Abstract
Urban forest is considered as the most important component of urban green infrastructure and can make vital contributions to urban biodiversity. Understanding the species composition and diversity of urban forest is important for urban biodiversity enrichment. In this study, we evaluated the urban forest woody plant diversity and spatial pattern in Changchun, northeast China. The differentiations in species composition and diversity among types of urban forests and gradients of urbanization were then explored. Principal Component Analysis (PCA) was performed to characterize the species distribution. Similarity Percentage (SIMPER) analysis was adopted to determine the species differentiation and the main contributed species among different urban forest types and different urbanization gradients. The results showed that urban forest species in Changchun were abundant with 88 species that belonged to 50 genera and 24 families. The three major species were Salix matsudana, Populus davidiana, and Pinus sylvestris var. mongolica. Native species were preferred in urban forest of Changchun. Significant differentiations in species composition were observed among different types of urban forests. Attached forest (AF) had the highest species richness and biodiversity, whereas production and management forest (PF) had the lowest ones. SIMPER results showed the highest species dissimilarity between AF and PF and lowest species dissimilarity between AF and landscape and relaxation forest (LF). For different urbanization gradients, species richness and diversity in the third ring were the highest and in the first ring were the lowest. SIMPER results showed the lowest species dissimilarity between the third ring and the fourth ring and highest species dissimilarity between the first ring and the fourth ring. Based on the 10/20/30 “rule of thumb”, the diversity must be urgently increased at the species, genus, and family levels for EF and PF. For different urbanization gradients, diversity should be increased at the species level in the first ring and the fifth ring, and should be increased at the family level in the second ring and the fifth ring.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urban forests are essential components of urban ecosystems and offer direct and indirect ecosystem services (Jim and Zhang 2013). These services include improvement in air quality (Yang et al. 2005), carbon sequestration (McPherson et al. 2013; Nowak et al. 2013), reduction of storm water runoff (Inkilainen et al. 2013; Xiao and McPherson 2011; Zhang et al. 2012), and mitigation of heat island effect (Li et al. 2013; Ren et al. 2013). More importantly, urban forests also provide habitats for various organisms and increase urban species diversity (Conway and Bourne 2013). Urban biodiversity plays a crucial role in the functioning and the stability maintenance of urban ecosystems (Zhang and Jim 2014a). A higher level of species diversity provides protection from catastrophic loss to pests and diseases and superior security against environmental shifts, such as those caused by climate change (Kendal et al. 2014). Advanced levels of urban species diversity could be also useful for further complex ecosystem function (Jim and Liu 2001). Therefore, species diversity is recognized as a key component of strategic urban forest management (Kenney et al. 2011).
Despite widespread recognition of the significance of urban forests biodiversity (Kendal et al. 2014; Sjoman et al. 2012), almost no studies have been found in Northeast China according to our knowledge. Most studies just focused on species diversity of street trees (Nagendra and Gopal 2010), public housing estates (Zhang and Jim 2014a), domestic gardens (Zhang and Jim 2014b), and landscape trees (Jim and Chen 2009). However, few studies have investigated comprehensive analysis and comparison of the species differentiation among different types of urban forests.
A number of other ecological studies have addressed the factors that influence urban species biodiversity, which included different types of land uses (Andersson et al. 2007; Colding 2007; Andersson and Colding 2014; Vakhlamova et al. 2014), urban building forms (Andersson and Colding 2014), and socioeconomic factors (Clarke et al. 2013; Golding et al. 2010; Hope et al. 2003). However, the increasing progress of urbanization and its associated land-cover change lead to species habitat fragmentation and biodiversity loss (McDonald et al. 2008; McKinney 2008; Goddard et al. 2010; Seto et al. 2012). There have been few studies on the effects of urbanization on species composition and diversity of urban forests, which mainly focused on several cities of Europe, North America, Australia, and Central Asia (Vakhlamova et al. 2014). Related research in China has received little attention, especially in Northeast China.
Changchun, as a typical city in northeast China, has experienced an accelerated urbanization since 1979. Increases in the urban population and urban impervious area have accelerated in recent decades (Huang et al. 2009), which resulted in dramatic changes of urban forest structures and species composition. Changes in species diversity along with the urbanization gradients remain unknown. An understanding of species composition and differentiation during the progress of urbanization is essential to develop strategies for improving urban forest management and enriching urban biodiversity. This study aims to (1) determine the species composition, richness, and importance value (IV) of the urban forests in Changchun, (2) determine the species differentiation among different types of urban forests and the main contributing species, and (3) understand the effects of urbanization on the species diversity and the differentiation.
Methods
Study area
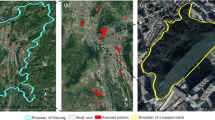
Changchun is the capital city of Jilin Province in northeast China. The climate is a continental monsoon climate of North Temperate Zone, with distinct seasons and rain, which is characterized by wet moderate climate (Ren et al. 2013). The annual average temperature is 4.8 °C and the annual average precipitation is 567 mm. Our study area is primarily located within the fifth ring road of Changchun, with an area of 524 km2 (125°07′ E to 125°26′ E, 43°44′ N to 44°02′N; Fig. 1) and a total population of 3.63 million. The forest resources of Changchun are not rich. The woodland area is lower than that of the provincial and the national average level. The forest land types are mainly composed of coniferous and broad-leaved deciduous trees.
Urbanization gradients definition and urban forest classification
Changchun City expanded gradually from the city center to the fringe, and the main urban area had increased from 90 km2 in 1978 to 365 km2 in 2010 (Huang et al. 2009; Li et al. 2012). Urbanization gradients were defined by different ring roads from the first ring to the fifth ring based on the urban development process. Urban forests were classified into five types based on their location, function, and management objectives (He et al. 2004; Liu and Li 2012): (1) road forest (RF), trees along railroads, highways, boulevards, roads, and streets, (2) attached forest (AF), trees next to buildings in school yards, campuses, hospitals, commercial and business districts, industrial areas and residential areas, (3) landscape and relaxation forest (LF), trees in the public parks, forest parks, historic sites, and scenic areas, (4) ecological and public welfare forest (EF), trees next to river bank and farmland, (5) production and management forest (PF), trees in the nurseries, orchards, plantations, and wood-lands.
Field survey
In this study, we adopted a stratified random sampling method to allocate the plot. The number of plots for different types of urban forests were further determined according to the tree cover of each forest type (Liu and Li 2012; Nowak et al. 2003). The total number of plots was 331, which included 123 plots for RF, 96 plots for AF, 80 plots for LF, 20 plots for EF, and 12 plots for PF (Fig. 2). Field surveys were conducted from July 2012 to October 2012, and each plot was 400 m2. Meanwhile, we recorded 25 plots for the first ring, 50 plots for the second ring, 106 plots for the third ring, 71 plots for the fourth ring, and 79 plots for the fifth ring for different urbanization gradients. All woody plants were recorded for each plot. The species were grouped into six classes according to the literatures (Jim 2013, 2014), namely, signature (>200 trees/species), dominant (100 to 199 trees/species), common (50 to 99 trees/species), occasional (10 to 49 trees/species), rare (2 to 9 trees/species), and solitary (1 tree/species).
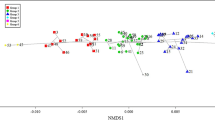
PCA showing richness and distribution of the forty best fitted species among different types of urban forests. Axis1 accounted for 60 % of the variables, and Axis2 accounted for 20 % of the variables. Refer to Appendix Table 8 for the abbreviated species names
Data analysis
Species diversity indices were calculated for each type of urban forest and each gradient of urbanization to compare the species diversity among five urban forest types and among different urbanization gradients. These indices include Berger - Parker species richness index (d), Shannon - Wiener species diversity index (H′), evenness index (J), and species Importance Value (IV).
-
Berger-Parker index: \( d=1/\frac{{\mathrm{n}}_{\max }}{N} \) (Berger and Parker 1970)
-
Shannon–Wiener index: \( {H}^{\prime }=-\sum_{i=1}^SR{A}_i\left( \ln\ R{A}_i\right) \) (Shannon and Weaver 1963; Magurran 1988)
-
Evenness index: \( J=\frac{H^{\prime }}{ \ln \kern0.15em (s)} \) (Pielou 1966)
-
Importance value: \( I{V}_i=\frac{\left(R{A}_i+R{D}_i+RF{Q}_i\right)\times 100}{3} \) (Ellenberg and Mueller-Dombois 1974)
Where S is the total number of species in each forest type or each urbanization gradient; n max is the number of the most common species; N is the total number of individuals; RA is relative abundance, that is, RA i represents number of trees for species i/total number of trees; RD is relative dominance, that is, RD i represents basal area at breast height for each species i/ total basal area; and RFQ is relative frequency, that is, RFQ i represents number of plots for species i/total number of plots.
Principal Component Analysis (PCA) was applied to assess the effects of urban forest type and urbanization on species composition (Austin 2013; McCune et al. 2002). Prior to PCA analysis, the species abundance was log-transformed. The final best solution, as 2-D recommended, represented the highest percentage of variance in species data.
Similarity Percentage (SIMPER) analysis was adopted to assess the species differentiation among different forest types and urbanization gradients. Prior to SIMPER analysis, the IVs of species were square root transformed and standardized by normalization. SIMPER analysis was used to examine the contribution of each species to the average Bray-Curtis dissimilarity between different types of urban forests and the contribution of each species to the average Bray-Curtis dissimilarity among different gradients of urbanization.
SIMPER analysis was performed with PRIMER version 5.0 (Primer-E Ltd., Roborough, UK). PCA ordination was performed with Canoco 5.0 (Centre for Biometry, Wageningen, The Netherlands).
Results
Total species composition and importance value
In this study, 7778 woody plants were surveyed and 88 species were identified, with 66 species of trees and 22 species of shrubs. These species belonged to 50 genera and 24 families. Among tree species, there were 51 broad-leaved deciduous species and 15 coniferous species. Table 1 showed that the contributions of individual species were highly heterogeneous. The 28 occasional species had the highest species number, which accounted for 31.81 % of the total species and only 10.29 % of trees. The dominant group ranked the second and contained 16 species and 2443 trees which comprised 18.18 % of species and 31.41 % of trees. The 14 species in common group contributed 15.91 % of species and 13.10 % of trees. The same species count in rare group only accounted for 1.18 % of trees. The signature category encompassed only 10 species but accounted for 43.94 % of trees. Among which S. matsudana, P. davidiana, P. sylvestris var. mongolica, Picea asperata, Armeniaca mandshurica, and Pinus tabulaefomis var. mukdensis were the most important six species. The solitary category had only six species and about 0.08 % of trees.
Species composition was heavily biased toward natives. A total of 80 species was native and furnished 7183 trees, contributing 90.91 % species and 92.35 % trees (Table 1). Only eight species were exotic with 595 trees, contributing to less than 10 % species and trees. The notable exotic species were Ulmus densa, Acer negundo, and Populus x Canadensis of the dominant group, Rhus typhina, Robini apseudoacacia, Pinus sylvestris var. sylvestriformis of occasional group, and Pinus banksiana, Amorpha fruticosa of rare group.
For RA, RFQ, RD, and IV, the signature group had the highest values, followed by dominant, common, occasional, rare, and solitary (Table 1). Only S. matsudana and P. davidiana had the IV of nearly 10 %, whereas the other species were all less than 5.0 % (Table 1).
Species composition, diversity, and dissimilarity by types of urban forests
The result of Principal Component Analysis (PCA) showed that the first and second axes accounted for 60 % and 20 % of total variance, respectively (Fig. 2). Species distribution was highly uneven among five types of urban forests. PCA showed that more species dwelt in AF and LF than in RF, EF, and PF (Fig. 2). AF had the highest species richness with 75 species, followed by LF, RF, and EF with 72, 56, and 38 species, respectively. Moreover, PF had the lowest species richness with only 16 species. Among the forty best fitted species (Fig. 2), the richness of Salix babylonica,Quercus mongolica, Acer mono, Syringa oblata, Picea asperata, Betula platyphylla, Ulmus pumila, A. mandshurica, P. tabulaefomis mukdensis, Amygdalus davidiana, Ligustrum quihoui, and Prunus ussuriensis in AF were higher than those in other types of urban forests. The richness of P. amurense, Amygdalus triloba multiplex, Malus baccata, Pinus koraiensis, Larix olgensis, Picea wilsonii, Larix gmelinii, Viburnum macrocephalum, Fraxinus mandshurica, Abies nephrolepis, Padus racemosa, Rosa xanthina, Syringa villosa, Tilia amurensis, and Acer triflorum were the highest in LF. The richness of Ulmus pumila cv. tenue, Caragana arborescens, Lonicera maackii, Robinia pseudoacacia, Weigela florida, Syringa reticulata, S. matsudana, Prunus ceraifera, Amygdalus triloba, Swida alba, and P. davidiana were the highest in RF (Fig. 2).
SIMPER analysis was used to assess the species differentiation by types of urban forests (Table 2). The results indicated that PF had the highest dissimilarity with the other four types with the percent of greater than fifty. The lowest dissimilarity was detected between AF and LF with the percent of 25.34. Meanwhile, species diversity indices of the different types of urban forests were also caculated (Table 3). The results of Shannon–Wiener index, Evenness index, and Berger–Parker index were all higher for AF, LF and RF than those for EF and PF. AF had the highest Shannon–Wiener index, Evenness index, and Berger-Parker index with 3.8, 0.8, and 14.93, respectively. In contrast, EF had the lowest Evenness index and Berger-Parker index with 0.77 and 2.83, respectively. However, PF had the lowest Shannon-Wiener index with 2.31.
Species composition, diversity, and dissimilarity by gradients of urbanization
Compared with different types of urban forests, species distribution among different urbanization gradients were less uneven. The species richness unexpectedly increased from the first ring to the third ring and then decreased from the third ring to the fifth ring. The third ring had the most species (76), closely followed by the fourth ring (63). However, the first ring had the lowest species with only 42 species (Fig. 3). The result of PCA showed that the two axes accounted for 70 % of total variance. Among the forty best fitted species (Fig. 3), P. wilsonii, L. olgensis, P. suffruticosa, P. sylvestris var. sylvestriformis (in the fourth quadrant of Fig. 3) had the higher richness in the first ring than those in other rings. A. davidiana, Malus asiatica, P. sylvestris mongolica had the highest richness in the fourth ring. Species which were in the third quadrant had the highest richness in the third ring.
PCA showing richness and distribution of the forty best fitted species among the different gradients of urbanization. Axis1 accounted for 48 % of the variables, and Axis2 accounted for 22 % of the variables. Refer to Appendix Table 8 for the abbreviated species names
Spatial differentiation of urban forest species by gradients of urbanization was also analyzed with SIMPER analysis (Table 4). The first ring had the highest species dissimilarity compared with other rings, which were 37.75 % with the second ring, 37.29 % with the third ring, 44.71 % with the fourth ring, and 43.76 % with the fifth ring. The lowest dissimilarity was detected between the third ring and the fourth ring with the percent of 21.90. Furthermore, the dissimilarity between the fourth and fifth ring and between the third and fifth ring both were lower than 25 %.
Species diversity indices were depicted in Table 5. The highest Shannon–Wiener index was found in the third ring with the value of 3.97, followed by the fourth ring (3.76), the second ring (3.70), the fifth ring (3.61), and the first ring (3.42). Berger–Parker index for different rings had the same trend with the Shannon–Wiener index. These had a similar trend with the species richness.
Discussion
Total species composition and exotic species
Species richness is an important indicator to measure biodiversity level. In this study, 88 species had been identified, which was less than 173 species of the nearby city of Shenyang (Liu and Li 2012). Moreover, other Chinese cities such as Guangzhou and Taipei had 254 and 164 species, respectively (Jim and Chen 2008; Jim and Liu 2001). The species richness of urban forests in Changchun was low. This phenomen may be closely related to the regional climate and geographical characteristics.
A high diversity of species had been considered as a key requirement for a healthy and sustainable urban tree population (Sjoman et al. 2012). To manage and improve species diversity, the 10/20/30 “rule of thumb” which has been widely accepted states that urban forests should comprise no more than 10 % of any particular species, 20 % of any one genus, or 30 % of any single family (Santamour 1990; Kendal et al. 2014). In our study, the RA of the species, genus, and family levels were in accordance with the “rule of thumb” (Fig. 4a). The four most signature species comprised 23.7 % of the total population (Table 1), which was lower than the landscape trees in public housing estates in Hong Kong where the four most signature species comprised 28 % of the population (Zhang and Jim 2014a). The population was also lower than that of the urban trees in Chicago and Bangkok where the top four species comprise nearly and over half of the total trees, respectively (McPherson et al. 1997; Thaiutsa et al. 2008). This finding indicated that a preference for certain groups was not evident in Changchun urban forests.
a Relative abundance of the most common trees at the species, genus and family levels for the total trees; b Relative abundance of the most common trees at the species, genus and family levels for different types of urban forests; c Relative abundance of the most common trees at the species, genus and family levels for different gradients of urbanization
Urban areas are hot spots for intentional and unintentional introduction and naturalization of exotic species (McKinney 2006). In this study, urban forest contained 90.91 % native species and 9.09 % exotic species (Table 1). The results were higher than 29.7 % native species in Hong Kong public housing estates (Zhang and Jim 2014a), 25 % of native species in Kigali (Seburanga et al. 2014), 61 % and 32 % of native tree species in residential neighborhoods and in newly developed sites of Rome, respectively (Celesti-Grapow et al. 2006), and 47 % of native taxa in central European cities (Lososova et al. 2012). Compared with the preceding findings, the results of this study reflected the rather earnest preference for native speices in the urban forests of Changchun.
Species diversity and differentiation among types of urban forests
Within a city, species composition varied significantly among different land uses and habitats (Jim and Liu 2001). Some researchers determined that the current diversity of species in cities and the loss in density of species were best explained by anthropogenic features (Aronson et al. 2014). The combination of urban forest management systems and habitat conditions and their changes through time had engendered distinctive forest types in terms of ecological, amenity, and environmental functions (Jim and Liu 2001). In this study, five types of urban forests had significant differences in species distribution. Individual attitudes and management practices strongly influenced the urban forest species diversity and composition in Changchun (Zhang and Jim 2014b). Tree planting and management of AF are usually decided by estate agencies or enterprises and institutions who will entrust the greening project to the landscaping company. As special ornamental tree species, colored leaf trees (e.g., Q. mongolica, B. platyphylla, A. mono, U. densa, A. mandshurica and P. ussuriensis) have been increasingly applied in AF to increase the degree of beauty. In addition, building age and house prices may be the main influenced factors for the diversity of residential areas. Moreover, the campus usually has a rich vairety of species which contributed to the high species diversity of AF.
As LF, trees planting and management are manily determined by regional municipal bureau of landscape and forestry. Ornamental tree species with beautiful crown and colorful leaf are more prefered by garden workers, for example, P. koraiensis, A. nephrolepis, L. olgensis, Phellodendron amurense, F. mandschurica. LF, where biodiversity hotspots of the city were, had similar species richness and diversity with AF. But its area was only one of the third of AF in Changchun. The similar ecological functions contributed to the lowest dissimilarity between AF and LF. SIMPER analysis indicated that P. dabidiana, P. x canadensis, P. sylvestris var. sylvestriformis, P. sylvestris var. mongolica, and F. mandshurica were the primary contributing species to the dissimilarity between AF and LF (Table 6). The RA of most common species, genus, and family for the AF and LF trees were all lower than the rule of thumb proposed by Santamour (1990) (Fig. 4b). This indicated that AF and LF trees exhibited a quite healthy diversity.
Road trees play an important role in mitigating the intensity of urban heat island effects, filtering noise and capturing higher amounts of air pollutants (Chow and Roth 2006; McPherson et al. 1997; Nagendra and Gopal 2010). Thus, the selection of RF trees must be based on the characteristics of environmental amilioration, sun shading, strong adaptation, and reduction of air and noise pollution (Jim and Liu 2001). Populus species were prefered by the urban forest managers and made up 20.9 % of the RF trees. Characteristics of Populus species with fast growing, and wide and compact crown, make them well-adapted for RF. Despite the RA of the most common species was lower than 10 %, the RA of the most common genus and family was higher than the rule of thumb proposed by Santamour (1990). These contributed to the lower Shannon–Wiener index, Evenness index, and Berger-Parker index than AF and LF (Table 2). SIMPER analysis indicated that P. dabidiana, P. x canadensis, L. gmelinii, Populus alba var. pyramidalis, Pinus tabuliformis were the main contributors for the dissimilarity between RF and AF (Table 6). P. dabidiana, P. amurense, P. koraiensis, Malus baccata, U. pumila cv. tenue were the main contributors for the dissimilarity between RF and LF (Table 6).
The trees of EF are mainly distributed along the river banks and farmland shelterbelts. Populus dabidiana was widely used in EF for its better windbreak and sand-fixation and soil and water conservation function. For PF, it was the nursery for some common species, e.g., Pinus, Picea, Populus, Salix, and several ornamental shrubs. The RA of most common species, genus, and family for the EF and PF were all apparently higher than the rule of thumb proposed by Santamour (1990) (Table 4b). Thus, EF and PF will be vulnerable to suffer from catastrophic diseases and insect pests in the same area at the same time (Kendal et al. 2014). Four signature species, namely, P. davidiana, P. tabulaefomis var. mukdensis, Q. mongolica, and A. davidiana, five dominant species, namely, U. pumila, A. negun, S. babylonica, Catalpa ovata, and P. x canadensis, and two occasional species, namely, M. prunifolia and Juniperus rigida, mainly accounted for the dissimilarity between PF and other four types of urban forests. Through the above discussion, understanding the species differentiation of types of urban forests is of great importance and necessity for species selection and optimization of different types of urban forests.
Urbanization effects on species composition and diversity
Positive correlation had been found between species richness and regional-level of urbanization (Ricketts and Imhoff 2003). Some researchers believed that urban areas had extremely high spatial habitat heterogeneity (Savard et al. 2000; Thompson et al. 2003). This level of spatial habitat heterogeneity can produce very high levels of beta diversity (Niemela 1999) and great species richness than those of surrounding rural areas (Wania et al. 2006). Some studies found that plant species richness was higher in suburban areas than in urban and rural areas (Kent et al. 1999; Zerbe et al. 2003). Suburban areas were extremely heterogeneous given that urban and rural landscape components intermix at these areas (Schmidt et al. 2014). In our study, the result showed that species richness and diversity in the urban area (from the first ring to the third ring) were higher than those in the fourth ring and the fifth ring, which was in accordance with the conclusion of the former researchers (Niemela 1999; Wania et al. 2006). Species richness and diversity were the lowest in the urban core (the first ring). This anomoly was probably due to low urban forest cover (Fig. 5) and high density of commercial district, which also contributed to the higher species dissimilarity with the remaining gradients. Two signature species, namely, P. davidiana and P. tabulaefomis var. mukdensis, six dominant species, namely, P. racemosa, S. babylonica, L. gmelinii, P. x canadensis, A. triflorum, and A. monoand, and three occasional species, namely, Paeonia suffruticosa, P. tabuliformis, and P. sylvestris var. sylvestriformis, were the main contributors to the dissimilarity between the first ring and the other rings (Table 7).
The RA in the first ring and the fifth ring had a slightly higher value than 10 % at the species level, whereas the second ring and the fifth ring had a slightly higher value than 30 % at the family level specified by the rule of thumb (Fig. 4c). These results indicated that diversity should be increased at the species level in the first ring and the fifth ring, and the species diversity should be increased at the family level in the second ring and the fifth ring. The species compositions in other gradients were relatively healthy. AF and LF were the dominant types of urban forests in the third ring which contributed to the highest species richness and diversity in different rings (Figs. 5 and 6). The species diversity was lower in the fourth and the fifth ring than that in the third ring (Table 4). This mainly because parts of these regions were in the process of development and construction, and the green infrastructure was still incomplete. Thus, there will be great potential to enrich the species diversity in these areas. An understanding of the species diversity and main contributors to the differentiation among gradients of urbanization is helpful to increase the diversity and maintain urban ecosystem stability by selecting appropriate species.
Conclusion and implications
Urban forest species diversity plays a vital role in the stability and function of urban ecosystem. This study determined the urban forest species composition, and explored the effects of forest type and urbanization on species composition and differentiation of urban forest in Changchun. The conclusion and implications were as follows:
-
(1)
The species richness of urban forest in Changchun is lower than the southern cities, which mainly was due to the regional cold climate and geographical characteristics. Native species were heavily preferred for the urban forest in Changchun.
-
(2)
Large species differentation existed among different types of urban forests. Anthropogenic attitudes and management practices strongly influenced the species diversity and composition. Replanting and updating unhealthy trees in old residential areas are effective measures to improve the species diversity of AF. Increasing the diversity of genus and family level is necessary for RF plantations. An appropriate and diverse mix of large and small sized tree species should increase the diversity in RF (Nagendra and Gopal 2010). For EF and PF, the diversity must be urgently increased at the species, genus, and family levels to improve the ecosystem stability and anti-interference ability.
-
(3)
The species composition had certain variability with the development of the urbanization. Distribution of types of urban forests is the vital factor contributed to the dissimilarity of species composition among different gradients of urbanization. It is noteworthy that, with the acceleration of urbanization, some areas had been denuded of tree cover for building construction and widening activities for the new metro and viaduct project (Jim and Liu 2001; Nagendra and Gopal 2010). Thus, a special attention must be urgently committed for such trees. Moreover, constructing large area of landscape forest and increaseing the native species in the fourth and the fifth ring are effective ways to enrich the urban biodiversity.
In conclusion, understanding the species differentiation among different types of urban forests and different gradients of urbanization are of great importance for species selection and optimization of the urban forests. Furthermore, it will be helpful to increase the urban biodiversity and improve the stability and capacity of resisting disturbance of urban ecosystem.
References
Andersson E, Colding J (2014) Understanding how built urban form influences biodiversity. Urban For Urban Green 13:221–226. doi:10.1016/j.ufug.2013.11.002
Andersson E, Barthel S, Ahrné K (2007) Measuring social- ecological dynamics behind the generation of ecosystem services. Ecol Appl 17:1267–1278. doi:10.1890/06-1116.1
Aronson MFJ et al (2014) A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proc R Soc B Biol Sci. doi:10.1098/rspb.2013.3330, 281
Austin MP (2013) Inconsistencies between theory and methodology: a recurrent problem in ordination studies. J Veg Sci 24:251–268. doi:10.1111/j.1654-1103.2012.01467.x
Berger WH, Parker FL (1970) Diversity of planktonic foraminifera in deep-sea sediments. Science 168:1345–1347
Celesti-Grapow L, Pysek P, Jarosik V, Blasi C (2006) Determinants of native and alien species richness in the urban flora of Rome. Divers Distrib 12:490–501. doi:10.1111/j.1366-9516.2006.00282.x
Chow WTL, Roth M (2006) Temporal dynamics of the urban heat island of Singapore. Int J Climatol 26:2243–2260. doi:10.1002/joc.1364
Clarke LW, Jenerette GD, Davila A (2013) The luxury of vegetation and the legacy of tree biodiversity in Los Angeles, CA. Landsc Urban Plan 116:48–59. doi:10.1016/j.landurbplan.2013.04.006
Colding J (2007) ‘Ecological land-use complementation’ for building resilience in urban ecosystems. Landsc Urban Plan 81:46–55. doi:10.1016/j.landurbplan.2006.10.016
Conway TM, Bourne KS (2013) A comparison of neighborhood characteristics related to canopy cover, stem density and species richness in an urban forest. Landsc Urban Plan 113:10–18. doi:10.1016/j.landurbplan.2013.01.005
Ellenberg D, Mueller-Dombois D (1974) Aims and methods of vegetation ecology. Wiley, New York, NY
Goddard MA, Dougill AJ, Benton TG (2010) Scaling up from gardens: biodiversity conservation in urban environments. Trends Ecol Evol 25:90–98. doi:10.1016/j.tree.2009.07.016
Golding J, Gusewell S, Kreft H, Kuzevanov VY, Lehvavirta S, Parmentier I, Pautasso M (2010) Species-richness patterns of the living collections of the world’s botanic gardens: a matter of socio-economics? Ann Bot 105:689–696. doi:10.1093/aob/mcq043
He XY, Liu CF, Chen W, Guan ZJ, Zhao GL (2004) Discussion of urban forest classification. Chin J Ecol 23:175–178
Hope D et al (2003) Socioeconomics drive urban plant diversity. Proc Natl Acad Sci U S A 100:8788–8792. doi:10.1073/pnas.1537557100
Huang X, Huang XJ, Chen C (2009) The characteristic, mechanism and regulation of urban spatial expansion of Changchun. Areal Res Dev 5:015
Inkilainen ENM, McHale MR, Blank GB, James AL, Nikinmaa E (2013) The role of the residential urban forest in regulating through fall: a case study in Raleigh, North Carolina, USA. Landsc Urban Plan 119:91–103. doi:10.1016/j.landurbplan.2013.07.002
Jim CY (2013) Drivers for colonization and sustainable management of tree-dominated stonewall ecosystems. Ecol Eng 57:324–335
Jim CY (2014) Passive warming of indoor space induced by tropical green roof in winter. Energy 68:272–282
Jim CY, Chen S (2008) Assessing natural and cultural determinants of urban forest quality in Nanjing (China). Phys Geogr 29:455–473
Jim CY, Chen WY (2009) Diversity and distribution of landscape trees in the compact Asian city of Taipei. Appl Geogr 29:577–587
Jim CY, Liu HT (2001) Species diversity of three major urban forest types in Guangzhou City, China. For Ecol Manag 146:99–114. doi:10.1016/s0378-1127(00)00449-7
Jim CY, Zhang H (2013) Species diversity and spatial differentiation of old-valuable trees in urban Hong Kong. Urban For Urban Green 12:171–182
Kendal D, Dobbs C, Lohr VI (2014) Global patterns of diversity in the urban forest: is there evidence to support the 10/20/30 rule? Urban For Urban Green 13:411–417. doi:10.1016/j.ufug.2014.04.004
Kenney WA, van Wassenaer PJ, Satel AL (2011) Criteria and indicators for strategic urban forest planning and management. Arboricult Urban For 37:108–117
Kent M, Stevens RA, Zhang L (1999) Urban plant ecology patterns and processes: a case study of the flora of the City of Plymouth, Devon UK. J Biogeogr 26:1281–1298. doi:10.1046/j.1365-2699.1999.00350.x
Li YM, Xiu CL, Wei Y, Sun PJ (2012) Analysis on mechanism and spatial-temporal features of urban sprawl: a case study of Changchun. Econ Geogr 32:59–64
Li XM, Zhou WQ, Ouyang ZY (2013) Relationship between land surface temperature and spatial pattern of green space: what are the effects of spatial resolution? Landsc Urban Plan 114:1–8. doi:10.1016/j.landurbplan.2013.02.005
Liu C, Li X (2012) Carbon storage and sequestration by urban forests in Shenyang, China. Urban For Urban Green 11:121–128
Lososova Z et al (2012) Native and alien floras in urban habitats: a comparison across 32 cities of central Europe. Glob Ecol Biogeogr 21:545–555. doi:10.1111/j.1466-8238.2011.00704.x
Magurran AE (1988) Ecological diversity and its measurement. Princeton University Press, Princeton, NJ
McCune B, Grace JB, Urban DL (2002) Analysis of ecological communities vol 28. MjM software design, Gleneden Beach, OR
McDonald RI, Kareiva P, Formana RTT (2008) The implications of current and future urbanization for global protected areas and biodiversity conservation. Biol Conserv 141:1695–1703. doi:10.1016/j.biocon.2008.04.025
McKinney ML (2006) Urbanization as a major cause of biotic homogenization. Biol Conserv 127:247–260. doi:10.1016/j.biocon.2005.09.005
McKinney M (2008) Effects of urbanization on species richness: a review of plants and animals. Urban Ecosyst 11:161–176. doi:10.1007/s11252-007-0045-4
McPherson EG, Nowak D, Heisler G, Grimmond S, Souch C, Grant R, Rowntree R (1997) Quantifying urban forest structure, function, and value: the Chicago urban forest climate project. Urban Ecosyst 1:49–61. doi:10.1023/A:1014350822458
McPherson EG, Xiao QF, Aguaron E (2013) A new approach to quantify and map carbon stored, sequestered and emissions avoided by urban forests. Landsc Urban Plan 120:70–84. doi:10.1016/j.landurbplan.2013.08.005
Nagendra H, Gopal D (2010) Street trees in Bangalore: density, diversity, composition and distribution. Urban For Urban Green 9:129–137. doi:10.1016/j.ufug.2009.12.005
Niemela J (1999) Ecology and urban planning. Biodivers Conserv 8:119–131. doi:10.1023/a:1008817325994
Nowak DJ, Crane DE, Stevens JC, Hoehn RE (2003) The urban forest effects (UFORE) model: field data collection manual V1b Newtown Square, PA: US Department of Agriculture, Forest Service, Northeastern Research Station
Nowak DJ, Greenfield EJ, Hoehn RE, Lapoint E (2013) Carbon storage and sequestration by trees in urban and community areas of the United States. Environ Pollut 178:229–236. doi:10.1016/j.envpol.2013.03.019
Pielou E (1966) The measurement of diversity in different types of biological collections. J Theor Biol 13:131–144
Ren Z, He X, Zheng H, Zhang D, Yu X, Shen G, Guo R (2013) Estimation of the relationship between urban park characteristics and park cool island intensity by remote sensing data and field measurement. Forests 4:868–886
Ricketts T, Imhoff M (2003) Biodiversity, urban areas, and agriculture: locating priority ecoregions for conservation. Ecol Soc 8:1
Santamour, FS (1990) Trees for urban planting: diversity, uniformity, and common sense. In: Proceedings of the seventh conference of the metropolitan tree improvement alliance (METRIA), pp. 57–65
Savard JPL, Clergeau P, Mennechez G (2000) Biodiversity concepts and urban ecosystems. Landsc Urban Plan 48:131–142. doi:10.1016/s0169-2046(00)00037-2
Schmidt KJ, Poppendieck HH, Jensen K (2014) Effects of urban structure on plant species richness in a large European city. Urban Ecosyst 17:427–444. doi:10.1007/s11252-013-0319-y
Seburanga JL, Kaplin BA, Zhang QX, Gatesire T (2014) Amenity trees and green space structure in urban settlements of Kigali, Rwanda. Urban For Urban Green 13:84–93. doi:10.1016/j.ufug.2013.08.001
Seto KC, Guneralp B, Hutyra LR (2012) Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc Natl Acad Sci U S A 109:16083–16088. doi:10.1073/pnas.1211658109
Shannon EC, Weaver W (1963) The mathematical theory of communication. University of Illinois Press, Urbana, IL
Sjoman H, Ostberg J, Buhler O (2012) Diversity and distribution of the urban tree population in ten major Nordic cities. Urban For Urban Green 11:31–39. doi:10.1016/j.ufug.2011.09.004
Thaiutsa B, Puangchit L, Kjelgren R, Arunpraparut W (2008) Urban green space, street tree and heritage large tree assessment in Bangkok, Thailand. Urban For Urban Green 7:219–229. doi:10.1016/j.ufug.2008.03.002
Thompson K, Austin KC, Smith RM, Warren PH, Angold PG, Gaston KJ (2003) Urban domestic gardens (I): putting small-scale plant diversity in context. J Veg Sci 14:71–78. doi:10.1111/j.1654-1103.2003.tb02129.x
Vakhlamova T, Rusterholz H-P, Kanibolotskaya Y, Baur B (2014) Changes in plant diversity along an urban–rural gradient in an expanding city in Kazakhstan, Western Siberia. Landsc Urban Plan 132:111–120. doi:10.1016/j.landurbplan.2014.08.014
Wania A, Kuhn I, Klotz S (2006) Plant richness patterns in agricultural and urban landscapes in Central Germany - spatial gradients of species richness. Landsc Urban Plan 75:97–110. doi:10.1016/j.landurbanplan.2004.12.006
Xiao QF, McPherson EG (2011) Rainfall interception of three trees in Oakland, California. Urban Ecosyst 14:755–769. doi:10.1007/s11252-011-0192-5
Yang J, McBride J, Zhou J, Sun Z (2005) The urban forest in Beijing and its role in air pollution reduction. Urban For Urban Green 3:65–78
Zerbe S, Maurer U, Schmitz S, Sukopp H (2003) Biodiversity in Berlin and its potential for nature conservation. Landsc Urban Plan 62:139–148. doi:10.1016/S0169-2046(02)00145-7
Zhang H, Jim CY (2014a) Contributions of landscape trees in public housing estates to urban biodiversity in Hong Kong. Urban For Urban Green 13(2):272–284. doi:10.1016/j.ufug.2013.12.009
Zhang H, Jim CY (2014b) Species diversity and performance assessment of trees in domestic gardens. Landsc Urban Plan 128:23–34. doi:10.1016/j.landurbplan.2014.04.017
Zhang BA, Xie GD, Zhang CQ, Zhang J (2012) The economic benefits of rainwater-runoff reduction by urban green spaces: a case study in Beijing, China. J Environ Manag 100:65–71. doi:10.1016/j.jenvman.2012.01.015
Acknowledgments
We are thankful for the help from Chao Gong in editing and correcting the manuscript, as well as the help from Bin Zhang and Zhilei Liu in the data analysis. This work is supported by the CAS/SAFEA International Partnership Program for Creative Research Teams (KZZD-EW-TZ-07-09) and the Excellent Young Scholars of Northeast Institute of Geography and Agroecology at the Chinese Academy of Sciences (DLSYQ13004). We also thank the anonymous reviewers for helpful comments that have improved our manuscript.
Conflict of interests
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Zhang, D., Zheng, H., He, X. et al. Effects of forest type and urbanization on species composition and diversity of urban forest in Changchun, Northeast China. Urban Ecosyst 19, 455–473 (2016). https://doi.org/10.1007/s11252-015-0473-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-015-0473-5