients with various kidney diseases, such as hypertensive kidney disease, specific glomerulopathies, or NDD-CKD [5–10] (Table 2).
In a cohort of 162 incident patients with biopsy-proven immunoglobulin A (IgA) nephropathy, the presence of elevated BMI at the time of the first biopsy was significantly associated with more severe pathological kidney lesions, increased proteinuria and subsequent development of CKD [6].
However, in contrast with the above findings, other studies suggest beneficial effects of obesity in CKD patients. In a longitudinal cohort study with a more than 5-year follow-up period, the relationship of BMI with earlier stages of CKD concerning cardiovascular mortality as well as the possible risk modification of proteinuria was investigated. Impressively, it has been shown that in proteinuric CKD patients, BMI was inversely correlated with the risk of death [8]. Prospective cohort studies with NDD-CKD participants investigated the association between BMI and mortality and showed that highly elevated BMI may reduce the risk of cardiovascular death [7, 9, 10] (Table 2).
Obesity in end-stage renal disease and dialysis patients
In a large cohort of 5,897 incident dialysis patients with hypertension from the Hypertension and Follow up Program (HDFP), significant associations of overweight and obesity with the development of ESRD were noted during a 5-year- follow-up, suggesting that obese adults with hypertension have an increased risk of ESRD [52].
In hemodialysis (HD) patients, already known key risk factors such as obesity, hypercholesterolemia, and hypertension are inversely related to mortality [53]. An increased number of epidemiological studies and analyses of large samples of dialysis patients (Table 3) indicated that high BMI is associated with improved survival [54–60]. Although studies in CKD patients undergoing peritoneal dialysis (PD) have yielded conflicting results, high BMI was shown to be an independent positive predictor of patient survival [61, 62]. In a recent large prospective study on a total of 5,592 patients, the association between BMI and mortality was examined. At the start of dialysis, patients were classified in four categories according to the BMI: underweight, normal range, overweight, and obese. Also, patient survival was analyzed according to five quintiles of body weight changes during the first year of HD treatment <−5.8%, −5.8 to −1.1%, −1.1 to 1.7% (reference category), +1.7 to +5.5%, and >+5.5%. Survival was significantly lower in patients presenting the lower quintile of body weight change (<−5.8% in 1 year), whereas overweight and obese patients carried a significant lower mortality risk than patients in the normal and lower BMI ranges [55].
These findings are in contrast to the well-known association between over-nutrition and poor outcome among the general population. The association between under-nutrition and adverse cardiovascular outcome in dialysis patients, which stands in contrast to that seen in non-ESRD individuals, has been referred to as “reverse epidemiology”. Even though the term is valid only for the inflammated patients, the etiology of this inverse association between conventional risk factors and clinical outcome in dialysis patients is not clear [63]. One possible explanation is that patients on HD with decreased nutritional reserve over time are not able to withstand inflammation, insufficient protein-calorie intakes, chronic acidosis, as well as vascular access failures, hospitalizations, and suboptimal small and middle solute clearance. Therefore, according to the aforementioned studies, HD patients with increased BMI may have a lower mortality risk [54, 55, 57, 60].
Obesity in kidney transplant patients
In the past years, a growing body of evidence has indicated that renal transplant recipients with elevated BMI have inferior patient and graft survival compared to patients with lower BMI [64–68] (Table 4). In a study including 292 renal transplant recipients, the impact of post-transplant weight gain on graft survival at the time of transplantation as well as 1 year post-transplant was explored. In multivariate analysis, patients with an increase in BMI of more than 5% at 1 year post-transplant had an increased risk of graft loss [64]. In a retrospective analysis of 51,927 primary, adult renal transplants registered in the United States Renal Data System (USRDS), BMI was significantly associated with outcomes after renal transplantation, independently of most of the known risk factors for patient and graft survival. Very high BMI was strongly associated with worse patient and graft survival [65]. In a retrospective study of 193 consecutive, adult renal transplant patients, with at least 6 months follow-up, those with a BMI ≥ 30 were less likely to experience graft loss. In this study, the rates of acute rejection were not increased in obese recipients, and the ratio of recipient-to-donor BMI did not influence graft survival. However, while mortality was not increased in the BMI > 30 group, morbidity, especially surgery-related, had an increased incidence [66]. On top of these, there is evidence that obesity in kidney transplant patients predicts increased cardiac risk, especially for chronic heart failure and atrial fibrillation [67].
It is now clear that the majority of the studies found a direct relationship between BMI and cardiovascular risk. Furthermore, a continuous positive relationship between BMI and increased glomerular filtration fraction has been documented throughout the entire range from normal-to-abnormal BMI values [69], while other studies suggest a J-shaped association [14, 16]. There is growing evidence that overweight and increased BMI in adolescents is predictive of CKD in adult life [15]. Even in men as young as 18 years old, unadjusted estimated creatinine clearance is progressively higher in individuals with increasing BMI [70]. When BMI is in the normal range, the increase in body weight is independently associated with an increased risk of CKD [12]. On the other hand, as mentioned above in NDD-CKD population, higher BMI is associated with kidney function deterioration but with lower mortality rates [7, 9, 10]. Furthermore, all of the data in patients undergoing maintenance HD support the concept that mortality risk is decreased with increasing BMI. In contrast, in renal transplant recipients, higher BMI is associated with worse patient and graft survival.
Central body fat versus BMI
The next logical question is whether central obesity or BMI per se could be a stronger predictor of CKD. It has been suggested that central obesity with waist circumference measurement may be a better predictor than BMI [71, 72]. However, there is evidence that the central distribution of body fat is related to a variety of physiological abnormalities. Central body fat is associated with hypertension, hyperlipidemia, hyperinsulinemia, and atherosclerosis [72]. Furthermore, in a study of nondiabetic subjects, elevated waist-to-hip ratio (WHR) increased the risk of diminished GFR, whether they were lean, overweight, or obese [71]. In a recent study, patients with ESRD with large WC and low BMI were at the highest risk of overall and cardiovascular mortality [73]. On the other hand, BMI per se does not differentiate between fat and muscle mass. This is best exemplified by data from a recent study, in which the relationship between measures of fat, muscle mass, and mortality was examined in 1,709 patients during a median follow-up of 2.5 years. Triceps skin-fold thickness was used to assess body fat, and mid-arm muscle circumference was used to assess muscle mass. In multivariate analysis and adjusted models, lower quartiles of triceps skin-fold thickness, mid-arm muscle circumference, and BMI were all significantly associated with higher all-cause mortality [74]. In practical terms, body composition in ESRD patients bears a complex relationship to all-cause mortality. However, it is important to point out that in ESRD patients [73] and in kidney transplant patients [68], higher waist circumference was more strongly associated with higher mortality after adjustment for BMI. Furthermore, higher BMI and waist circumference display opposite associations with mortality, and waist circumference appears to be a better marker of obesity than BMI [68, 73].
Kidney histopathology in obesity
It is clear from the foregoing discussion the adverse effect of obesity in patients with primary or with specific kidney disease [6, 52, 64–66]. In kidney biopsies of obese patients, the type of kidney disease that has become recognized is the so-called obesity-related glomerulopathy with or without focal segmental glomerulosclerosis [49]. The frequency has been increased from 0.62 to 1.0% during the last 5 years (P = 0.02) [75]. The biopsy specimens shows glomerulomegaly, increased mesangial matrix and proliferation, podocyte hypertrophy, lesions of segmental sclerosis and food process effacement, thickening of the glomerular basement membrane with interstitial fibrosis [49, 76, 77].
It should be acknowledged that the individual risk of developing glomerulopathy connected to obesity is very low. It is proposed that glomerular hyperfiltration/hypertrophy is per se not pathogenic in the absence of an enhanced glomerular blood pressure transmission, and the modest preglomerular vasodilatation that is likely present in the large majority of obese individuals is not sufficient to result in such increased blood pressure transmission [78]. In extremely obese people with normal kidney function prior to bariatric surgery, the majority had increased mesangial matrix mesangial cell proliferation, podocyte hypertrophy, and glomerulopathy. The risk factors of developing glomerular lesions were weight related [77]. These findings resembled those described at early stages of diabetic nephropathy and are observed even before the appearance of microalbuminuria [79].
Mechanism of kidney failure in obesity
Obesity, renin–angiotensin–aldosterone system activation and hypertension
The renin–angiotensin–aldosterone system (RAAS) is a well-coordinated hormonal mechanism that regulates adrenal, cardiovascular as well as kidney function by controlling fluid and electrolyte balance. A number of mechanisms have been proposed to be responsible for modifying blood pressure and renal function via homeostasis of renal salt and water, alteration in sympathetic nerve activity, and changes in vascular tone [80]. Furthermore, the RAAS regulates vasomotor tone, cellular proliferation, and structure in the kidney.
In obese individuals, commonly the RAAS is activated. It has been demonstrated that weight loss is accompanied by reductions in plasma renin activity (PRA) and aldosterone, and PRA reductions, irrespective of sodium intake [81]. For example, a 5% reduction in body weight can lead to a meaningfully reduced RAAS in plasma and adipose tissue, which may contribute to the reduced blood pressure [82].
The pathogenesis of obesity-related hypertension and kidney function impairment is highly complex. Vasoconstriction and sodium retention are promoted by several acting together factors [83]. Various factors need to be accounted for when you consider potential mechanism of kidney impairment related to obesity. Evidence is growing that local RAAS seems to be differently regulated in subcutaneous adipose tissue from obese patients with hypertension in comparison with normotensive obese patients and controls [84]. In particular, it has demonstrated the presence—and different levels of expression—of the various components of the RAAS system (angiotensinogen-AGT), angiotensin II type receptor (AT1), and angiotensin-converting enzyme (ACE) in human subcutaneous adipose tissue and in visceral adipose tissue. These observations highlight the different role and regulation of the system in the two tissues. Its high expression in visceral adipose tissue suggests that its regulation and function are involved in all conditions where visceral adiposity is present [85]. Angiotensin II (AgII), the final effector of the RAAS, is locally produced. The function of adipose RAAS is not well known; besides participating, together with other hormones and substances, in adipocyte differentiation and fat tissue growth, it could also be involved in the pathogenesis of the complications of obesity-related renal failure and obesity-related hypertension [86]. Positive and significant correlation was found between the expression of AGT in visceral adipose tissue and BMI. These data suggest that angiotensinogen may be determinant of fat distribution and may be involved in the plurimetabolic syndrome of central obesity [87].
Adipose tissue, leptin, adiponectin, and fetuin-A
Adipose tissue is a prolific organ that secrets several immunomodulators and bioactive molecules [88, 89]. Leptin is a hormone with significant pleitropic actions on several systems [90, 91]. The role of leptin in the kidney is not completely defined. It seems to be a potential salt-regulating factor and may function pathophysiologically as a common link to obesity and hypertension [90]. It is also recognized that conditions of hyperleptinemia have been linked to renal structural changes that were particularly associated with obesity [92, 93]. Leptin is partly cleared by the kidney, and hyperleptinemia has been demonstrated in patients with kidney disease [94] and is elevated in HD patients [95]. Furthermore, in PD patients, the elevated body fat mass appears to contribute to more elevated leptin levels [96].
Insulin resistance, oxidative stress, and inflammation have all been implicated in albuminuria and renal dysfunction, although the picture is not entirely clear [97]. Elegant studies implicate low adiponectin in the pathogenesis of renal disease in obesity. Adiponectin is secreted by adipocytes [98] and circulates in plasma as various complexes, such as high molecular weight, low molecular weight, and trimeric forms. High molecular weight levels are in great quantity in women and fails gradually in obesity [98]. Low adiponectin levels are associated with higher prevalence of inflammation, type 2 diabetes, and atherosclerosis [98]. Furthermore, low plasma adiponectin was related to increased urinary albumin excretion in African-Americans, a group disposed to CKD and obesity [99]. Treatment with adiponectin reverses the fusion of podocyte foot processes in adiponectin-negative (-) mice and improved urinary albuminuria, glomerular hypertrophy, and tubulointerstitial fibrosis [99]. These changes were associated with the anti-inflammatory role of adiponectin in the kidney in accordance with its similar action in the vasculature and the other organs [100].
Another point of importance is the association of the glycoprotein fetuin-A with obesity in patients with CKD. Higher levels of fetuin-A are associated with obesity and insulin resistance both in the general population [101, 102] and in patients with CKD [103]. In particular, fetuin-A was recognized as a promoter of insulin resistance, because it inhibits the insulin receptor tyrosine kinase in the muscles and the hepatocytes. This action results in insulin resistance due to inhibition of the insulin signal transduction at the target tissue [104, 105]. It is also recognized that the high levels of fetuin-A have the same effects as the low levels of adiponectin because the two proteins have the same results but in the opposite direction [106]. There is a specific effect of fetuin-A on adiponectin. It is noteworthy that treatment with fetuin-A lowered adiponectin levels probably because fetuin-A inhibits the generation of adiponectin in the adipose tissue and furthermore fetuin-A suppresses mRNA encoding in cultured human adipocytes [107].
Potential interventions to prevent injury
In the aforementioned recent meta-analysis by Wang et al. was estimated that more than one-forth (24.2% in men vs. 33.9% in women) of CKD cases in the United States and one-fifth in industrialized countries (16.5% vs. 26.3%) could be prevented by eliminating overweight and obesity [46]. Undoubtedly, preventing obesity not only must be considered a public health priority, but it needs to be controlled as a chronic disease in an effort so that the ultimate goal is realized. It is reasonably the first attempt to be dietary. Curing obesity must be an ultimate goal as a modifiable risk factor in early nephropathy [108]. Weight loss improves the glomerular hemodynamic abnormalities associated with over obesity in subjects without overt kidney disease. Elevated GFR and renal blood flow (RBF) decreased after weight loss, and this reflect mainly decreased in a single-nephron plasma flow and a single-nephron GFR [109]. A lower pressure is transmitted to the glomerular capillaries due to decreased systemic arterial pressure and an increase in afferent arteriole resistance resulting in a diminished GFR [110]. Furthermore, the fractional excretion of albumin decreased following weight loss, possibly by altering glomerular permselectivity of affecting glomerular cell metabolism and the transforming growth factor (TGF-b) production [111–113]. Weight reduction results in a significant decrease in arterial pressure mediated in part through a reduction in peripheral resistance [114], and this could be translated as prevention of renal and cardiovascular disease. Potential interventions with antihypertensive treatment that target RAAS may improve the kidney outcome in obese patients. In a recent study, the effects of treatment with Ag II type-1 receptor blocker (ARB) on the regulation of adipocytokines were determined. The ARB Olmesartan significantly blunted the age- and body weight-associated falls in plasma adiponectin both in genetically and in diet-induced obese mice, without affecting body weight, but had no effect on plasma adiponectin levels in lean mice. These results indicated that blockade of Ang II receptor ameliorates adipocytokine dysregulation and that such action is mediated, at least in part, by targeting oxidative stress in obese adipose tissue. Ang II signaling and subsequent oxidative stress in adipose tissue may be potential targets for the prevention of atherosclerotic cardiovascular disease in metabolic syndrome and also in metabolic syndrome-based CKD [115].
Conclusions
Obesity has reached epidemic proportions and continues to be a growing problem worldwide. What is more, it has increased the incidence of diabetes, hypertension, and heart disease and is associated with an increased risk for CKD worsening in this group of patients. Furthermore, it predisposes toward kidney disease independently of diabetes and hypertension. In fact, the presence of proteinuria is actually the initial clinical evidence of involving kidney and obesity. However, a significant percentage of CKD cases could be prevented by eliminating overweight and obesity. Hopefully, potential interventions that target RAAS may improve the kidney outcome in obese hypertensive patients.
References
Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM (2006) Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 295:1549–1555
Hall JE, Crook ED, Jones DW, Wofford MR, Dubbert PM (2002) Mechanisms of obesity-associated cardiovascular and renal disease. Am J Med Sci 324:127–137
Dubbert PM, Carithers T, Sumner AE et al (2002) Obesity, physical inactivity, and risk for cardiovascular disease. Am J Med Sci 324:116–126
Health implications of obesity (1985) National institutes of health consensus development conference statement. Ann Intern Med 103:147–151
Kramer HJ, Saranathan A, Luke A et al (2006) Increasing body mass index and obesity in the incident ESRD population. J Am Soc Nephrol 17:1453–1459
Bonnet F, Deprele C, Sassolas A et al (2001) Excessive body weight as a new independent risk factor for clinical and pathological progression in primary IgA nephritis. Am J Kidney Dis 37:720–727
Evans M, Fryzek JP, Elinder CG et al (2005) The natural history of chronic renal failure: results from an unselected, population-based, inception cohort in Sweden. Am J Kidney Dis 46:863–870
Obermayr RP, Temml C, Gutjahr G et al (2009) Body mass index modifies the risk of cardiovascular death in proteinuric chronic kidney disease. Nephrol Dial Transplant 24:2421–2428
Kovesdy CP, Anderson JE, Kalantar-Zadeh K (2007) Paradoxical association between body mass index and mortality in men with CKD not yet on dialysis. Am J Kidney Dis 49:581–591
Kwan BC, Murtaugh MA, Beddhu S (2007) Associations of body size with metabolic syndrome and mortality in moderate chronic kidney disease. Clin J Am Soc Nephrol 2:992–998
Foster MC, Hwang SJ, Larson MG et al (2008) Overweight, obesity, and the development of stage 3 CKD: the Framingham Heart Study. Am J Kidney Dis 52(1):39–48
Ryu S, Chang Y, Woo HY et al (2008) Changes in body weight predict CKD in healthy men. J Am Soc Nephrol 21:1798–1805
Iseki K, Ikemiya Y, Kinjo K, Inoue T, Iseki C, Takishita S (2004) Body mass index and the risk of development of end-stage renal disease in a screened cohort. Kidney Int 65:1870–1876
Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS (2006) Body mass index and risk for end-stage renal disease. Ann Intern Med 144:21–28
Ejerblad E, Fored CM, Lindblad P, Fryzek J, McLaughlin JK, Nyren O (2006) Obesity and risk for chronic renal failure. J Am Soc Nephrol 17:1695–1702
Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D (2004) Predictors of new-onset kidney disease in a community-based population. JAMA 291:844–850
Munkhaugen J, Lydersen S, Wideroe TE, Hallan S (2009) Prehypertension, obesity, and risk of kidney disease: 20-year follow-up of the HUNT I study in Norway. Am J Kidney Dis 54:638–646
Elsayed EF, Sarnak MJ, Tighiouart H et al (2008) Waist-to-hip ratio, body mass index, and subsequent kidney disease and death. Am J Kidney Dis 52:29–38
Gelber RP, Kurth T, Kausz AT et al (2005) Association between body mass index and CKD in apparently healthy men. Am J Kidney Dis 46:871–880
Praga M (2002) Obesity—a neglected culprit in renal disease. Nephrol Dial Transplant 17:1157–1159
Ogden CL, Yanovski SZ, Carroll MD, Flegal KM (2007) The epidemiology of obesity. Gastroenterology 132:2087–2102
Coresh J, Selvin E, Stevens LA et al (2007) Prevalence of chronic kidney disease in the United States. JAMA 298:2038–2047
Flegal KM, Carroll MD, Ogden CL, Johnson CL (2002) Prevalence and trends in obesity among US adults, 1999–2000. JAMA 288:1723–1727
Flegal KM, Carrol MD, Ogden CL, Curtin LR (2010) Prevalence and trends in obesity among US adults, 1999–2008. J Am Med Assoc 303:235–241
Hallan S, de MR, Carlsen S, Dekker FW, Aasarod K, Holmen J (2006) Obesity, smoking, and physical inactivity as risk factors for CKD: are men more vulnerable? Am J Kidney Dis 47:396–405
Rennie KL, Jebb SA (2005) Prevalence of obesity in Great Britain. Obes Rev 6:11–12
Garrison RJ, Kannel WB, Stokes J III, Castelli WP (1987) Incidence and precursors of hypertension in young adults: the Framingham Offspring Study. Prev Med 16:235–251
Wofford MR, Hall JE (2004) Pathophysiology and treatment of obesity hypertension. Curr Pharm Des 10:3621–3637
US RDS 2009 Annual Data Report: Atlas of End-Stage Renal Disease in the United States in: National Institutes of Health, National Institutes of Diabetes and Digestive and Kidney Disease. Viewed: 12.8.2010
Paeratakul S, Lovejoy JC, Ryan DH, Bray GA (2002) The relation of gender, race and socioeconomic status to obesity and obesity comorbidities in a sample of US adults. Int J Obes Relat Metab Disord 26:1205–1210
Cossrow N, Falkner B (2004) Race/ethnic issues in obesity and obesity-related comorbidities. J Clin Endocrinol Metab 89:2590–2594
Perneger TV, Whelton PK, Klag MJ (1995) Race and end-stage renal disease. Socioeconomic status and access to health care as mediating factors. Arch Intern Med 155:1201–1208
Perneger TV, Klag MJ, Whelton PK (1995) Race and socioeconomic status in hypertension and renal disease. Curr Opin Nephrol Hypertens 4:235–239
Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Stamler J (1997) End-stage renal disease in African–American and white men. 16-year MRFIT findings. JAMA 277:1293–1298
Chang VW, Asch DA, Werner RM (2010) Quality of care among obese patients. JAMA 303:1274–1281
Drewnowski A, Specter SE (2004) Poverty and obesity: the role of energy density and energy costs. Am J Clin Nutr 79:6–16
Xue JL, Ma JZ, Louis TA, Collins AJ (2001) Forecast of the number of patients with end-stage renal disease in the United States to the year 2010. J Am Soc Nephrol 12:2753–2758
Schoolwerth AC, Engelgau MM, Hostetter TH et al (2006) Chronic kidney disease: a public health problem that needs a public health action plan. Prev Chronic Dis 3:A57
USRD 2008 ADR/reference tables. Available: http://www.usrds.org-2008.htm.Viewed 12/11/09
Dirks JH, de ZD, Agarwal SK et al (2005) Prevention of chronic kidney and vascular disease: toward global health equity—the Bellagio 2004 declaration. Kidney Int Suppl 98:S1–S6
Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS (2003) Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 41:1–12
Wilson PW, D’Agostino RB, Sullivan L, Parise H, Kannel WB (2002) Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med 162:1867–1872
Liu K, Ruth KJ, Flack JM, Jones-Webb R, Burke G, Savage PJ et al (1996) Blood pressure in young blacks and whites: relevance of obesity and lifestyle factors in determining differences. The CARDIA study. Coronary artery risk development in young adults. Circulation 93:60–66
Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC (1994) Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care 17:961–969
Burrows NR, Wang J, Geiss LS, Venkat Narayan KM, Engelgau MM (2005) Incidence of end-stage renal disease among persons with diabetes—United States, 1990–2002 MMWR. Morb Mortal Wkly Rep 54:1097–1100
Wang Y, Chen X, Song Y, Caballero B, Cheskin LJ (2008) Association between obesity and kidney disease: a systematic review and meta-analysis. Kidney Int 73:19–33
Ritz E (2008) Obesity and CKD: how to assess the risk? Am J Kidney Dis 52:1–6
Fesler P, Safar ME, du CG, Ribstein J, Mimran A (2007) Pulse pressure is an independent determinant of renal function decline during treatment of essential hypertension. J Hypertens 25:1915–1920
Kambham N, Markowitz GS, Valeri AM, Lin J, D’Agati VD (2001) Obesity-related glomerulopathy: an emerging epidemic. Kidney Int 59:1498–1509
Kramer H, Reboussin D, Bertoni AG et al (2009) Obesity and albuminuria among adults with type 2 diabetes: the look AHEAD (Action for Health in Diabetes) study. Diabetes Care 32:851–853
Afshinnia F, Wilt TJ, Duval S, Esmaeili A, Ibrahim HN (2010) Weight loss and proteinuria: systematic review of clinical trials and comparative cohorts. Nephrol Dial Transplant 25:1173–1183
Kramer H, Luke A, Bidani A, Cao G, Cooper R, McGee D (2005) Obesity and prevalent and incident CKD: the hypertension detection and follow-up program. Am J Kidney Dis 46:587–594
Goodkin DA, Bragg-Gresham JL, Koenig KG et al (2003) Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: the dialysis outcomes and practice patterns study (DOPPS). J Am Soc Nephrol 14:3270–3277
Leavey SF, McCullough K, Hecking E, Goodkin D, Port FK, Young EW (2001) Body mass index and mortality in ‘healthier’ as compared with ‘sicker’ haemodialysis patients: results from the dialysis outcomes and practice patterns study (DOPPS). Nephrol Dial Transplant 16:2386–2394
Chazot C, Gassia JP, Di BA, Cesare S, Ponce P, Marcelli D (2009) Is there any survival advantage of obesity in Southern European haemodialysis patients? Nephrol Dial Transplant 24:2871–2876
Degoulet P, Legrain M, Reach I et al (1982) Mortality risk factors in patients treated by chronic hemodialysis. Report of the diaphane collaborative study. Nephron 31:103–110
Johansen KL, Young B, Kaysen GA, Chertow GM (2004) Association of body size with outcomes among patients beginning dialysis. Am J Clin Nutr 80:324–332
Kaizu Y, Tsunega Y, Yoneyama T et al (1998) Overweight as another nutritional risk factor for the long-term survival of non-diabetic hemodialysis patients. Clin Nephrol 50:44–50
Port FK, Ashby VB, Dhingra RK, Roys EC, Wolfe RA (2002) Dialysis dose and body mass index are strongly associated with survival in hemodialysis patients. J Am Soc Nephrol 13:1061–1066
Kopple JD (1997) Nutritional status as a predictor of morbidity and mortality in maintenance dialysis patients. ASAIO J 43:246–250
Churchill D, Wayne Teylor D, Keshaviah P (1996) Adequacy of dialysis and nutrition in continuous peritoneal dialysis: association with clinical outcomes. Canada-USA (CANUSA) peritoneal dialysis study group. J Am Soc Nephrol 7:198–207
Johnson DW, Herzig KA, Purdie DM et al (2000) Is obesity a favorable prognostic factor in peritoneal dialysis patients? Perit Dial Int 20:715–721
Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD (2003) Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int 63:793–808
Ducloux D, Kazory A, Simula-Faivre D, Chalopin JM (2005) One-year post-transplant weight gain is a risk factor for graft loss. Am J Transplant 5:2922–2928
Meier-Kriesche HU, Arndorfer JA, Kaplan B (2002) The impact of body mass index on renal transplant outcomes: a significant independent risk factor for graft failure and patient death. Transplantation 15(73):70–74
Massarweh NN, Clayton JL, Mangum CA, Florman SS, Slakey DP (2005) High body mass index and short- and long-term renal allograft survival in adults. Transplantation 80:1430–1434
Lentine KL, Rocca-Rey LA, Bacchi G et al (2008) Obesity and cardiac risk after kidney transplantation: experience at one center and comprehensive literature review. Transplantation 86:303–312
Kovesdy CP, Czira ME, Rudas A et al (2010) Body mass index, waist circumference and mortality in kidney transplant recipients. Am J Transplant 10:2644–2651
Bosma RJ, van der Heide JJ, Oosterop EJ, de Jong PE, Navis G (2004) Body mass index is associated with altered renal hemodynamics in non-obese healthy subjects. Kidney Int 65:259–265
Tomaszewski M, Charchar FJ, Maric C et al (2007) Glomerular hyperfiltration: a new marker of metabolic risk. Kidney Int 71:816–821
Pinto-Sietsma SJ, Navis G, Janssen WM, de ZD, Gans RO, de Jong PE (2003) A central body fat distribution is related to renal function impairment, even in lean subjects. Am J Kidney Dis 41:733–741
Haffner SM, Stern MP, Hazuda HP, Pugh J, Patterson JK (1987) Do upper-body and centralized adiposity measure different aspects of regional body-fat distribution? Relationship to non-insulin-dependent diabetes mellitus, lipids, and lipoproteins. Diabetes 36:43–51
Postorino M, Marino C, Tripepi G, Zoccali C (2009) Abdominal obesity and all-cause and cardiovascular mortality in end-stage renal disease. J Am Coll Cardiol 14(53):1265–1272
Huang CX, Tighiouart H, Beddhu S et al (2010) Both low muscle mass and low fat are associated with higher all-cause mortality in hemodialysis patients. Kidney Int 77:624–629
Chen HM, Li SJ, Chen HP, Wang QW, Li LS, Liu ZH (2008) Obesity-related glomerulopathy in China: a case series of 90 patients. Am J Kidney Dis 52:58–65
Weisinger JR, Kempson RL, Eldridge FL, Swenson RS (1974) The nephrotic syndrome: a complication of massive obesity. Ann Intern Med 81:440–447
Serra A, Romero R, Lopez D et al (2008) Renal injury in the extremely obese patients with normal renal function. Kidney Int 73:947–955
Griffin KA, Kramer H, Bidani AK (2008) Adverse renal consequences of obesity. Am J Physiol Renal Physiol 294:F685–F696
Goumenos DS, Kawar B, El NM et al (2009) Early histological changes in the kidney of people with morbid obesity. Nephrol Dial Transplant 24:3732–3738
Carey RM, Siragy HM (2003) Newly recognized components of the renin-angiotensin system: potential roles in cardiovascular and renal regulation. Endocr Rev 24:261–271
Tuck ML, Sowers J, Dornfeld L, Kledzik G, Maxwell M (1981) The effect of weight reduction on blood pressure, plasma renin activity, and plasma aldosterone levels in obese patients. N Engl J Med 304:930–933
Engeli S, Bohnke J, Gorzelniak K et al (2005) Weight loss and the renin-angiotensin-aldosterone system. Hypertension 45:356–362
Montani JP, Antic V, Yang Z, Dulloo A (2002) Pathways from obesity to hypertension: from the perspective of a vicious triangle. Int J Obes Relat Metab Disord 26(Suppl 2):S28–S38
Faloia E, Gatti C, Camilloni MA et al (2002) Comparison of circulating and local adipose tissue renin-angiotensin system in normotensive and hypertensive obese subjects. J Endocrinol Invest 25:309–314
Giacchetti G, Faloia E, Mariniello B et al (2002) Overexpression of the renin-angiotensin system in human visceral adipose tissue in normal and overweight subjects. Am J Hypertens 15:381–388
Faloia E, Camilloni MA, Giacchetti G, Mantero F (2000) Adipose tissue as an endocrine organ? A review of some recent data. Eat Weight Disord 5:116–123
Giacchetti G, Faloia E, Sardu C et al (2000) Gene expression of angiotensinogen in adipose tissue of obese patients. Int J Obes Relat Metab Disord 24(Suppl 2):S142–S143
Hajer GR, van Haeften TW, Visseren FL (2008) Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J 29:2959–2971
Hutley L, Prins JB (2005) Fat as an endocrine organ: relationship to the metabolic syndrome. Am J Med Sci 330:280–289
Guha PK, Villarreal D, Reams GP, Freeman RH (2003) Role of leptin in the regulation of body fluid volume and pressures. Am J Ther 10:211–218
Sharma V, McNeill JH (2005) The emerging roles of leptin and ghrelin in cardiovascular physiology and pathophysiology. Curr Vasc Pharmacol 3:169–180
Wolf G, Ziyadeh FN (2006) Leptin and renal fibrosis. Contrib Nephrol 151:175–183
Wolf G, Chen S, Han DC, Ziyadeh FN (2002) Leptin and renal disease. Am J Kidney Dis 39:1–11
Stenvinkel P, Lonnqvist F, Schalling M (1999) Molecular studies of leptin: implications for renal disease. Nephrol Dial Transplant 14:1103–1112
Sharma K, Considine RV, Michael B et al (1997) Plasma leptin is partly cleared by the kidney and is elevated in hemodialysis patients. Kidney Int 51:1980–1985
Johansen KL, Mulligan K, Tai V, Schambelan M (1998) Leptin, body composition, and indices of malnutrition in patients on dialysis. J Am Soc Nephrol 9:1080–1084
Locatelli F, Pozzoni P, Del Vecchio L (2006) Renal manifestations in the metabolic syndrome. J Am Soc Nephrol 17(4 Suppl 2):S81–S85
Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K (2006) Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 116:1784–1792
Sharma K, Ramachandrarao S, Qiu G et al (2008) Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest 118:1645–1656
Ouedraogo R, Gong Y, Berzins B et al (2007) Adiponectin deficiency increases leukocyte-endothelium interactions via upregulation of endothelial cell adhesion molecules in vivo. J Clin Invest 117:1718–1726
Ix JH, Shlipak MG, Brandenburg VM, Ali S, Ketteler M, Whooley MA (2006) Association between human fetuin-A and the metabolic syndrome: data from the heart and soul study. Circulation 11(113):1760–1767
Stefan N, Hennige AM, Staiger H et al (2006) Alpha2-Heremans-Schmid glycoprotein/fetuin-A is associated with insulin resistance and fat accumulation in the liver in humans. Diabetes Care 29:853–857
Axelsson J, Wang X, Ketteler M et al (2008) Is fetuin-A/alpha2-Heremans-Schmid glycoprotein associated with the metabolic syndrome in patients with chronic kidney disease? Am J Nephrol 28:669–676
Rauth G, Poschke O, Fink E et al (1992) The nucleotide and partial amino acid sequences of rat fetuin. Identity with the natural tyrosine kinase inhibitor of the rat insulin receptor. Eur J Biochem 204:523–529
Srinivas PR, Wagner AS, Reddy LV et al (1993) Serum alpha 2-HS-glycoprotein is an inhibitor of the human insulin receptor at the tyrosine kinase level. Mol Endocrinol 7:1445–1455
Ix JH, Sharma K (2010) Mechanisms linking obesity, chronic kidney disease, and fatty liver disease: the roles of fetuin-A, adiponectin, and AMPK. J Am Soc Nephrol 21:406–412
Hennige AM, Staiger H, Wicke C et al (2008) Fetuin-A induces cytokine expression and suppresses adiponectin production. PLoS One 3:e1765
Shen WW, Chen HM, Chen H, Xu F, Li LS, Liu ZH (2010) Obesity-related glomerulopathy: body mass index and proteinuria. Clin J Am Soc Nephrol 5:1401–1409
Chagnac A, Weinstein T, Herman M, Hirsh J, Gafter U, Ori Y (2003) The effects of weight loss on renal function in patients with severe obesity. J Am Soc Nephrol 14:1480–1486
Deen WM, Robertson CR, Brenner BM (1972) A model of glomerular ultrafiltration in the rat. Am J Physiol 223:1178–1183
Hirakata M, Kaname S, Chung UG et al (1997) Tyrosine kinase dependent expression of TGF-beta induced by stretch in mesangial cells. Kidney Int 51:1028–1036
Li Q, Muragaki Y, Hatamura I, Ueno H, Ooshima A (1998) Stretch-induced collagen synthesis in cultured smooth muscle cells from rabbit aortic media and a possible involvement of angiotensin II and transforming growth factor-beta. J Vasc Res 35:93–103
Russo LM, Osicka TM, Bonnet F, Jerums G, Comper WD (2002) Albuminuria in hypertension is linked to altered lysosomal activity and TGF-beta1 expression. Hypertension 39:281–286
Jacobs DB, Sowers JR, Hmeidan A, Niyogi T, Simpson L, Standley PR (1993) Effects of weight reduction on cellular cation metabolism and vascular resistance. Hypertension 21:308–314
Kurata A, Nishizawa H, Kihara S et al (2006) Blockade of Angiotensin II type-1 receptor reduces oxidative stress in adipose tissue and ameliorates adipocytokine dysregulation. Kidney Int 70:1717–1724
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kalaitzidis, R.G., Siamopoulos, K.C. The role of obesity in kidney disease: recent findings and potential mechanisms. Int Urol Nephrol 43, 771–784 (2011). https://doi.org/10.1007/s11255-011-9974-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-011-9974-1