Abstract
We evaluated the richness, composition and abundance of bird communities in three urban forests of Mediterranean France during winter and spring. The urban forests differed in size, composition, structure, age of vegetation, and location relative to the city centre. Estimated species richness across all three urban forest parks was 45 species. Twenty six (26) species were recorded in both winter and spring, whereas ten species were recorded only in spring, and six were recorded only in winter. Distribution, turnover, and type of bird foraging guild were related to characteristics of each urban forest and season. During spring migration, more species were recorded in sampling units (250 × 250 m) within the largest and most natural urban forest located in the outskirts of Montpellier, whereas during winter, more species were recorded in the most urbanized park (i.e., a botanical garden dominated by exotic vegetation), which was located in the city centre. Insectivorous birds were more abundant in winter, whereas seedeaters associated with wooded habitats were recorded more frequently in spring. Our results suggested that different kinds of urban forests are important modulators of urban bird communities and they are necessary to maintain the diversity of migratory and resident birds as well as increasing the environmental quality of urban areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is an increasing interest in bird communities in urban and suburban habitats as they are amongst the most visible components of urban biodiversity. Urban bird communities are affected by natural constraints and by the characteristics and intensity of urbanization (Bessingers and Osborne 1982, Cousins 1982). When a natural habitat is replaced by human neighbourhoods, the richness, abundance and composition of bird communities are modified and there is a shift to a set of urban-adapted species found in cities around the world (Jokimäki et al. 1996; Blair 2004; Stratford and Robinson 2005; Clergeau et al. 2006). Several studies have shown a general increase in bird density and a decrease in species richness in urban bird communities compared to native habitats (Clergeau et al. 1998; Melles et al. 2003). However, there are not many studies which have examined bird communities in habitat patches (Jokimäki 1999; Mörtberg and Wallentinus 2000; Fernández-Juricic and Jokimäki 2001) surrounded by human development, such as parks and squares, or simply undeveloped parcels.
The green areas in a city are the most attractive to birds (Sandstrom et al. 2006). The size of urban green areas and the structure and composition of their vegetation are principally determined by humans. In contrast, urban birds are not directly controlled by man; their presence is driven by immigration or ecological succession in urban green areas (Andrzejewski 1982). In particular, urban woodlands could be important for explaining urban bird richness and diversity (Fernández-Juricic 2000; Clergeau et al. 2001, Cornelis & Hermy 2004). Urban woodlands host a large number of urban bird species because they offer higher diversity and higher quality than other urban habitats (McCollin, 1998; Caula et al. 2010). Some researchers have pointed out that the bird assemblage of an urban woodland is influenced by the regional species pool, the characteristics of the surrounding urban matrix, the size and perimeter of the urban woodland, the structure (physiognomy) and composition (floristic) of the vegetation, the occurrence of reproduction sites and the presence of water bodies (Suhonen & Jokimäki, 1988, Sewell and Catterall 1998, Jokimäki 1999). Some studies have addressed seasonal variations in the composition urban bird communities (Clergeau et al. 1998, Caula et al. 2008) but the role of urban forests remains unclear.
Our aim was to understand better the role of urban forests in seasonal dynamics and in the conservation of urban avifauna. In this paper we will compare the bird community composition and richness in three urban forest areas of different size, vegetation composition and localization in the urban matrix, during spring and winter, in order to understand how bird communities respond a) to changes in the characteristics of urban woodland and (b) seasonal variation.
Material and methods
Study areas
Montpellier, like most European cities, consists of a gradient of human disturbance decreasing from downtown to the periphery. However, urban vegetation can be divided into several categories. The green areas consist of public parks and squares, tree-lined streets and tramway tracks, green areas surrounding public or industrial buildings, private gardens and patches of natural habitats or cultivated areas within the urban landscape scattered across a wide range of built-up areas.
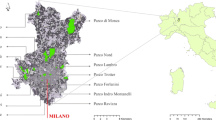
Montpellier city is located 12 km north of the Mediterranean Sea (43°40′N, 3°50′E) and has 741 ha of public green spaces within the total area of 5700 ha. The study was conducted in three of the largest urban parks in the district of Montpellier (pop 244 500; INSEE 2002): Botanical Garden (BG), Garrigues Lake forest (GF) and Montmaur forest (MF) (Fig. 1). These three green spaces differ from each other in their surface areas, location in the urban matrix, as well as in the composition, structure and age of their vegetation.
Location of the three urban forest in Montpellier district. Montmaur forest (27 ha) suburban woodland with native vegetation. Garrigues lake forest (9 ha) with a large artificial body of water and vegetation composed of native and exotic species. Botanical Garden (4.6 ha) located in town centre has 2771 cultivated exotic plants species
The Botanical Garden was created in 1593. It is located town centre in a high density building sector. Its area is 4.6 ha which includes a permanent water body. It has 2771 cultivated exotic plant species in an open area and 679 plant species in a greenhouse. The main tree species present are: broad-leaved taxa, e.g. Acer pseudoplatanus, A. neapolitanum, A. opalus, Celtis sinensis, Olea europea, Fraxinus excelsior, Melia azedarach, Mimosa pigra, Quercus pseudococcifera and Q. calliprinos; several different conifers, e.g. Cupressus goveniana, C. macrocarpa, C. sempervirens, Pinus gerardiana, P. halepensis, P. nigra, P. pinea, Picea abies and P. pungens and palms, e.g. Butia capitata, B. yatay, Phoenix dactylifera and Trithrinax sp. Apart from the tree species, the main woody taxa are shrubs, e.g. Ligustrum lucidum, L. vulgaris, Laurus nobilis, Morus boniniensis, M. alba, Myrtus communis, Pyracantha sp., Crataegus sp.,and Lavandula sp. and a large quantity of Phyllostachys bambusoides (bamboo).
Garrigues Lake forest is situated on the western periphery of the city, with an area of almost 9 ha and a large artificial body of water. It is surrounded by a low density built-up area. The woody vegetation of Garrigues Lake forest is composed of some typical native Mediterranean forest species and other exotic taxa: the most frequent species are Pinus halepensis, Quercus sp., Buxus sempervirens, Prunus mahaleb, Celtis australis, Acer campestre, A. platinoides, Alnus glutinosa and some individuals of Platanus orientalis, Laurus nobilis, Cornus sanguinea, Populus sp., Salix sp., Ulmus sp. and Fraxinus sp.
The Montmaur forest, donated to the municipality in 1910 by French zoologist Henri de Lunaret, is 27 ha of suburban woodland with native vegetation. It is situated on the eastern periphery of the city surrounded by a low density built-up area—individual houses, small collective buildings with small parks and gardens, scattered vineyards and cropland. The tree and shrub species belong to the sclerophyll forest of the Mediterranean basin. The understory layer (3 to 5 m in height) is dense and is dominated by Quercus ilex, and Q. coccifera, with some individuals of Buxus sempervirens, Frangula agnus, Arbutus unedo, Ulex parviflorus, Phillyrea sp. Rhamnus sp. and Pistacia lenticus, while Pinus halepensis, Cupressus sempervirens, Q. pubescens, Acer monspessulanum and Celtis australis are abundant in the canopy layer.
Avian survey
Sampling units of 250 × 250 m were defined in each study sector in relation to its size and heterogeneity: one sampling unit in the Botanic Garden of Montpellier (4.6 ha), two sampling units in Garrigues Forest (9 ha) and three sampling units in Montmaur Forest (27 ha). In 2005–2006, we recorded the birds within each sampling unit at the time of year when the communities were most stable: in winter and spring (Ralph et al. 1996). Each sampling unit was visited four times in each season, two early and two late visits. The bird counts were carried out in the morning, starting 15 min after sunrise on days with no wind, rain or heavy cloud cover. During each visit we walked through the whole of each sampling square for 30 min. We recorded all birds seen and heard. The identification of species was based on Hume et al. (2004).
Data analysis
We calculated corrected estimates of species richness in the three parks with the COMDYN software (Hines et al. 1999). COMDYN accounts for heterogeneity in species detectability by using the jackknife estimator. The occurrence of bird species in spring and winter was compared in each sampling unit in the three sites.
To describe the variation of the distribution of each species in the urban forests with season, we defined occurrence as the number of sampling units where a species was found in each seasons. We ranked species with respect to the occurrences in the sampling units of urban parks in spring and winter. The species were classified in guilds (Hume et al. 2004, Milesi et al. 2002, Martí 2004) and their occurrences were analyzed by seasons. Although the use of guilds has been criticized, different supra-specific grouping (e.g. guilds, functional groups) are widely adopted in the evaluation, management and monitoring of ecosystems (Milesi et al. 2002). Functional classification in bird species can be based on the structure and composition of vegetation and the resources available for feeding and nesting (Rodewald and Smith, 1998).
We investigated how forests differences and season contributed to species richness by means of a multivariate analysis with interaction (Alatalo and Alatalo, 1977). This technique supposes a community of “s” species living in a two dimensional resource space (parks and seasons) and calculates the relative contribution of both variables to species richness. A habitat independency analysis was carried out, of each bird species with every park in both seasons. We analyzed the distribution of species in the parks with a corresponding analysis for each season (CA, CANOCO Version 4.52 2003).
Results
Species richness and diversity
The estimated species richness in the three urban forest parks was 45 species (SE 3.38). In the Botanical Garden 25 species were recorded, whereas 31 species were recorded in the Garrigues Lake Forest and 35 species in the Montmaur Forest. The species density was BG = 5.4 species/ha, GF = 3.4 species /ha and MF = 1.29 species/ha.
A total of 26 species was recorded in both seasons (Table 1), and only 10 species only Spring, of which seven were migratory. Six species were only recorded in winter. Among the 26 species recorded in both seasons, six were abundant all year round, four species had higher occurrences in spring, six had higher occurrences in winter and 10 species had lower occurrences in both seasons.
Seasonal dynamics of species
In the MF sampling unit species richness was significantly higher in spring than in winter (Fig. 2) and in the BG it was significantly lower in spring than in winter (Wilcoxon test W(N 6, d.f. 1) = 7, p < 0.05). In BG, a higher percentage of species (50 %) was recorded all year round and the number of species only seen in winter was twice that only seen in spring (S: 15 % vs W: 35 %) (Fig. 3). In MF, between 29 % and 46 % of the species were present throughout the year and the increase in species in spring was 3–5 times that in winter (S: 41–57 % vs W: 7–18 %).
The multivariate analysis (Alatalo and Alatalo, 1977) shows that the degree of overlap of species in space (parks) and time (seasons) is 38.7 %, i.e. on average 16.3 species were recorded in the three parks in winter and spring (Table 2). The differences between parks and seasons contributed 61.3 % of the variation in bird species richness in our samples. The “park” variable is the greatest source of variation, its effect on species richness (42.3 %) being twice the effect of seasons (19.3 %). GF had the greatest and BG the lowest species turnover during the year.
The habitat independency analysis establishes the fidelity of species to each site (Fig. 4). More species remained in MF in spring (38 %), whereas more species were faithful to BG in winter (34.6 %). There were eight species, mainly of forest habitat, which remain loyal to the MF and GF only in spring (Table 3). Regardless of the season, half the species loyal to BG were secondary cavity nesters. Among these, C. brachydactyla and S. vulgaris were faithful in both spring and winter.
In spring, the correspondence analysis of bird communities shows that the three sites are segregated on the first two axes (Fig. 5). A gradient going from MF bird species (negative score) to GF bird species (positive score) was segregated from the BG species group. The gradient has most of the woodland species, such as Luscinia megarhynchos, Erithacus rubecula, Fringilla coelebs, Picus viridis, Regulus ignicapillus and Dendrocopus minor. In winter, although Axis I and Axis II clearly segregated the samples, the segregation was less marked and the contrast between sites was lower in winter than in spring.
Scatter diagrams of correspondence analysis in spring and winter. Spring: sampling unit N = 6, bird species N = 28. Cumulative percentage variance of species data by Axis I and Axis II = 67.9 %. Winter: sampling unit N = 6, bird species N = 26. Cumulative percentage variance of species data by Axis I and Axis II = 63.7 %
Seasonal dynamics of guilds
In all three parks, there is a higher percentage of insectivorous species in Spring (Fig. 6). They decrease in winter, mainly because the insectivorous migratory species left the city. The BG and GF had a higher percentage of species nesting in secondary cavities, in spring while there was a decreased in the winter. In spring, in the BG a higher percentage of mixed habitat species was recorded, in GF a higher percentage of open habitat species, whereas MF and GF had a higher percentage of woodland species. In winter the percentages of mixed and woodland habitat species were similar in the three parks.
Species proportion of each guild in spring and winter. Major Food: i = insectivores, f = frugivorous, s = seedeaters, o = omnivorous. Breeding site: sc = secondary cavity, m = mixte, t = tree, s = shrub, ex = excavator. Habitat: Mix = generalist, Wood = woodland, open/scrub/urban = shrubland-openland and urban
The six species of similar occurrence in both seasons were habitat and food generalists. Species that increased in abundance in spring were seedeaters associated with wooded or mixed habitats, whereas in winter these types of species were not recorded in GF and diminished in MF (Table 4). Five of the six species with greater abundance and wide distribution in winter were predominantly insectivorous associated principally with woodland habitats. In spring, four of these species were restricted to MF while only two species, Parus caeruleus and Phoenicurus ochruros, that breed in secondary cavities, remained mainly in BG. Ten species exclusively recorded in spring were insectivores, but with dissimilar breeding habits and habitats. Five of the six species recorded exclusively in winter were insectivores of woodland habitat. The sixth, Larus cachinnans is an opportunistic species frequently observed in winter in coastal cities and also in areas away from the sea (Álvarez and Méndez 1995).
Discussions and conclusions
Species richness and diversity
In this study, we compared three urban woodlands that differ in size, location in the urban matrix, and in vegetation composition and structure. We found that these factors interact, and also with the seasons, contributing to the spatio-temporal diversity of the avian urban woodland community of Montpellier.
Bird richness might increase with woodland patch size because the largest areas support larger bird populations of different species, resistant to demographic stochastic and competition limitations (MacArthur and Wilson 1967, Jokimäki 1999). Large urban woodlands usually have greater habitat diversity and can support larger samples from the regional bird pool (Gilmore, 1985). Donnelly and Marzluff (2004) found that larger urban woodlands of Seattle (USA) contained richer and less even bird communities than smaller ones. Similar results were reported by Fernández-Juricic and Jokimäki (2001) for urban forests in Spain and Finland. However, these conditions may be modified by many factors, such as human-induced disturbance, recreational use and seasonal variation (Fernández-Juricic 2000). Following these antecedents, we hypothesized that in Montpellier the parks with smaller areas would have lower richness; nevertheless we found that the relationship between these two variables in all three urban woodlands of Montpellier was dependant on the seasonality. There was lower bird richness in the annual total in the smallest urban park studied (BG) than in the other two larger parks (GF and MF). However, in winter we recorded higher richness in BG than in the other two urban woodlands, probably due to the urban microclimate protection, greater diversity of trees and shrubs and the availability of urban waste which contained more varied resources. This pattern changed in spring, when we recorded more bird species in MF than in BG. Despite the lower richness in spring, BG maintains the highest density of birds all year round. GF had the lowest richness in spring and winter probably because the pedestrian traffic is higher than in BG and MF.
Seasonal dynamics of species
Corcuera and Zavala-Hurtado (2006) affirmed that the distribution of urban birds tended to be individualistic as a response to changes in the quality and level of resources. In Montpellier, the “site characteristics” variable was more important for explaining bird richness on an annual basis. On the whole, there was a greater similarity in the composition of the avian communities in GF and MF than with that recorded in BG. However, seasonality plays a significant role in the temporal distribution of birds in urban woodlands. Distributional changes of the birds are expected between seasons due to changes in resource availability within urban habitats. BG had a different seasonal composition to GF and MF. In spring, woodland species (i.e. Luscinia megarhynchos, Upupa epops, Oriolus oriolus), both resident and migrant, were only recorded in GF and MF, whereas resident woodland species only used the BG in winter (i.e. Erithacus rubecula, Fringilla coelebs, Parus caeruleus).
As birds select habitats at multiple scales and the selection changes over time (Bersier and Meyer 1994), bird response to habitat composition may be both at a large scale (urbanization intensity) and at a small scale (local vegetation). Habitat selection and habitat fidelity are functions of bird activities: the opportunity of finding a sexual partner and food availability, nesting and breeding success, and risk of predation (Mörtberg, 2003). In Montpellier, the differences in species number, distribution and fidelity to the parks between winter and spring suggest that many birds use MF in spring, BG in winter and GF in both. We explain this by habitat and spatial restriction of breeding or by changes in foraging behaviour with the season. This is consistent with the results of Caula et al. (2008) who reported that the scarcity of resources in winter is related to a more even distribution of urban bird species than in the spring.
Donnelly and Marzluff (2004) showed that woodland birds living in urban areas are attracted by woody vegetation, while most of the nests constructed in isolated trees, exotic shrubs or buildings failed as a result of predation or starvation. Native vegetation and local bird species have coevolved together and so urban woodland rich in native vegetation would offer a large number of habitats available for nesting and breeding for a wide range of birds and would increase the richness of guilds during the different seasons of the year (Ortega-Álvarez and MacGregor-Fors 2009). For example, in the breeding season, birds breeding in trees and holes, and seedeaters, chose parks of Montpellier where predominantly woody native species were found, whereas only a low percentage of these guilds were present during the rest of the year.
However, in botanic gardens, where native and exotic plants grow together, there are a larger number of habitats and more niches, offering more or better quality food and refuges to the avian community than in the suburban green areas during unfavorable seasons (winter) (Van Heezik et al. 2008). In spite of its small size and its location within a densely built-up urban matrix (downtown) and with the lowest number of bird species recorded in Montpellier due to the scarcity of vegetation (Caula et al. 2008), BG has similar richness and a higher density of birds than GF and MF that are located in residential neighborhoods with more vegetation. Botanical gardens, with numerous exotic plant species, play a part in the attractiveness of urban areas for birds in winter as compared with the surrounding cropland or forests (Nuorteva 1971, DeGraaf & Wentworth 1986, Jokimäki et al. 1996, Jokimäki & Suhonen 1998, Fernández-Juricic and Jokimäki 2001, Caula et al. 2010). Moreover, cities are heat islands (EPA 2009), i.e. they are significantly warmer than their surrounding rural and natural areas due to the modification of the land surface with materials which retain heat effectively. Urban woodlands immersed in an intensively urbanized matrix can act as a sink for generalist and opportunist birds mainly from densely built-up areas, and also specialist ones that live in woodland, cropland and green areas around the city when climatic conditions are adverse (Chamberlain et al. 2004). Probably as consequence of the availability of important and predictable resources, including feeding by humans, and a favourable microclimate in the downtown area, BG has the greatest number of bird species per sampling unit in winter.
Seasonal dynamics of guilds
The distribution of the three major trophy guilds, insectivores, seedeaters and mixed feeding, in the urban parks was season-dependent. In general generalist species, e.g. Parus major, Pica pica, Sylvia atricapilla and S. melanocephala tended to remain in all three woodlands all year round, whereas the specialist species, e.g. seedeaters (i.e. Columba palumbus, Carduelis carduelis and Serinus serinus) and insectivores (i.e. Turdus merula, Erithacus rubecula, Fringilla coelebs and Parus caeruleus) were less constant in their locations during the study period. The insectivores were well represented in all three urban woodlands in spring but decreased in winter. This result is partially explained because the migratory species only recorded in spring in the forests of Montpellier are insectivorous. The seedeaters, with higher occurrences in spring, also decreased in winter, especially in MF and GF. Seeds of the most abundant Mediterranean forest tree species are one of the more easily accessible foods in late summer and early autumn (Quézel and Médail 2003). Later in the year, the resource is less frequent and eventually disappears. The abundance of seedeaters varied with the annual variation in resources, maximizing their foraging efficiency (Moorcroft et al. 2002). This strategy is illustrated by an increase in the abundance of birds with of mixed feeding (seeds and insects) in winter in all the sampled parks.
In the nesting period, woodland nesting birds chose MF and GF for breeding. In MF, 3/4 of the total birds living there in spring constructed their nests in woody vegetation (shrubs and trees), whereas this proportion decreased in BG. In the BG and GF species nesting in secondary cavities were more numerous probably due to the use of man-made structures for nesting. The birds choose to nest and breed in safe places with a high availability of resources where the probability of breeding success is greater.
Conclusions
In summary, in BG there is a higher percentage of generalist and secondary cavity-nesting species and greater species loyalty to the park all year around. The bird community is more stable, with more species remaining throughout the year, and the seasonal turnover is lower, than in GF and MF. The BG is an important habitat for woodland species in winter. The MF and GF have more woodland species in spring and a higher seasonal turnover. The native woodlands are an important habitat for specialist species in spring.
Urban woodlands have an important conservation value for the avian community of Montpellier. MF and GF are habitats for some endangered birds, such as the declining species Picus viridis (European Green Woodpecker) and Phoenicurus phoenicurus (Redstart), and vulnerable and migratory species, such as Upupa epops (Hoopoe) and Hirundo rustica (Barn Swallow); Delichon urbicum (House Martin), a vulnerable and migratory species, was also recorded in BG (birds endangered classification following Birdlife International, 2004). Urban woodlands are reported as important biodiversity hotspots in cities (Fernández-Juricic and Jokimäki 2001). Woody habitats in an urban matrix are quite valuable for bird conservation, since they allow the presence of certain native, migratory and/or birds without special adaptations for urban life. Moreover, greater bird diversity is seen in urban settings with mixed species woodlands (native and exotic; trees and shrubs; deciduous and coniferous) than in single species woodland stands as more species with different habitat requirements can make use of the greater diversity in floristic composition.
Our study focused on the composition of bird communities in urban woodland. We found that the compositions of these communities are dynamic and spatially and temporally structured by selection forces such as feeding and breeding. Urban woodlands are important for maintaining bird biodiversity and benefit both migratory and resident species. They also improve the environmental quality of the urban systems. Many vital ecological services, including the provision of habitats for birds and other wildlife species, as well as providing recreational areas for humans, can be maintained by protecting existing urban woodland areas and extending them. The development and maintenance of forested areas or woodland within densely built-up urban areas and the establishment of urban green corridors among different types of green spaces could be an important way to provide resources, protection and a microclimate for birds in cities, in both spring and winter.
References
Alatalo R, Alatalo R (1977) Components of diversity: multivariate analysis with interaction. Ecology 58:900–906
Álvarez Laó, C, Méndez Iglesias, M (1995) Alimentación de la gaviota patiamarilla (larus cachinnans) en dos localidades costeras asturianas, Chioglossa. Vol. Esp. 1: 23–30. La Coruña
Andrzejewski R (1982) Problems and prospects of faunistical investigations in towns. In: Luniak M, Pisarski B (eds) Animals in urban enviromental. Polish Academy of Sciences, Wroclaw, pp 9–16
Bersier L-F, Meyer DR (1994) Bird assemblages in mosaic forests: the relative importance of vegetation structure and floristic composition along the successional gradient. Acta Oecologica 15:561–576
Bessingers R, Osborne DR (1982) Effects of urbanization on avian community organization. Condor 84:75–83
Blair RB (2004) The effects of urban sprawl on birds at multiple levels of biological organization. Ecol Soc 9:21
Caula S, Marty P, Martin JL (2008) Seasonal variation in species composition of an urban bird community in Mediterranean France. Landscape and Urban Planning 87:1–9
Caula S, Sirami C, Marty P, Martin JL (2010) Value of an urban habitat for the native Mediterranean avifauna. Urban Ecosyst 13:73–89
Chamberlain DE, Cannon AR, Toms MP (2004) Associations of garden birds with gradients in garden habitat and local habitat. Ecography 27:589–600
Clergeau P, Savard JPL, Mennechez G, Falardeau G (1998) Bird abundance and diversity along an urban–rural gradient: a comparative study between two cities on different continents. Condor 100:413–425
Clergeau P, Jokimaki J, Savard JPL (2001) Are urban bird communities influenced by the bird diversity of adjacent landscapes? J Appl Ecol 38:1122–1135
Clergeau P, Croci S, Jokimaki J, Kaisanlahti-Jokimaki M, Dinetti M (2006) Avifauna homogenisation by urbanisation: analysis at different European latitudes. Biological Conservation special issue 127:336–344
Corcuera, Zavala-Hurtado (2006) The influence of vegetation on bird distribution in dry forests and oak woodlands of western Mexico. Rev Biol Trop 54(2):657–672
Cornelis J, Hermy M (2004) Biodiversity relationships in urban and suburban parks in Flanders. Landscape and Urban Planning 69(4):385–401
Cousins SH (1982) Species size distributions of birds and snails in an urban area. In: Bornkamm R, Lee JA, Seaward MRD (eds) Urban Ecology. Blackwell University Press, Oxford, pp 99–109
DeGraaf RM, Wentworth JM (1986) Avian guild structure and habitat associations in suburban bird com munities. Urban Ecology 9:399–412
Donnelly R, Marzluff JM (2004) Importance of reserve size and landscape context to urban bird conservation. Conserv Biol 18:733–745. doi:10.1111/j.1523-1739.2004.00032.x
Environmental Protection Agency of United States (EPA) (2009) Urban Climate– Climate Study and UHI. 2009-02-09. http://www.epa.gov/hiri/about/index.htm. Retrieved 2011-01-15
Fernández-Juricic E (2000) Bird community composition patterns in urban parks of Madrid: the role of age, size and isolation. Ecol Res 15:373–383. doi:10.1046/j.1440-1703.2000.00358.x
Fernández-Juricic E, Jokimäki J (2001) A habitat island approach to conserving birds in urban landscapes: case studies from southern and northern Europe. Biodivers Conserv 10:2023–2043
Gilmore AS (1985) The influence of vegetation structure on the density of insectivorous birds. Pp. 21–31 in Birds of Eucalypt Forests and Woodlands: Ecology, Conservation, Management. A. Keast
Hines JE, Boulinier T, Nichols JD, Sauer JR, Pollock KH (1999) COMDYN: software to study the dynamics of animal communities using a capture- recapture approach. Bird Study 46(suppl):S209–S217
Hume R, Lesaffre G, Duquet M (2004) Oiseaux de France et d’Europe. Larousse/S.E.J.E.R.. ISBN 2-03-560311-0
Jokimäki J, Suhonen J, Inki K, Jokinen S (1996) Biogeographical comparison of winter bird assemblages in urban environments in Finland. J Biogeogr 23:379–386
Jokimäki J, Suhonen J (1998) Distribution and habitat selection of wintering birds in urban environments. Landscape and Urban Planning 39:253–263
Jokimäki J (1999) Occurrence of breeding bird species in urban parks: effects of park structure and broad-scale variables. Urban Ecosystems 3:21–34
MacArthur RH, Wilson EO (1967) The theory of island biogeography. Princeton University Press, Princeton
Martí R (2004) Atlas de las aves reproductoras de España. M.de Medio Ambiente. Pp: 733 ISBN: 8480145501
McCollin (1998) Forest edges and habitat selection in birds: a functional approach. Ecography 21(3):247–260
Melles S, Glenn S, Martin K (2003) Urban bird diversity and landscape complexity: Species– environment associations along a multiscale habitat gradient. Conservation Ecology 7(1):5. [online] URL: http://www.consecol.org/vol7/iss1/art5
Milesi FA, Marone L, López de Casenave J, Cueto VR, Mezquida ET (2002) Gremios de manejo como indicadores de las condiciones del ambiente: un estudio de caso con aves y perturbaciones del hábitat en el Monte Central, Argentina. Ecología Austral 12:149–161
Moorcroft D, Whittingham MJ, Bradbury RB, Wilson JD (2002) The selection of stubble fields by wintering granivorous birds reflects vegetation cover and food abundance. J Appl Ecol 39:535–547
Mörtberg U, Wallentinus HG (2000) Red-listed forest bird species in an urban environment—Assessment of green space corridors. Landscape and Urban planning 50(4):215–226
Mörtberg U (2003) Resident bird species in urban forest remnants; landscape and habitat perspectives. Landsc Ecol 16(3):193–203. doi:10.1023/A:1011190902041
Nuorteva P (1971) Annoying mass occurrence of Phormia terraenovae R. D. (Diptera, Calliphoridae) in the surroundings of a rendering plant in southwestern Finland. Annales Zoologici Fennici 8:336–339
Ortega-Álvarez R, MacGregor-Fors I (2009) Living in the big city: effects of urban land-use on bird community structure, diversity, and composition. Landscape and Urban Planning 90:189–195
Quézel P, Médail F (2003) Ecologie et biogéographie des forêts du bassin méditerranéen. Elsevier (Collection Environnement), Paris, 573 p
Ralph CJ, Geupel GR, Pyle P, Martin TE, DeSante DF, Milá B (1996) Manual de métodos de campo para el monitoreo de aves terrestres. Gen. Tech. Rep. PSW-GTR-159. Albany. Pacific Southwest research Station. Forest Service. U.S. Department of Agriculture, 46p
Rodewald PG, Smith KG (1998) Short-term effects of understory and overstory management on breeding birds in Arkansas oak-hickory forest. J Wildlife Manage 62:1411–1417
Sandstrom UG, Angelstam P, Mikusinski G (2006) Ecological diversity of birds in relation to the structure of urban green space. Landscape and Urban Planning 77(1–2):39–53
Sewell S, Catterall CP (1998) Bushland modification and styles of urban development: their impacts on birds in south east Queensland. WildlifeResearch 25:41–64
Suhonen J, Jokimäki J (1988) A biogeographical comparison of the breeding bird species assemblages in twenty Finnish urban parks. Ornis Fennica 65(2):76–83
Stratford JA, Robinson DW (2005) Gulliver travels to the fragmented tropics: geographic variation in mechanisms of avian extinction. Frontiers in Ecology and the Environment March 3(2):85–92
Tucker GM, Evans MI (1997) Habitats for birds in Europe. A conservation strategy for the wilder environment. Birdlife Conservation Series Nr 6 Bird Life International, Cambridge
Van Heezik Y, Ludwig K, Whitwell S, McLean IG (2008) Nest survival of birds in an urban environment in New Zealand. New Zeal J Ecol 32(2):155–165
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Caula, S., de Villalobos, A.E. & Marty, P. Seasonal dynamics of bird communities in urban forests of a Mediterranean city (Montpellier, Southern France). Urban Ecosyst 17, 11–26 (2014). https://doi.org/10.1007/s11252-013-0295-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-013-0295-2