Abstract
Objective
Depression, the most common psychological disorder among patients with end-stage renal disease (ESRD), is associated with poor survival. The prevalence of depression and its relation with the malnutrition–inflammation complex syndrome (MICS) have not yet been clearly defined in Chinese continuous ambulatory peritoneal dialysis (CAPD) patients.
Patients and methods
A total of 142 patients on CAPD were enrolled in the First Affiliated Hospital of Sun Yat-Sen University. The Hamilton Depression Scale (HAMD) and the malnutrition–inflammation score (MIS) were used for depression and MICS evaluation, respectively. Clinical, socioeconomic, and malnutrition–inflammation factors were compared among patients with and without depression. Binary regression analysis was performed to investigate the independent association between depression and MICS.
Results
The mean HAMD and MIS scores were 7.12 ± 5.28 and 4.45 ± 3.56, respectively. According to HAMD, 37 patients (26.1%) had depression and 70 patients (49.3%) had potential depression. Older age, longer dialysis vintage, worse residual renal function, lower employment and reimbursement status, and higher comorbidity index were positively correlated with depression. Compared to non-depressed patients, the depressed ones also showed lower levels of serum albumin and higher levels of C-reactive protein (CRP). Correlation results showed that the HAMD scores were significantly and positively correlated with MIS (r = 0.46, P < 0.01). Moreover, the incidence of peritonitis was significantly higher in depressed compared to non-depressed patients. Binary regression analysis showed that MIS was the only independent risk factor for depression.
Conclusion
Depression is commonly encountered in Chinese CAPD patients. A close relationship exists between depression and MICS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Depression is well established as a common mental health problem in people with end-stage renal disease (ESRD). The prevalence of depression in ESRD patients varies widely from 25 to 50% in different studies, where different populations have been assessed with different assessment tools [1–3]. To date, most studies concerning depression in dialysis patients are from Western countries [4, 5]. There are few data on depression in Chinese ESRD patients.
The occurrence of depression has been shown to be associated with increased mortality [6, 7]. However, the biological mechanisms by which depression might increase the risk of mortality are not very clear. It is well known that both protein-energy malnutrition and inflammation are strongly associated with poor clinical outcome in dialysis patients [8, 9]. In addition, these two conditions are highly prevalent and are found to be closely related to each other. Together they are referred to as the malnutrition–inflammation complex syndrome (MICS) [10, 11]. Some previous studies have shown that depression is associated with decreased food intake [12] and activated inflammatory response [5, 13]. However, there is still insufficient data about the relation between depression and MICS, especially in peritoneal dialysis (PD) patients.
We hypothesized that depression is associated with MICS, which might, in turn, mediate the relationship between depression and mortality in ESRD patients. The present study was therefore designed to look at the prevalence of depression and its relationship with MICS in Chinese patients undergoing continuous ambulatory peritoneal dialysis (CAPD).
Materials and methods
Patients
One hundred and forty-two patients of >18 year-old undergoing CAPD for at least 3 months in the First Affiliated Hospital of Sun Yat-Sen University from November 2005 to November 2006 were enrolled in the study. All patients received CAPD via a Tenckhoff coiled catheter and complied very well to medication and dialysis schedule. The patients used Baxter’s Ultra Bag system (Baxter Healthcare, Deerfield, Illinois, USA). Most CAPD patients were prescribed 2-L exchanges four times daily. The causes of chronic renal failure were chronic glomerulonephritis in 89 (62.7%) patients, diabetic nephropathy in 27 (19.0%), hypertension in 17 (12.0%), stone disease in 3 (2.1%), polycystic kidney disease in 3 (2.1%), and unknown in 3 (2.1%). Baseline demographic, socioeconomic, medical, and comorbidity profiles of these patients were recorded. Presence of comorbid conditions was obtained by chart review. A modified version of the Charlson’s comorbidity index, without the age and kidney disease components, was used to assess the severity of comorbidities. Cardiovascular diseases (CVD) were defined as coronary artery disease (angina pectoris and old myocardial infarction), chronic heart failure, past cerebral infarction, and peripheral vascular diseases (dissecting aneurysm and intermittent claudication). None of the 142 patients had any psychiatric history and any acute illness during the previous 3 months. Patients were excluded from the study if one or more of the following conditions were present: (1) age less than 18 years; (2) dialysis vintage less than 3 months; (3) acute illness, including infection, and (4) recent cardiovascular or cerebrovascular events within the previous 3 months.
All episodes of peritonitis occurring during the 6-month period after each HAMD assessment were recorded. Any episode of peritonitis that occurred beyond this period was not included in the analysis. Peritonitis was defined as the presence of cloudy peritoneal dialysis effluent with more than 100 white blood cells/mm3 and more than 50% polymorphonuclear cells.
Assessment of depression
All patients were evaluated for the presence of depression with the Hamilton Depression Rating Scale (HAMD), a structured 17-question interview. Depression was classified by HAMD-17 score in four degrees: no depression (score < 4), potential depression (score 4-9), mild-to-moderate depression (score 10–13), and severe depression (score ≥ 14).
Assessment of malnutrition–inflammation complex syndrome (MICS)
We used the 10-component malnutrition–inflammation score (MIS), which is based on the 7-component conventional Subjective Global Assessment (SGA) of Nutrition plus 3 other elements (body mass index (BMI), serum albumin, and serum transferrin). Each MIS component has 4 levels of severity, from 0 (normal) to 3 (very severe). The sum of all 10 MIS components ranges from 0 to 30, denoting the increasing degree of severity.
Nutrition-related anthropometric parameters were also determined. These included BMI, triceps skinfold, mid-arm circumference (MAC), and mid-arm muscle circumference (MAMC).
Non-fasting blood samples were collected, and biochemical parameters were determined using an automatic biochemical analyzer. The measurements of serum C-reactive protein (CRP), albumin, creatinine (Cr), blood urea nitrogen (BUN), total cholesterol, triglyceramide, uric acid, calcium and phosphorus concentrations were recorded. Albumin concentration was determined by the bromocresol green method. CRP was measured by immunoturbidimetric method. Serum intact parathyroid hormone (iPTH) was determined using a commercial ELISA kit. Residual renal function (RRF), total creatinine clearance (CCI), and protein catabolic rate normalized to body weight (nPCR) were measured by Baxter PD Adequest, version 1.4, a computer-based kinetic modeling program for PD (Baxter Healthcare Corporation, McGaw Park, IL, USA). A high nPCR presumably reflects a high protein intake.
Statistical methods
Statistical analyses were performed with SPSS statistical software (SPSS Inc. Chicago, IL, version 12.0 for Windows). Differences between the groups with and without depression were analyzed by Mann–Whitney U test and the χ2 test. Correlation between variables was analyzed by Spearman’s correlation analysis. Binary logistic regression analysis was performed to investigate independent association between depression and MICS. Statistical significance was assigned to P values less than 0.05.
Results
The mean age of the 142 patients recruited for this study was 53 (range 18–87) years, and their mean duration on PD was 24.27 (range 3–117.8) months. Among the patients, 42.3% were women, and 19% had diabetes mellitus. Mean MIS score was 4.45 ± 3.56 (0–20). Mean HAMD score was 7.12 ± 5.28. Patients were divided into 3 groups according to HAMD scores: no depression, 35 (24.6%) patients; potential depression, 70 (49.3%) patients; and definite depression, 37 (26.1%) patients, including 22 patients (15.5%) with mild-to-moderate depression, and 15 patients (10.6%) with severe depression. For the sake of comparison, we combined patients without depression and with potential depression as non-depressed patients. The comparison of demographic, socioeconomic, and malnutrition–inflammation profile between non-depressed and depressed patients is shown in Table 1. Depressed patients had significantly older age, longer dialysis duration, worse residual renal function (RRF), lower current employment and reimbursement rate, and higher comorbidity index compared with non-depressed patients. Depressed patients also showed a higher percentage of unmarried status (21.6%) compared with non-depressed patients (5.7%); however, the differences had no statistical significance (P = 0.078). Other factors, including gender, education, self-administration of PD, total Kt/V, CCL, blood urea nitrogen, and creatinine concentrations were comparable between depressed and non-depressed patients.
Moreover, compared to non-depressed patients, depressed patients had significantly lower serum albumin (36.63 ± 4.24 vs. 39.64 ± 5.11 g/L) and hemoglobin (95.67 ± 20.85 vs. 102.97 ± 20.01 g/L) levels. Patients with depression also had non-significant trends toward lower nPCR and nutrition-related anthropometric parameters (BMI, triceps skinfold, biceps skinfold, MAC, and MAMC). On the other hand, compared to non-depressed patients, a higher CRP level (21.89 ± 19.55 vs. 2.01 ± 1.89 mg/L) and MIS score (6.62 ± 3.63 vs. 3.75 ± 2.26) were observed in depressed patients (Table 1).
Correlations for HAMD scores and measures of demographic, nutritional, and inflammatory parameters are shown in Table 2. The results showed a significant positive correlation between HAMD scores and MIS and serum CRP level, but there was an inverse correlation between HAMD scores and serum albumin, and nPCR levels.
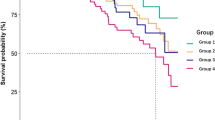
We also compared incidences of peritonitis among patients with different degrees of depression during the 6-month follow-up. There were 25 episodes of peritonitis in all the patients: 4 episodes occurred in patients without depression, 11 episodes occurred in patients with potential depression, and 10 episodes occurred in patients with depression. When combined patients without depression and with potential depression as non-depressed patients, the incidence of peritonitis in depressed patients was significantly higher than that in non-depressed patients (1/22.2 vs. 1/50.0 episodes per patient-months) (Fig. 1).
To investigate whether the association between depression and MICS is independent from other co-variables, binary logistic regression analysis was performed (use the presence of depression as an outcome variable) and the result showed that the MIS score was the only independent risk factor for depression (Table 3).
Discussion
Despite many years of efforts and significant improvement in dialysis technique and patient care, the current mortality rate is still unacceptably high, at approximately 20% per year [14]. As a domiciliary therapy, PD requires patients to organize their own care. Therefore, it can be expected that depression plays a crucial role in influencing the complication rate and outcomes associated with this therapy. To our knowledge, we are the first to study the incidence of depression among Chinese PD patients. In the current study, the prevalence of definite depression was 26.1%, with 10.6% reporting severe depression. The result was in good agreement with other similar studies. Kalender et al. [5] reported a Structured Clinical Interview depression (SCID) prevalence of 26.2%, and in another study, a prevalence of 19.2% was reported, using a stringent cutoff of the Beck Depression Inventory (BDI) [15]. However, we noticed a high incidence of potential depressive patients (49.3%) among our cohort, indicating that depression is a common but under-diagnosed problem in Chinese PD patients.
The current study demonstrated a close association between depression and MICS. Depression has been shown to be linked with adverse outcomes in PD patients, including increased incidence of peritonitis, poor nutritional status, increased hospitalization, withdrawal from dialysis, and even increased mortality [2, 3, 16, 17]. Previous data suggested that MICS might play a pivotal role. Depression is commonly associated with decreased food intake [18]. Koo et al. demonstrated that depression is closely related to nutritional status and could be an independent risk factor for malnutrition [12]. Our current study found that depression and malnutrition were closely related in PD patients; depressed patients had significantly lower serum albumin levels than non-depressed patients. There is also evidence that depression is accompanied by activation of the pro-inflammatory cytokines, which may have a role in causation of depression [13, 19], and may lead to increased protein catabolism, poor oral intake, and malnutrition. Smith put forward the “macrophage theory of depression” in 1991, in which excessive secretion of macrophage-derived cytokines was proposed as the cause of depression [20]. Cytokines such as IL-1, IL-6, and TNF-α are potent modulators of corticotrophin-releasing hormone, which produces heightened hypothalamic–pituitary–adrenal axis activity characterized by increases in adrenocorticotropin hormone and cortisol, both of which are reported to be elevated in major depression [21], while anti-depressant treatment can significantly decrease TNF-α, CRP, and leukocyte count, as well as HAMD and BDI scores [22, 23]. However, Kalender et al. [15, 18] found no relation between serum cytokines and depression in PD patients, but higher serum CRP and ferritin concentrations were observed in depressive patients. We did not evaluate serum inflammatory factor levels in this study. We did, however, measure serum CRP and found a significant positive correlation between HAMD score and serum CRP level. In addition, no patient identified with depression by HAMD received any anti-depression treatment in the current study; therefore, we did not assess the effect of anti-depressant therapy on depression and MICS.
The MIS, an indicator of MICS, is a comprehensive scoring system that significantly correlates with hospitalization and mortality rates, as well as measures of nutrition, inflammation, and anemia in maintenance dialysis patients [24–27]. Several studies have shown that MIS is superior to conventional SGA and to individual laboratory values as a predictor of dialysis outcome [25–28]. We used the MIS tool to assess the extent of MICS and found a significant positive correlation between HAMD score and MIS among the dialysis patients, as reported previously by Micozkadioglu et al. [29] in patients on hemodialysis, which was the first investigation to examine the relationship between depression and MICS.
The initial univariate analysis indicated that depression was most common in patients with older age, longer duration of PD, worse RRF, lower current employment and reimbursement status, and higher comorbidity index, which is in agreement with previous studies [30–32]. However, subsequent multivariate analysis did not support the independent association between depression and these factors. Whereas dialysis adequacy and serum Cr, calcium, and phosphorus concentrations were not found to influence HAMD score in the present study.
Among the dialysis patients, the association between depression and comorbidities such as diabetes mellitus and CAD is uncertain. De Groot [33] demonstrated a significant and consistent association between diabetes complications and depressive symptoms. Mahajan et al. [32] found that depression correlated positively with cardiovascular disease, but not with cerebrovascular disease, peripheral vascular disease, or diabetes. In the current study, although comorbidity index score was significantly higher in depressed patients compared to non-depressed patients, when looking at the incidence of cardiovascular disease, the differences were not statistically significant, which was probably due to the relatively few patients in this study. Peritonitis, the leading cause of patient morbidity and dropout from long-term PD therapy, was also examined in the current study, and demonstrated a positive correlation between depression and the risk of peritonitis. This result was in accordance with other studies’ reports [34].
It should be emphasized that, although a significant association between depression and MICS was demonstrated, data in the current cross-sectional study were not enough to identify the causal relationship between depression and MICS. Longitudinal research is needed to clarify the cause of depression and its influence on clinical outcome.
In conclusion, the current study revealed a high prevalence of depression among Chinese PD patients, which is neither recognized by their physicians nor treated. A close relationship between depression and MICS was also found in the current study. Further longitudinal studies are needed to examine the causal relationship between depression and MICS.
References
Cukor D, Cohen SD, Peterson RA et al (2007) Psychosocial aspects of chronic disease: ESRD as a paradigmatic illness. J Am Soc Nephrol 18(12):3042–3055
Chen YS, Wu SC, Wang SY et al (2003) Depression in chronic haemodialysed patients. Nephrology (Carlton) 8(3):121–126
Kimmel PL, Emont SL, Newmann JM et al (2003) ESRD patient quality of life: symptoms, spiritual beliefs, psychosocial factors, and ethnicity. Am J Kidney Dis 42(4):713–721
Ibrahim S, El Salamony O (2008) Depression, quality of life and malnutrition-inflammation scores in hemodialysis patients. Am J Nephrol 28(5):784–791
Kalender B, Dervisoglu E, Sengul E et al (2007) Depression, nutritional status, and serum cytokines in peritoneal dialysis patients: is there a relationship? Perit Dial Int 27(5):593–595
Finkelstein FO, Finkelstein SH (2000) Depression in chronic dialysis patients: assessment and treatment. Nephrol Dial Transplant 15(12):1911–1913
Kimmel PL, Peterson RA, Weihs KL et al (2000) Multiple measurements of depression predict mortality in a longitudinal study of chronic hemodialysis outpatients. Kidney Int 57(5):2093–2098
Herselman M, Moosa MR, Kotze TJ et al (2000) Protein-energy malnutrition as a risk factor for increased morbidity in long-term hemodialysis patients. J Ren Nutr 10(1):7–15
Ravnskov U (2004) Inflammation, cholesterol levels, and risk of mortality among patients receiving dialysis. Jama 291(15):1833–1834 author reply 1834–1835
Kalantar-Zadeh K, Ikizler TA, Block G et al (2003) Malnutrition-inflammation complex syndrome in dialysis patients: causes and consequences. Am J Kidney Dis 42(5):864–881
Stenvinkel P, Heimburger O, Lindholm B et al (2000) Are there two types of malnutrition in chronic renal failure? Evidence for relationships between malnutrition, inflammation and atherosclerosis (MIA syndrome). Nephrol Dial Transplant 15(7):953–960
Koo JR, Yoon JW, Kim SG et al (2003) Association of depression with malnutrition in chronic hemodialysis patients. Am J Kidney Dis 41(5):1037–1042
Dervisoglu E, Kir HM, Kalender B et al (2008) Depressive symptoms and proinflammatory cytokine levels in chronic renal failure patients. Nephron Clin Pract 108(4):c272–c277
Devereaux PJ, Schunemann HJ, Ravindran N et al (2002) Comparison of mortality between private for-profit and private not-for-profit hemodialysis centers: a systematic review and meta-analysis. Jama 288(19):2449–2457
Kalender B, Ozdemir AC, Koroglu G (2006) Association of depression with markers of nutrition and inflammation in chronic kidney disease and end-stage renal disease. Nephron Clin Pract 102(3–4):c115–c121
Shayamsunder AK, Patel SS, Jain V et al (2005) Sleepiness, sleeplessness, and pain in end-stage renal disease: distressing symptoms for patients. Semin Dial 18(2):109–118
Einwohner R, Bernardini J, Fried L et al (2004) The effect of depressive symptoms on survival in peritoneal dialysis patients. Perit Dial Int 24(3):256–263
Kalantar-Zadeh K, Supasyndh O, Lehn RS et al (2003) Normalized protein nitrogen appearance is correlated with hospitalization and mortality in hemodialysis patients with Kt/V greater than 1.20. J. Ren Nutr 13(1):15–25
Bossola M, Tazza L, Luciani G (2009) Mechanisms and treatment of anorexia in end-stage renal disease patients on hemodialysis. J Ren Nutr 19(1):2–9
Smith RS (1991) The macrophage theory of depression. Med Hypotheses 35(4):298–306
O’Brien SM, Scott LV, Dinan TG (2004) Cytokines: abnormalities in major depression and implications for pharmacological treatment. Hum Psychopharmacol 19(6):397–403
Lee SK, Lee HS, Lee TB et al (2004) The effects of antidepressant treatment on serum cytokines and nutritional status in hemodialysis patients. J Korean Med Sci 19(3):384–389
Tuglu C, Kara SH, Caliyurt O et al (2003) Increased serum tumor necrosis factor-alpha levels and treatment response in major depressive disorder. Psychopharmacology (Berl) 170(4):429–433
Ho LC, Wang HH, Peng YS et al (2008) Clinical utility of malnutrition-inflammation score in maintenance hemodialysis patients: focus on identifying the best cut-off point. Am J Nephrol 28(5):840–846
Rambod M, Kovesdy CP, Kalantar-Zadeh K (2008) Malnutrition-Inflammation Score for risk stratification of patients with CKD: is it the promised gold standard? Nat Clin Pract Nephrol 4(7):354–355
Afsar B, Sezer S, Ozdemir FN et al (2006) Malnutrition-inflammation score is a useful tool in peritoneal dialysis patients. Perit Dial Int 26(6):705–711
Kalantar-Zadeh K, Kopple JD, Block G et al (2001) A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis 38(6):1251–1263
Afsar B, Sezer S, Elsurer R et al (2008) Malnutrition-inflammation score in peritoneal dialysis: growing reliability. Perit Dial Int 28(2):207
Micozkadioglu H, Micozkadioglu I, Zumrutdal A et al (2006) Relationship between depressive affect and malnutrition-inflammation complex syndrome in haemodialysis patients. Nephrology (Carlton) 11(6):502–505
Chung SH, Heimburger O, Stenvinkel P et al (2003) Association between residual renal function, inflammation and patient survival in new peritoneal dialysis patients. Nephrol Dial Transplant 18(3):590–597
Wang AY, Sea MM, Ip R et al (2001) Independent effects of residual renal function and dialysis adequacy on actual dietary protein, calorie, and other nutrient intake in patients on continuous ambulatory peritoneal dialysis. J Am Soc Nephrol 12(11):2450–2457
Mahajan S, Tiwari SC, Kalra V et al (2007) Analysis of depression and its effect on outcome among adult Indian peritoneal dialysis patients. Perit Dial Int 27(1):94–96
de Groot M, Anderson R, Freedland KE et al (2001) Association of depression and diabetes complications: a meta-analysis. Psychosom Med 63(4):619–630
Troidle L, Watnick S, Wuerth DB et al (2003) Depression and its association with peritonitis in long-term peritoneal dialysis patients. Am J Kidney Dis 42(2):350–354
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors Zhi-Jian Li and Xin An contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, ZJ., An, X., Mao, HP. et al. Association between depression and malnutrition–inflammation complex syndrome in patients with continuous ambulatory peritoneal dialysis. Int Urol Nephrol 43, 875–882 (2011). https://doi.org/10.1007/s11255-011-9917-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-011-9917-x