Abstract
We report the case of a 61-year-old man with nephrotic syndrome due to glomerulonephritis and chronic brucellosis complicated by dissecting aortic aneurysm. The patient worked as a veterinarian and was diagnosed for chronic but non-active brucellosis with positive serum test for Brucella melitensis in the past. Administration of cyclosporine in combination with low dose prednisone resulted at least in proteinuria reduction and partial remission for 3 years. Dissecting aortic aneurysm was treated by insertion of a stent-graft, that resulted in canalization of blood flow and retraction of aneurysm wall later in the course in our patient.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Brucellosis is a zoonotic disease with a wide spectrum of clinical findings. Gastrointestinal, cardiovascular, genitourinary, hematopoetic, nervous, skeletal, pulmonary, cutaneous, and ocular manifestations have been reported [1]. The natural history of human brucellosis is characterized by relapse after a variable period of clinical latency. This relapsing course of the disease is related to intracellular localization of Brucella [2].
We report the case of a 61-year-old man with nephrotic syndrome due to glomerulonephritis and brucellosis complicated by dissecting aortic aneurysm, which was treated by endovascular stent-graft repair.
2 Case report
A 61-year-old man, who worked as a veterinarian, was referred in April 2003 to the Department of Nephrology, Wroclaw Medical University for peripheral edema, fatigue, and proteinuria. For many years, he has suffered from hypertension treated with an ACE inhibitor and an alpha-adrenergic blocker. The patient worked as a veterinarian during 1967–1975 and had direct contact with bovine brucellosis (Bang’s disease). In 1975, the patient was diagnosed with brucellosis with positive serum test (standard tube agglutination) for Brucella melitensis.
On physical examination, vital signs were as follows: blood pressure (BP) 180/110 mmHg, pulse rate 105/min, and temperature 37°C. He had pretibial and sacral region edema but otherwise normal physical examination. Laboratory analysis showed erythrocyte sedimentation rate of 93 mm/h, normal C-reactive protein, total serum protein of 40.9 g/l, serum albumin of 17 g/l, total cholesterol of 11.0 mmol/l, antineutrophil antibody (ANCA) titer of 26.8 IU/ml (upper limit) and negative anti-dsDNA and ANA. Serum complement activity (CH50, C3) was decreased. Kidney and liver function, and complete blood count were all normal. Urinalysis showed hematuria, andantineu 24-hour urinary protein was 8.29 g/d. The urine was sterile on culture. Abdominal ultrasonography was normal. The segmental pressure measurements, echocardiography and chest X-ray did not reveal any signs of systemic atherosclerosis.
The patient was hospitalized with the diagnosis of nephrotic syndrome. Recent diagnosis of chronic non-active brucellosis is based on positive Burnet’s intradermal allergy test, negative Brucella complement binding test and negative brucella agglutination test. The renal biopsy showed mesangiocapillary glomerulonephritis. The light microscopy of renal biopsy specimen revealed cellular proliferation in the mesangium, lobular accentuation, mesangial nodules and thickening of the basement membranes. Immunohistochemical examination was positive for IgG and revealed C3 in diffuse granular pattern in the mesangium and the peripheral capillary walls.
The PCR was applied to assess the presence/absence of Brucella spp. in the renal biopsy specimen of the patient. However, no trace of Brucella DNA was found in the renal biopsy of the patient.
2.1 Detection of Brucella spp. using PCR in the renal biopsy specimen
The forward TGGCTCGGTTGCCAATATCAA and reverse CGCGCTTGCCTTTCAGGTCTG primers were used to specifically detect Brucella DNA in the biopsy lysate with amplification product of 223 bp. The primers were previously shown [3] to detect single cells of B. melitensis and B. abortus with specificity restricted to Brucella sp. The PCR reaction was performed starting with 95°C, 15 min for polymerase activation and sample denaturation with subsequent thermal cycling 40 × (94°C, 15 s/58°C, 60 s/72°C, 60 s) and final elongation at 72°C, 10 min. The mixture contained 0.025 U/μl HotStarTaq DNA polymerase (Qiagen), 0.5 μM each primer, 200 μM dNTP, 1.5 mM MgCl2, in 20 mM Tris-HCl, pH 8.8, 10 mM KCl, 10 mM (NH4)2SO4, 0.1% Triton X-100. The Brucella melitensis biovar abortus PCM 2160 strain from Polish Collection of Microorganisms, Institute of Immunology and Experimental Therapy in Wrocław, was used as a standard for verification of the PCR conditions. The biopsy was removed from paraffin by standard xylene/ethanol procedure, followed by Qiagen Protease (56°C, O/N) and T4 lysozyme (37°C, 30 min) digestions. With the experiments performed it was shown that the procedure is capable of detecting even few copies of Brucella DNA in the presence of biopsy lysate containing ∼105 copies of human DNA. No trace of Brucella DNA was found in the renal biopsy of the patient.
Steroid therapy was started with intravenous methylprednisolone pulses (500 mg/day for three consecutive days) followed by oral prednisolone 1 mg/kg per day. In addition, furosemide and an ACE inhibitor were administered. After 4 weeks of treatment edema resolved, serum total protein increased to 50 g/l and 24-hour protein decreased to 6 g/d.
The patient was readmitted in September 2003 due to severe nephrotic syndrome with massive edema of the lower extremities, abdomen and the face. Laboratory findings revealed normal kidney function, anemia with hemoglobin of 10.2 g/dl, erythrocyte sedimentation rate of 67 mm/h, total serum protein of 35.8 g/l, serum albumin level 16 g/l, and 24-hour protein over 20 g/d. ANA and ANCA titers were negative; complement level was decreased. At that time he was treated with 60 mg of oral prednisone and furosemide. Cyclosporine A (CsA) was started due to steroid resistance with target serum concentration of 100–150 ng/ml. The patient improved and was discharged with total protein level of 51 g/l and 24-hour proteinuria of 6 g/d. Slow prednisone taper was planned.
Ambulatory follow-up showed compensated nephrotic syndrome (slight peripheral edemas, total serum protein between 50–55 g/l, proteinuria of 4–6 g/d) between November 2003 and October 2004.
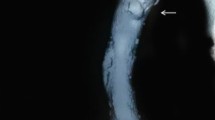
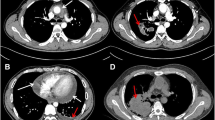
In February 2005, the patient was admitted with strong thoracic pain and back pain and with a BP of 220/120 mmHg. Laboratory findings were remarkable for hemoglobin level 10 g/dl, C-reactive protein (CRP) 275 mg/l, total protein 53 g/l, proteinuria of 5.5 g/d. Thoracic X-ray showed widening of upper mediastinum. Transthoracic and abdominal ultrasound examination showed dissecting aortic aneurysm from the descending thoracic part to the abdominal part down to iliac artery (DeBakey type 3). The decision for endovascular stent-graft repair was made. Under fluoroscopic guidance, the stent-graft was positioned above and below the aneurysmal segment resulting in restoration of the blood flow and disappearance of symptoms (Figs. 1, 2). A febrile state appeared in the postintervention period, most probably due to streptococcal/staphylococcal infection as a complication of intravascular procedure. Even though, many blood cultures were negative, there was an evident link between the procedure and febrile state appearance, and the patient was given IV vancomycin + ceftazidime for 14 days, which resulted in normalization of temperature and inflammatory indices (CRP and leukocyte count). The patient was discharged with normal temperature, BP 135/85 mmHg, serum creatinine concentration of 1.4 mg/dl, total serum protein 59 g/l, albumin 29 g/l, CRP 35 mg/l, proteinuria of 3 g/d.
The long-term follow-up showed normal kidney function, well-controlled BP and partial remission of nephrotic syndrome. In October 2005, proteinuria reached 2.4 g/d and in May 2006 1.5 g/d with simultaneous increase of serum protein concentration; 56 g/l and 78 g/l, respectively.
3 Discussion
Renal involvement in brucellosis is uncommon. Only anecdotal reports of nephropathies exist in the literature. Brucella nephropathy may be secondary to glomerulonephritis (GN), interstitial nephritis, renal vasculitis and granuloma or abscess formation. Membranous glomerulopathy, mesangial proliferative GN, IgA nephropathy and mesangiocapillary GN have been reported [4–10].
Patients with Brucella glomerulonephritis almost always present with urinary sediment abnormalities, proteinuria, and/or azotemia. In our patient, nephrotic syndrome with proteinuria, hematuria, hypoproteinemia and massive edema were detected.
Mesangiocapillary GN may occur primary or secondary to chronic bacterial infections, hematological malignancies or autoimmune diseases [11]. The detection of complement components in the glomeruli in most patients is suggestive of an immune complex-associated pathogenesis. It is likely that chronic antigenemia seen in infectious diseases is especially related to mesangiocapillary glomerulonephritis [11, 12]. Similar to our case situation, low serum complement activity (CH50 and C3 level) at the time of diagnosis are seen in approximately half of the patients with mesangiocapillary GN. In light of all these data, and as our patient had no other etiologic factor that could explain the nephrotic syndrome, we assume that GN in this case was secondary to immune complexes associated with Brucella.
Because of normal CRP, normal temperature, absence of Brucella spp. DNA in the renal biopsy specimen and relatively long time from diagnosis of brucellosis we did not give antimicrobial therapy directed to Brucella spp. Steroid therapy was initiated and CsA had to be introduced after 6 months due to steroid resistance. Administration of CsA in combination with low dose prednisone resulted in proteinuria reduction and recently in partial remission of nephrotic syndrome.
The formation of aneurysms in brucellosis may be due to direct bacterial invasion, embolic occlusion, or injury due to deposition of immune complexes [13]. More than 60% of such aneurysms are infectious in origin [14]. Blain et al. reported a case with thoracic aortic aneurysm and lumbar spondylodiscitis due to Brucella melitensis [15]. Another case, which had a mycotic aneurysm of the ascending aorta complicating discrete subaortic stenosis due to Brucella, was reported by Kumar et al. [16].
The long-standing and uncontrolled hypertension is known causative factor of aortic dissection. However, it is less likely in our patient, that high BP caused dissection of the aneurysm, since BP was well controlled by calcium blocker, ACE inhibitor, and alpha-adrenergic blocker.
Current treatment of dissecting aortic aneurysm is based on endovascular repair or open surgical treatment. Endovascular repair consists of the placement of a stent-graft across the aneurysm. Two randomized trials that compared conventional and endovascular repair showed a lower operative mortality rate for endovascular repair and less frequent complications than with conventional techniques [17]. Insertion of the stent-graft resulted in canalization of blood flow and retraction of aneurysm wall later in the course in our patient.
Antibiotic therapy may decrease symptoms, reduce complications, and prevent relapses of chronic brucellosis [2]. The successful treatment of brucellosis usually requires a prolonged therapy with a combination of antibiotics. Optimal treatment regimens for brucellosis should include at least one agent with good intracellular penetration. Even after following the combination antibiotic regimens, relapse occurs in as many as 5–40% of patients with acute brucellosis in the following year. The type of an antibiotic used, duration of treatment, and combination may have some effect on the relapse rate [2, 18]. A doxycycline–streptomycin combination has long been recommended as the first-line treatment for human brucellosis and is associated with a relapse rate close to 5%. A doxycycline–rifampicin combination seems to be the best alternative to the doxycycline–streptomycin combination [18].
In summary we report a patient with glomerulonephritis and aortic aneurysm probably caused by chronic brucellosis. To our knowledge, this is the first reported case of simultaneous renal disease, aortic aneurysm and chronic brucellosis.
References
Doganay M, Aygen B (2003) Human brucellosis: an overview. Int J Infect Dis 7:173–182
Solera J, Martínez-Alfaro E, Espinosa A, Castillejos ML, Geijo P, Rodríguez-Zapata M (1998) Multivariate model for predicting relapse in human brucellosis. J Infect 36:85–92
Baily GG, Krahn JB, Drasar BS, Stoker NG (1992) Detection of Brucella melitensis and Brucella abortus by DNA amplification. J Trop Med Hyg 95:271–275
Abu Romeh SH, Kozma GN, Johny KV, Sabha M (1987) Brucella endocarditis causing acute renal failure. Nephron 46:388–389
Haririan A, Ghadiri G, Broumand B (1993) Brucella glomerulonephritis. Nephrol Dial Transplant 8:375–376
Eugene M, Gauvain JB, Roux C et al (1987) A case of acute brucellosis with membranous glomerulopathy. Clin Nephrol 28:158–159
Sahin I, Arabaci F, Eminbeyli L, Ilhan M, Onbasi K, Avni Sahin H (2005) Renal involvement in brucellosis. Nephrol Dial Transplant 20(Suppl 5):288 (Abstract)
Elzouki AY, Akthar M, Mirza K (1996) Brucella endocarditis associated with glomerulonephritis and renal vasculitis. Pediatr Nephrol 10:748–751
Dunea G, Kark RM, Lannigan R, D’Alessio D, Muehrcke RC (1969) Brucella nephritis. Ann Intern Med 70:783–790
Altiparmak MR, Pamuk GE, Pamuk ON, Tabak F (2002) Brucella glomerulonephritis: review of the literature and report on the first patient with brucellosis and mesangiocapillary glomerulonephritis. Scand J Infect Dis 34:477–480
Massry SH, Glassock RJ (eds) (1995) Textbook of nephrology. Williams & Wilkins, Baltimore, MD pp. 734–739
Schrier RW, Gottschalk CW (eds) (1993) Diseases of the kidney. Little, Brown and Company, Boston, MA pp. 1815–1838
Mansur AJ, Grinberg M, Leao PP et al (1986) Extracranial mycotic aneurysms in infective endocarditis. Clin Cardiol 9:65–72
Ohmi M, Kikuchi Y, Ito A et al (1990) Superior mesenteric artery aneurysm secondary to infective endocarditis. J Cardiovasc Surg (Torino) 31:115–117
Blain H, Laraki R, levy-Soussan M et al (1997) Aneurysm of thoracic aorta and spondylodiscitis disclosing brucellosis. Rev Med Interne 18:876–881
Kumar N, Prabhakar G, Kandeel M et al (1993) Brucella mycotic aneurysm of ascending aorta complicating discrete subaortic stenosis. Am Heart J 125:1780–1782
Sakalihasan N, Limet R, Defawe OD (2005) Abdominal aortic aneurysm. Lancet 365:1577–1589
Ariza J (1996) Brucellosis. Curr Opin Infect Dis 9:126–131
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kusztal, M., Dorobisz, A., Kuzniar, J. et al. Dissecting aneurysm of the thoracic aorta in a patient with nephrotic syndrome and brucellosis. Int Urol Nephrol 39, 641–645 (2007). https://doi.org/10.1007/s11255-006-9090-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-006-9090-9