Abstract
Urbanisation is a major land use change that introduces novel sources of disturbance and risk into an ecosystem. Successful urban species modify their fear behaviour in response to the new conditions, as evolutionary mismatches between fear responses and environmental conditions are likely to have negative fitness consequences. Here we tested the effect of urbanisation on the fear response of a successful urban coloniser, the Australian Brush-turkey (Alectura lathami), using flight initiation distance (FID) as a measure of boldness. We predicted that Brush-turkeys in areas of natural vegetation would have longer FIDs than birds in more urbanised environments, and that males would have shorter FIDs than females. We recorded the FIDs of 80 Brush-turkeys across the Sydney region. We found that Brush-turkeys in natural vegetation and urban bushland reserves had longer FIDs than birds in urban streets and lawns. However, there was no difference in FID between sites in natural vegetation and urban bushland reserves. There was no difference in FID between male and female Brush-turkeys, between birds engaged in different behaviours, or between birds approached in the breeding and non-breeding seasons. Our results identified that Brush-turkeys displayed reduced fear behaviour in response to humans in more urban environments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urbanisation is a dramatic land use modification that radically alters the physical structure and ecosystem function in the landscapes it affects, with far reaching consequences for the biotic community. Biodiversity declines typically follow urbanisation, leaving a depleted and homogenised suite of species (Clergeau et al. 2006; McKinney 2002; McKinney 2006). For species that remain, the urban environment presents new challenges and opportunities. Urban dwelling animals must contend with built structures replacing natural vegetation, fragmentation and isolation of remaining greenspaces, increased levels of pollutants, potential competition with exotic species, and exposure to anthropogenic sources of disturbance (McKinney 2002; McKinney 2006; Shochat et al. 2006; Taylor and Hochuli 2017). However, species capable of persisting in urban areas may be able to take advantage of vacant niches, new resources, high primary productivity, heterogeneous green spaces, and potential release from natural competitors and predators (Callaghan et al. 2019a; Crooks and Soule 1999; Martin et al. 2010; Møller and Ibáñez-Álamo 2012; Shochat et al. 2006). With urbanisation increasing worldwide (Seto et al. 2012), it is vital to understand the mechanisms by which species become urbanised or fail to persist in order to better manage biodiversity in cities.
Species modify their behaviour in response to environmental change to avoid evolutionary mismatches with potential negative fitness consequences (Sih 2013). For example, failure to avoid novel predators can lead to population declines in native species (Dickman 1996; Sih et al. 2010). Due to the often extreme differences between urban and natural environments, animals in urban areas are, in general, predicted to behave differently to those in natural areas. Successful urban colonising species are expected to have highly plastic or generalist behaviours to increase their chances of survival in variable environments (Callaghan et al. 2019b; Tryjanowski et al. 2016). Behavioural changes in response to urbanisation have been detected in many aspects of animal behaviour across varied taxa (Lowry et al. 2013; Sol et al. 2013). These include changes in foraging behaviour (Sol et al. 2011), reproduction (Beck and Heinsohn 2006), communication (Slabbekoorn and Peet 2003), and habitat use (Stroud et al. 2019).
Fear behaviour has also been shown to be affected by urbanisation. Many novel sources of disturbance exist in cities including foot and vehicular traffic, dog walking, and anthropogenic noise (Banks and Bryant 2007; Mikula 2014). Trade-offs between avoiding disturbance and finding or using resources are key drivers of animal behaviour decisions (Cooper and Frederick 2007; Lima and Dill 1990). Fear behaviour is expected to be optimised as an inappropriate fear response is likely to incur a fitness cost (Fern et al. 2003). Early threat avoidance diverts time and energy away from foraging and reproductive activities, while late avoidance may result in a higher risk of predation (Brown and Kotler 2004; Cooper and Frederick 2007; LaManna and Martin 2016). Human approach is a significant source of disturbance in urban environments and acts as a potential predation risk (Frid and Dill 2002). Responding appropriately to human approach is therefore likely to be a pre-requisite for survival in urban environments.
Flight initiation distance (FID) is a commonly used metric for assessing the fear response of birds. The distance at which the focal animal moves to avoid an approaching human observer is treated as an indicator of boldness and disturbance tolerance (Weston et al. 2012). FID correlates with other aspects of fear behaviour, including scanning rate and alert distance, making it a useful indicator of an animal’s overall weariness (Blumstein 2006). FID is known to vary with the starting distance of the observer, with the life history of the species, and with the abundance or scarcity of resources (Blumstein 2003)(Møller et al. 2013). Species with an omnivorous or carnivorous diet, larger bodied species, and species with a later age of first reproduction tend to have longer FIDs (Blumstein 2006; Weston et al. 2012). Sex may also affect fear behaviour due to life history differences between males and females (Lagos and Herberstein 2017; Magnhagen 1991). Intra-specific variation in FID has previously been used to detect habituation to human disturbance (Fleming and Bateman 2017; Lin et al. 2012; Mikula 2014; Moller 2008; Rodriguez-Prieto et al. 2009), and has been suggested as a tool to support conservation decision making (Weston et al. 2012) and for studying shifts in fear behaviour due to environmental change (Møller et al. 2013).
The Australian Brush-turkey (Alectura lathami) is a ground foraging woodland and forest bird belonging to the family Megapodiidae. Megapodes are unique among birds due to their use of environmental sources of heat to incubate their eggs (Jones and Göth 2008). Brush-turkeys were once rare across the east coast of Australia due to overhunting but have become an increasingly common sight in urban and suburban areas over the last few decades. They have recolonised much of their former range, including major cities including Sydney and Brisbane (Göth et al. 2006; Jones and Everding 1991; Jones et al. 2004). Brush-turkeys have a number of traits which are considered to benefit urban exploiting species. These include a broad climatic range, a high tolerance to disturbance (Blumstein 2003; Weston et al. 2012), and an omnivorous diet (Callaghan et al. 2019b). However, their ground foraging habit, poor flying ability, obligate ground nesting, and the absence of parental care for their chicks are atypical characteristics for an urban exploiting bird (Clergeau et al. 2006; Croci et al. 2008; Moller 2009). The Brush-turkey expansion into urban areas makes them an interesting species for examination of how the behavioural traits of a colonising species adapt in response to urbanisation. The behaviour of male and female Brush-turkeys differ markedly during the breeding season (Jones 1988). Males are likely to experience different sources of disturbance while constructing and defending their nest mounds (Jones and Everding 1991). This presents an opportunity to examine the effect of sex and behavioural states on fear behaviour.
Here we tested the hypothesis that Brush-turkeys in urban areas would be more tolerant of disturbance from humans (shorter FID) than conspecifics in less modified areas due to habituation to human traffic. We tested the effect of habitat type, and within site habitat traits on FID to determine how Brush-turkeys were responding to habitat variation across the land use gradient. We also tested the hypothesis that Brush-turkey males would have shorter FIDs than females, and that male but not female FIDs would vary seasonally. Lastly we tested the hypothesis that Brush-turkeys FIDs would vary along with the initial behaviour of the focal bird.
Methods
Study area and species
The Brush-turkey is a large bird (Females: 1.85-2.67 kg, Males: 2.15-2.75 kg) with a ground foraging habit, making them observable and approachable on foot (Jones and Göth 2008). We recorded the FID of 80 Brush-turkeys at multiple sites across the Sydney region, New South Wales, Australia (Fig. 1) from April to October 2018. Sydney, Australia’s largest city, with a population of over four million with most residents living in expansive suburbs of low to medium density development. The suburbs of Sydney contain an array of greenspaces of managed parks and remnant natural vegetation, while the greater Sydney region is surrounded by national parks containing large stretches of natural vegetation (Keith 2004).
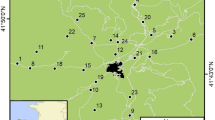
Map showing the distribution of Brush-turkeys sightings in Sydney (hatched area), map of Australia showing the location of Sydney (green box), and 17 sites in Northern Sydney where Brush-turkey FIDs were measured (green circles). FID sites were located in the core area of the Brush-turkey distribution in Sydney. National parkland is shaded in green. Brush-turkey sightings data obtained from the Atlas of Living Australia (Atlas of Living Australia Occurrence Download 2019)
When assessing each FID the site was broadly classified as either urban or natural based on land use. Landscape categories for sites, rather than a gradient approach, were used in order to capture the combination of physical landscape traits and patterns of human use of each site. Natural areas were defined as areas of contiguous relatively undisturbed native vegetation, set aside for conservation outside the urban matrix, and contained within a National Park. Urban sites were defined as being situated within an urban matrix of built on land. For urban sites, the surrounding area within a 10 m radius of the focal bird was further classified as a street, lawn, garden, or reserve. Sub categories of urban sites were defined based on the amount of impervious surface, extent of remnant vegetation, and patterns of human use associated with them. Streets were defined as areas covered with impervious surface with no vegetation cover, and with regular foot and vehicular traffic. Lawns were defined as areas of open grass cover, with little to no canopy or shrub cover. Gardens were defined as areas of privately managed mixed lawn and shrub cover. Reserves were defined as areas of remnant native vegetation situated within the urban matrix, and contained varying levels of grass, shrub and canopy cover.
Measuring FID
FIDs were measured using a standard method (Blumstein (2003). After locating a focal bird, we recorded its sex using visual cues, and classified behaviours into: foraging, nest construction, walking, or idle (Table 1). Date was record, noting the breeding (late July-early Feb.) or non-breeding seasons (late Feb.-early July) (Jones and Göth 2008). Birds engaged in vigilant, alarmed, or aggressive behaviours were not approached. We recorded our starting distance either using a laser rangefinder (Bosch PLR 40 C) or visual estimation, then approached the subject at a rate of 1 m/s (approximately equivalent to two small steps per second). As Brush-turkeys tend to walk or run rather than fly, we recorded the FID, using a laser rangefinder, as the distance at which the focal bird moved to avoid the observer through any form of locomotion. When the focal bird was already moving, an obvious change in the direction of movement or gait was used to determine FID.
Focal birds were not marked and resampling was avoided by approaching only one bird in each location per day. Where Brush-turkeys were located with additional birds, only the FID of a single focal bird was recorded. Brush-turkeys are not an obligate flocking species (Jones and Göth 2008), as such the number of birds present was not assessed. While it is impossible to completely rule out resampling the same individual on different sampling days, the number of birds sampled at each site makes this unlikely, and a small degree of resampling is not expected to influence our results (Runyan et al. 2004).
After each assessment of FID, we scored canopy cover (%), shrub cover (%), grass cover (%), average canopy height (m), average shrub height (m), the number of trees within 10 m of the focal bird’s starting location, and the distance to the nearest tree and shrub from the focal bird’s starting location (m).
Data analysis
Each FID was sorted into one of five pre-defined site categories (street n = 9, lawn n = 5, garden n = 21, urban reserve n = 28, national park n = 17). All analyses were conducted in SPSS version 24. We compared observer start distance among site types using a one-way analysis of variance (ANOVA). As FID was expected to co-vary with start distance (Blumstein 2003), we analysed the effects of site type, sex, season and starting behaviour on FID using analyses of co-variance (ANCOVA), with observer start distance as a covariate. Spearman’s rank correlation was used to compare between FID and all measured habitat traits.
Results
Of the 80 FIDs recorded from individual Brush-turkeys across the Sydney region, 46 were recorded during breeding and 34 were recorded during the non-breeding season. Of the birds approached, 38 were females and 42 were males. We did not detect any significant difference between breeding (mean = 4.235 m, sd = 2.368 m) and non-breeding (mean = 2.999 m, sd = 1.998 m) seasons (ANCOVA, F(1, 78) = 0.804 p = 0.804). We did not detect any significant difference in FID between males (mean = 4.117 m, sd = 2.461 m) and females (mean = 3.26 m, sd = 2.02 m) (ANCOVA, F(1, 78) = 0.432, p > 0.514). Observer start distance varied among habitat type (F(4, 75) = 5.713, p < 0.001). Start distances in national parks were significantly longer than in gardens (p < 0.001) and streets (p = 0.04), but not lawns or reserves (Fig. 2). Start distances in reserves were significantly longer than in gardens (p = 0.015), but not streets and lawns (Fig. 2). There were no significant differences in start distances among streets, lawns or gardens (p > 0.05).
Flight initiation distance (FID) plotted against start distance for all site types. Start distance varied among site types and had a positive linear relationship with FID. Data are pooled for sex and behaviour classes. Icons indicate site type: Blue crosses = garden, grey circles = street, orange triangles = lawn, brown diamonds = reserve, and green squares = natural
After accounting for the effect of start distance as a covariate, we found a significant difference in FID among habitat type (ANCOVA, F(4, 75) = 17.89, p < 0.001). Shorter FIDs were recorded in the highly modified habitats (streets, lawns, and parks) compared to the less modified habitats (reserves and national parks; Fig. 3). Post-hoc Tukey tests revealed no significant difference in average FID among streets, lawns or gardens, or between reserves and national parks (all p > 0.05). There were no significant interactions among behaviour, site type, sex, and season. FIDs were positively correlated with canopy cover (ρ = 0.243, p = 0.03), canopy height (ρ = 0.382, p = 0.001). No other measured habitat trait correlated with FID (ANOVA, p > 0.05). At the time of approach, 18 focal birds were engaged in mound construction, 36 were foraging, 8 were idle, and 18 were walking; we found no effect of bird behaviour on FID (ANCOVA, p > 0.05).
Box-plot of flight initiation distance (FID) habitats assessed. Brush-turkeys in more modified habitats had shorter FIDs than birds in less modified habitats. Error bars show 1.5 x inter-quartile range. Crosses indicate the mean and horizontal lines in the centre of each box indicate the median. Data are pooled for sex and behaviour classes
Discussion
Evaluating how animal behaviour changes in response to urbanisation informs our understanding of how species respond to human induced environmental change. We hypothesised that Brush-turkeys in more highly modified urban habitats would be more tolerant of human disturbance, due to the higher levels of disturbance and human exposure expected in these habitats. We found significantly shorter FIDs in the streets, gardens, and lawns, compared to reserves and national parks. This suggests that Brush-turkeys in more modified habitats have a reduced fear response to human approach and are more tolerant of human disturbance, supporting our hypothesis. As expected, the start distance of the observer also affected FID, however this effect was weaker than the effect of different habitats. Contrary to our hypothesis, neither the sex, season, nor the initial behaviour of the focal bird had any effect on FID.
Shorter FIDs in highly modified habitats likely allow urban dwelling Brush-turkeys to forage and reproduce more efficiently despite higher levels of human disturbance. FID has been shown vary among individuals, populations, and species. Brush-turkeys are thought to be among the least sensitive birds to disturbance relative to body size (Blumstein 2006; Weston et al. 2012). Their reported high disturbance tolerance may make Brush-turkeys better equipped for living in urban environments. Urban dwelling species are commonly found to have shorter FIDs than related rural dwelling species (Moller 2009) suggesting that short FIDs are a common adaptation for success in urban species. While some bold species may be pre-adapted for urban life (McDonnell and Hahs 2015), others develop shorter FIDs following exposure to urbanisation. Brush-turkey FIDs in our study were significantly shorter in more modified, less complex urban habitats, when compared to natural habitat or urban bushland reserves. This may indicate that either the Brush-turkeys in our study developed shorter FIDs following exposure to urbanisation or that bolder and less risk-averse birds are driving the colonisation of urban areas. Short flight distances have been found in urban populations of several bird species when compared to rural populations (Mikula 2014; Rodriguez-Prieto et al. 2009; Van Donselaar et al. 2018). This suggests that Brush-turkey fear behaviour is responding to the changed conditions found in urban habitats, and that shorter FIDs are an acquired trait for the urban Brush-turkey population in Sydney.
Changes in fear behaviour can occur either through learning, plasticity, or adaptation to a new stimulus (Sol et al. 2013). Habituation has been suggested as a mechanism behind shorter FIDs for a suite of urban bird species (Mikula 2014; Rodriguez-Prieto et al. 2009). From our field observations we can say that Brush-turkeys in urban environments experience higher levels of human disturbance than those in natural areas, although this was not quantified. Flexible fear behaviour may be a requirement for species to persist in highly modified and human dominated landscapes like cities (Moller 2009). For Brush-turkeys, within urban areas, encounter rates with humans are higher in streets, lawns, and gardens compared to reserves, presenting a potential source of habituation. Alternatively, dynamic behavioural decisions in response to perceived risks and rewards (Fleming and Bateman 2017; Rodriguez-Prieto et al. 2009) may account for shorter Brush-turkey FIDs in urban areas. Brush-turkeys may respond to humans differently on a fine spatial scale based on an assessment of potential risks and rewards. A human approach in one kind of environment may be treated differently to an approach in another depending on prior experience with humans in each (Fernandez-Juricic et al. 2003; Fleming and Bateman 2017; Van Donselaar et al. 2018). For example, humans observed around lawns or gardens may represent a scavenging opportunity, eliciting a reduced fear response compared to other habitats. Longer FIDs within urban reserves compared to other habitat types within the urban landscape could be reflective of behavioural decision making, habituation, or the interaction of both processes. Further research would be needed to tease apart the effects of each on Brush-turkeys in urban areas.
Brush-turkey males and females typically exhibit markedly different behaviours, especially during the breeding season. Males will spend a large proportion of their time engaged in the construction and maintenance of a nest mound, in addition to their usual foraging behaviour (Jones 1988). They are often reluctant to abandon mound building behaviour when approached (Jones and Göth 2008), and have been suggested to be more likely to habituate to disturbance than females as males remain near their mound rather than relocating (Jones and Everding 1991). This was not evident in our study, as we found no difference in FID between males and females, including with respect to the breeding season. Furthermore, we found no difference in FID between males engaged in mound construction compared to other behaviours. A likely explanation is that males and females are exposed to sufficient levels of human disturbance for habituation despite their different behavioural patterns.
Group size has a potential confounding effect on FID measurements. The dilution hypothesis argues that individual risk decreases as group size increases, resulting in shorter individual FIDs, however the many eyes hypothesis suggests that multiple observers increases overall group vigilance resulting in longer individual FIDs (Lima and Dill 1990). Evidence for the effect of group size is varied and appears to depend on the species and site specific context (Deboelpaep et al. 2018; Morelli et al. 2019). The effect of group size on Brush-turkey FID in this study cannot be determined as group sizes were not recorded. We believe it is unlikely that group size is a major determinant of Brush-turkey FIDs as they are not an obligate flocking species and foraging groups are formed and disintegrate on an ad hoc basis. The effect of group size on fear behaviour may also be smaller than the effect of habituation to human disturbance (Deboelpaep et al. 2018). However further studies are needed to determine how Brush-turkey foraging behaviour is affected by social cues. Selection bias may also have affected our results if bolder birds were more likely to be spotted by observers. We do not think this was the case as the upper range of FIDs recorded were still within easy spotting distance of a trained observer making it unlikely any birds escaped detection.
We found that Brush-turkeys expressed reduced fear behaviour in response to a human approach in less complex, more urbanised, habitats, and that this response was not affected by sex, season, or behavioural state. Our findings suggest that Brush-turkeys appear capable of adjusting their behaviour in response to the varying disturbance conditions within the heterogeneous urban landscape. Whether due to habituation, plasticity, or adaptation, reduced fear of human approach likely facilitates the ongoing colonisation of urban and suburban areas by this species. Studying the fear behaviour of a successful urban coloniser, like the Australian Brush-turkey, provides insight into how species respond to a gradient of environmental change as well as which traits distinguish species that thrive in urban areas from those which are less successful.
References
Atlas of Living Australia Occurrence Download (2019) https://biocache.ala.org.au/occurrences/search?q=lsid%3Aurn%3Alsid%3Abiodiversity.org.au%3Aafd.taxon%3A036fcf6e-6acc-4872-b5ce-e28904c50986&fq=(state%3A%22Australian%20Capital%20Territory%22%20OR%20state%3A%22New%20South%20Wales%22%20OR%20state%3A%22Queensland%22)&fq=cl1048%3A%22Sydney%20Basin%22&fq=occurrence_decade_i%3A%222010%22. Accessed 10/9/2019
Banks PB, Bryant JV (2007) Four-legged friend or foe? Dog walking displaces native birds from natural areas. Biol Lett 3:611
Beck NR, Heinsohn R (2006) Group composition and reproductive success of cooperatively breeding white-winged choughs (Corcorax melanorhamphos) in urban and non-urban habitat. Austral Ecology 31:588–596. https://doi.org/10.1111/j.1442-9993.2006.01589.x
Blumstein DT (2003) Flight-initiation distance in birds is dependent on intruder starting distance. J Wildl Manag 67:852–857. https://doi.org/10.2307/3802692
Blumstein DT (2006) Developing an evolutionary ecology of fear: how life history and natural history traits affect disturbance tolerance in birds. Anim Behav 71:389–399. https://doi.org/10.1016/j.anbehav.2005.05.010
Brown JS, Kotler BP (2004) Hazardous duty pay and the foraging cost of predation. Ecol Lett 7:999–1014. https://doi.org/10.1111/j.1461-0248.2004.00661.x
Callaghan CT, Bino G, Major RE, Martin JM, Lyons MB, Kingsford RT (2019a) Heterogeneous urban green areas are bird diversity hotspots: insights using continental-scale citizen science data. Landsc Ecol 34:1231–1246. https://doi.org/10.1007/s10980-019-00851-6
Callaghan CT, Major RE, Wilshire JH, Martin JM, Kingsford RT, Cornwell WK (2019b) Generalists are the most urban-tolerant of birds: a phylogenetically controlled analysis of ecological and life history traits using a novel continuous measure of bird responses to urbanization. Oikos. https://doi.org/10.1111/oik.06158
Clergeau P, Croci S, Jokimäki J, Kaisanlahti-Jokimäki M-L, Dinetti M (2006) Avifauna homogenisation by urbanisation: analysis at different European latitudes. Biol Conserv 127:336–344. https://doi.org/10.1016/j.biocon.2005.06.035
Cooper WE, Frederick WG (2007) Optimal flight initiation distance. J Theor Biol 244:59–67. https://doi.org/10.1016/j.jtbi.2006.07.011
Croci S, Butet A, Clergeau P (2008) Does urbanization filter birds on the basis of their biological traits. Condor 110:223–240. https://doi.org/10.1525/cond.2008.8409
Crooks KR, Soule ME (1999) Mesopredator release and avifaunal extinctions in a fragmented system. Nature 400:563–566. https://doi.org/10.1038/23028
Deboelpaep E, Keleman P-J, Vanschoenwinkel B, Koedam N (2018) Gallant geese, fearful flocks? Flock size and heterospecifics alter the escape behaviour of an invasive goose. Belg J Zool 148:135–147. https://doi.org/10.26496/bjz.2018.23
Dickman CR (1996) Impact of exotic generalist predators on the native fauna of Australia. Wildl Biol 2:185–195
Fern et al (2003) Testing the risk-disturbance hypothesis in a fragmented landscape: nonlinear responses of house sparrows to humans. Condor 105:316–326
Fernandez-Juricic E, Sallent A, Sanz R, Rodriguez-Prieto I (2003) Testing the risk-disturbance hypothesis in a fragmented landscape: nonlinear responses of house sparrows to humans. Condor 105:316–326
Fleming PA, Bateman PW (2017) Scavenging opportunities modulate escape responses over a small geographic scale. Ethology 123:205–212. https://doi.org/10.1111/eth.12587
Frid A, Dill L (2002) Human-caused disturbance stimuli as a form of predation risk. Conserv Ecol 6:11
Göth A, Nicol KP, Ross G, Shields JJ (2006) Present and past distribution of Australian brush-turkeys Alectura lathami in New South Wales - implications for management. Pac Conserv Biol 12:22–30
Jones DN (1988) Construction and maintenance of the incubation mounds of the Australian brush-Turkey Alectura lathami. EMU 88:210–218. https://doi.org/10.1071/MU9880210
Jones DN, Everding SE (1991) Australian brush-turkeys in a suburban environment: implications for conflict and conservation. Wildl Res 18:285–297. https://doi.org/10.1071/WR9910285
Jones DN, Göth A (2008) Mound-builders. CSIRO Pub, Collingwood, Vic
Jones DN, Sonnenburg R, Sinden KE (2004) Presence and distribution of Australian Brushturkeys in the greater Brisbane region. Sunbird 34:1–9
Keith DA (2004) Ocean shores to desert dunes: the native vegetation of New South Wales and the ACT. Department of Environment and Conservation (NSW), Hurstville
Lagos PA, Herberstein ME (2017) Are males more scared of predators? Differential change in metabolic rate between males and females under predation risk. Physiol Behav 173:110–115. https://doi.org/10.1016/j.physbeh.2017.02.002
LaManna JA, Martin TE (2016) Costs of fear: behavioural and life-history responses to risk and their demographic consequences vary across species. Ecol Lett 19:403–413. https://doi.org/10.1111/ele.12573
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640. https://doi.org/10.1139/z90-092
Lin T, Coppack T, Lin Q-x, Kulemeyer C, Schmidt A, Behm H, Luo T (2012) Does avian flight initiation distance indicate tolerance towards urban disturbance? Ecol Indic 15:30–35. https://doi.org/10.1016/j.ecolind.2011.09.018
Lowry H, Lill A, Wong BBM (2013) Behavioural responses of wildlife to urban environments. Biol Rev 88:537–549. https://doi.org/10.1111/brv.12012
Magnhagen C (1991) Predation risk as a cost of reproduction. Trends Ecol Evol 6:183–186. https://doi.org/10.1016/0169-5347(91)90210-O
Martin J, French K, Major R (2010) Population and breeding trends of an urban coloniser: the Australian white ibis. Wildl Res 37:230–239. https://doi.org/10.1071/WR10047
McDonnell MJ, Hahs AK (2015) Adaptation and Adaptedness of organisms to urban environments. Annu Rev Ecol Evol Syst 46:261–280. https://doi.org/10.1146/annurev-ecolsys-112414-054258
McKinney ML (2002) Urbanization, biodiversity, and conservation. BioScience 52:883–890. https://doi.org/10.1641/0006-3568(2002)052[0883:UBAC]2.0.CO;2
McKinney ML (2006) Urbanization as a major cause of biotic homogenization. Biol Conserv 127:247–260. https://doi.org/10.1016/j.biocon.2005.09.005
Mikula P (2014) Pedestrian density influences flight distances of urban birds. Ardea 102:53–60. https://doi.org/10.5253/078.102.0105
Moller AP (2008) Flight distance of urban birds, predation, and selection for urban life. Behav Ecol Sociobiol 63:63–75
Moller AP (2009) Successful City dwellers: a comparative study of the ecological characteristics of urban birds in the Western Palearctic. Oecologia 159:849–858
Møller AP, Ibáñez-Álamo JD (2012) Escape behaviour of birds provides evidence of predation being involved in urbanization. Anim Behav 84:341–348. https://doi.org/10.1016/j.anbehav.2012.04.030
Møller AP, Grim T, Ibáñez-Álamo JD, Markó G, Tryjanowski P (2013) Change in flight initiation distance between urban and rural habitats following a cold winter. Behav Ecol 24:1211–1217. https://doi.org/10.1093/beheco/art054
Morelli F et al (2019) Contagious fear: escape behavior increases with flock size in European gregarious birds. Ecol Evol 9:6096–6104. https://doi.org/10.1002/ece3.5193
Rodriguez-Prieto I, Fernández-Juricic E, Martín J, Regis Y (2009) Antipredator behavior in blackbirds: habituation complements risk allocation. Behav Ecol 20:371–377. https://doi.org/10.1093/beheco/arn151
Runyan AM, Blumstein DT, Russell (2004) Do individual differences influence flight initiation distance? J Wildl Manag 68:1124–1129. https://doi.org/10.2193/0022-541X(2004)068[1124:DIDIFI]2.0.CO;2
Seto KC, Güneralp B, Hutyra LR (2012) Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc Natl Acad Sci U S A 109:16083–16088
Shochat E, Warren PS, Faeth SH, McIntyre NE, Hope D (2006) From patterns to emerging processes in mechanistic urban ecology. Trends Ecol Evol 21:186–191. https://doi.org/10.1016/j.tree.2005.11.019
Sih A (2013) Understanding variation in behavioural responses to human-induced rapid environmental change: a conceptual overview. Anim Behav 85:1077–1088. https://doi.org/10.1016/j.anbehav.2013.02.017
Sih A et al (2010) Predator–prey naïveté, antipredator behavior, and the ecology of predator invasions. Oikos 119:610–621. https://doi.org/10.1111/j.1600-0706.2009.18039.x
Slabbekoorn H, Peet M (2003) Birds sing at a higher pitch in urban noise. Nature 424:267
Sol D, Griffin AS, Bartomeus I, Boyce H (2011) Exploring or avoiding novel food resources? The novelty conflict in an invasive bird. PLoS One 6. https://doi.org/10.1371/journal.pone.0019535
Sol D, Lapiedra O, González-Lagos C (2013) Behavioural adjustments for a life in the city. Anim Behav 85:1101–1112. https://doi.org/10.1016/j.anbehav.2013.01.023
Stroud JT et al (2019) Behavioral shifts with urbanization may facilitate biological invasion of a widespread lizard. Urban Ecosyst. https://doi.org/10.1007/s11252-019-0831-9
Taylor L, Hochuli DF (2017) Defining greenspace: multiple uses across multiple disciplines. Landsc Urban Plan 158:25–38. https://doi.org/10.1016/j.landurbplan.2016.09.024
Tryjanowski P et al (2016) Urbanization affects neophilia and risk-taking at bird-feeders. Sci Rep (Nature Publisher Group) 6:28575. https://doi.org/10.1038/srep28575
Van Donselaar JL, Atma JL, Kruyf ZA, LaCroix HN, Proppe DS (2018) Urbanization alters fear behavior in black-capped chickadees. Urban Ecosyst 21:1043–1051. https://doi.org/10.1007/s11252-018-0783-5
Weston MA, McLeod EM, Blumstein DT, Guay P-J (2012) A review of flight-initiation distances and their application to managing disturbance to Australian birds. Emu 112:269–286. https://doi.org/10.1071/MU12026
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Research was undertaken under scientific licence SL101960 authorised by the National Parks and Wildlife Service, NSW, Australia and animal ethics approval 4a817 authorised by the Taronga Conservation Society.
Conflict of interest
The authors declare they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Hall, M.J., Burns, A.L., Martin, J.M. et al. Flight initiation distance changes across landscapes and habitats in a successful urban coloniser. Urban Ecosyst 23, 785–791 (2020). https://doi.org/10.1007/s11252-020-00969-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-020-00969-5