Abstract
Exotic plant species very often comprise a large proportion of urban floras. Because herbivorous insects depend on the presence of suitable host plants to maintain their populations, it is imperative to elucidate the relative importance of native and exotic hosts to understand the response of herbivorous guilds to urbanization. By using a plant-herbivore system composed of Asteraceae hosts and flower-head endophagous insects, we investigated whether the diversity and composition of herbivorous insects differs between native and exotic host-plant species in an urban environment. Although we found only seven exotic Asteraceae among the 30 species recorded, the overall abundance of exotics was considerably greater than that of native host plants. Overall, the exotic host species supported a small subset of the herbivore assemblage found on the native ones. The number of herbivore species per host species was significantly higher among the native plants, but we did not find a difference in herbivore abundance. Moreover, the higher taxonomic composition of herbivores on exotic Asteraceae was reduced, being composed of only three genera and two families from a total of 16 genera and six families of herbivores. These results provide support for the idea that plants outside of their original geographic distribution have lower loads of enemies than phylogenetically related native species. Our findings indicate that native host plants in urban areas play a critical role in supporting the native herbivorous insect fauna.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urbanization is one of the most severe human modifications of the natural landscapes (Grimm et al. 2008), altering markedly both abiotic conditions (e.g., soil properties and microclimate) and resource availability (e.g., space, water and nutrients) for native species. Such alterations mean that species’ adaptations to original or neighboring natural habitats do not translate into higher competitive abilities over exotic species. Furthermore, the abundance and composition of species within urban regions is greatly managed and controlled by humans, especially for plants because they are a primary component of urban aesthetics and have been used for gardening and food supply in many urban regions. Consequently, exotic species very often comprise a large proportion of urban floras (Pysek 1998; Santos et al. 2010), decreasing from more toward less urbanized regions (McDonnell and Pickett 1990; McIntyre 2000; McKinney 2010).
Because most herbivorous insects feed on a few closely related plant species (Futuyma and Mitter 1996; Jaenike 1990; Strong et al. 1984), any change in the local set of available host-plant species can strongly affect both insect composition and diversity. Thus, it is of particular interest to ascertain whether exotic plants support similar assemblages of herbivorous insects compared to the native host plants in urban habitats. A better understanding of this point is critical for the formulation of more specific hypotheses regarding plant-insect interactions in urban habitats. Interestingly, there seems to be no general pattern in the response of herbivorous insect assemblages to urbanization gradients, with empirical studies reporting negative, neutral and nil impacts of urbanization on the abundance and diversity of distinct insect taxa and guilds (see McIntyre 2000; McKinney 2010; Raupp et al. 2010 and references therein). Because herbivores depend on the presence of suitable host plants to maintain their populations, it is imperative to elucidate the relative importance of native and exotic hosts to the response of herbivorous guilds to urbanization.
In this study, we used a plant-herbivore system comprised by Asteraceae hosts and flower-head endophagous insects to investigate whether exotic plants are used by herbivorous insects in an urban environment. Asteraceae is the largest plant family in the world and figures among the most common plant families in urban environments (Pysek 1998). A diverse assemblage of endophagous insects feed on flower heads during their larval stage, consuming sap, flowers, ovaries and fruits (Gagné 1994; Headrick and Goeden 1998; Zwölfer and Romstöck-Völkl 1991). Flower-head assemblages are comprised mainly of dipterans (Tephritidae, Agromyzidae and Cecidomyiidae), microlepidopterans (families) and coleopterans (mainly Apionidae in the Neotropics, and Curculionidae in the Holarctic) (Almeida et al. 2006; Fonseca et al. 2005; Gagné 1994; Headrick and Goeden 1998; Prado et al. 2002; Zwölfer and Romstöck-Völkl 1991).
Our aim was to investigate whether the diversity and composition of herbivorous insects differ between native and exotic host-plant species in an urban environment. Specifically, we addressed the following questions: (1) Do native Asteraceae species support a richer assemblage of flower head feeding herbivores than exotic confamilial species? (2) Are higher taxa of herbivores more diverse in native than in exotic host-plant species? and (3) Are the abundance and incidence of host plants equally important for the abundance and richness of herbivores in native and exotic host species?
Material and methods
Study area and sampling procedure
This study was carried out in four green areas (lawns) on the Campus of the State University of Campinas, located in the city of Campinas, State of São Paulo, Brazil (Fig. 1). The local climate is characterized by dry winters and rainy summers, with a mean annual temperature of 21.6°C and annual mean precipitation of 1,378 mm3. The minimum and maximum distances between any two sites are 300 and 1,150 m. Sites were covered mainly by grasses (Brachiaria sp., Pennisetum sp. and Paspalum sp.), and they used to be mowed practically at the same time (within a three-week time interval) with an average frequency of four times a year.
We sampled each site in three different periods: June and September, 2005, and February, 2006. These months include the peak flowering periods of the most representative tribes of Asteraceae according to previous phenological records in the region (Almeida et al. 2005; Batalha and Mantovani 2000; Batalha and Martins 2004; Fonseca et al. 2005; Marchini et al. 2001). According to literature and personal observation, most exotic Asteraceae flower during 8 months or more, while the flowering ranges of the majority of native Asteraceae are shorter than 4 months. For each individual plant, we randomly collected up to 80 ml of flower heads. We determined the maximum biomass to be removed rather than a minimum because there was great variation in the individual and total biomass of flower heads per individual among plant species, as well as abudance of individuals, with some hosts having only a single recorded individual and a few flower heads, while other had more than 1,000 recorded individuals in the four areas. The abundance of each species within the sites was classified according to the following classes: I) 1; II) 2; III) 3 to 10; IV) 11 to 30; V) 31 to 100; VI) 101 to 300; VII) 301 to 1,000; VIII) > 1,000 individuals. In the laboratory, flower head samples were kept in plastic containers covered with a fine mesh lid, where emergence of adult insects was checked once or twice weekly for 2 months or until emergence rates became insignificant. All insects were reliably identified at least to the genus level, and all host plants identified to species, based on our reference collections and the Unicamp herbarium.
Data analyses
To estimate whether each plant species was available to its assemblage of herbivorous insects, we produced an index of availability (hereafter “availability”) for each host species, composed of the multiplication of plant species incidence (the number of areas in which a species was found; ranging from 1 to 4), mean abundance (mean value for the classes of local abundance) and flowering span (number of flowering periods). To evaluate whether native and exotic species differ in their incidences, abundances, flowering ranges and availability, we performed non-parametric randomization tests (NPRT). We found a strong positive linear correlation of weight of sampled flower heads (a measure of sampling effort) with the mean abundance of host plant species (r Pearson = 0.700; P < 0.001) and with host species availability (r Pearson = 0.930; P < 0.001).
The NPRT procedure was also used to test for differences in the following variables between native and exotic host species: (1) abundance of insect individuals per unit weight of flower heads for each host species, (2) number of herbivore species per host plant, and (3) number of herbivore species per unit weight of sampled flower heads per host species. The number of insect individuals per total flower-head weight is a measure of insect density per host plant. In contrast, the number of herbivore species per host plant is relatively small and does not increase linearly with sampling effort. Thus, our correction for sampling artifacts related to differences in sampling effort cannot be interpreted as a suitable measure of herbivore species density per host species (the species density concept sensu Gotelli and Colwell 2001). We used this correction simply to provide a benchmark for the effects of sampling on the contrasts between the number of insects associated to native and exotic Asteraceae. Only plants with at least 1 g of sampled flower heads were used to compare the herbivore assemblages on native and exotic Asteraceae. Finally, we explored the relationships between host plant availability and the abundance and richness of their herbivorous assemblages through Spearman rank correlations. All randomizations were performed with the software Resampling Stats v.3 (Blank 2010).
Results
We found 30 species of Asteraceae (flowering or fruiting) from 29 genera and 11 tribes (Table 1). Among these species, four are native to Asia or Europe (Crepis japonica, Emilia sonchifolia, Sonchus oleraceus, and Taraxacum officinale), and three are from North or Central America (Conyza canadensis, Parthenium hysterophorus, and Tridax procumbens). Although exotic species comprised a quarter of the recorded Asteraceae species, both their index of availability and local abundance were significantly higher than those for native Asteraceae (Fig. 2; NPRT: P = 0.014 and P = 0.024 for availability and mean local abundance, respectively).
Rank order of overall plant availability (based on an index of availability) for the 30 Asteraceae species found in four green areas (lawns) of the main Campus of the State University of Campinas. Species' name abbreviations as in Table 1
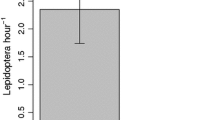
We reared 419 adult insects, comprising 26 species of flower head-feeding endophages belonging to 16 genera and six families (Table 2). All of the insect species were recorded on at least one native host species, but only four of the 26 species were found on the exotic hosts. The number of herbivore species per host species was significantly higher among the native plants (Fig. 3, NPRT: P = 0.023). This finding did not change when we controlled for differences in sampling effort using the number of insect species per weight of sampled flower head (NPRT: P = 0.034). Herbivore abundance (adjusted per weight of sampled flower heads) did not differ between native and exotic host-plants (NPRT: P = 0.063).
Overall, the exotic host plants supported a small subset of the herbivore assemblage found on the native plants (Table 2). From the total set of flower head endophages recorded in this study, only three species of agromyzid flies and one species of pyralid moth were recorded consuming flower heads of exotic Asteraceae. Interestingly, these four herbivore species were recorded in the same number or in a higher number of native host plants (always from distinct tribes). The pyralid moth was the only insect species whose abundance was higher on exotic (n = 54) than on native host plants (n = 10).
Host plant availability and its related variables (incidence, local abundance and flowering span) showed weak to moderate positive relationships with the richness and abundance of the herbivorous assemblages (Table 3). Comparisons between the correlations for native and for exotic plant species are limited to correlation coefficients because degrees of freedom for the latter is very small. Apart from the number of flowering periods, all other plant related variables showed stronger correlations for the native plants than for the exotic ones (Table 3).
Discussion
We showed that despite being generally more abundant and widespread, exotic Asteraceae species supported a small subset of the assemblage of flower head-feeding insects associated with native confamilial species in an urban environment. Moreover, the higher taxonomic composition of herbivores on exotic Asteraceae was reduced, being comprised of only three genera and two families from a total of 16 genera and six families of herbivores. These results provide support for the idea that plants out of their original geographic distribution have lower loads (abundance and richness) of enemies than phylogenetically related native species (Fenner and Lee 2001; Keane and Crawley 2002), a basic assumption of the “Enemy Release Hypothesis”.
Although similar findings have been reported for natural environments (e.g., Fenner and Lee 2001), richer herbivore assemblages on native host plants is not a given. In fact, some studies have found no differences (Frenzel and Brandl 2003; Zuefle et al. 2007) or higher richness of herbivorous insects on exotic plants (Novotny et al. 2006). These contrasting results may be due to the feeding specialization of the studied herbivorous guild and the phylogenetic relatedness between native and exotic plants. If the native assemblage of herbivores is mostly comprised of specialist insects exploiting closely related plant species, then most exotic plants will be consumed by no (or few) native herbivores. However, if the studied herbivorous guild is comprised of generalist insects capable of feeding on most plants from the same family or order, then there is no reason to expect higher herbivore richness on native plants. Actually, exotic plants could support a richer herbivore assemblage if their defenses are less effective against the phytophagous insects when compared with native plants that have long coevolutionary histories with their herbivores (see Raupp et al. 2010; Gandhi and Herms 2010).
Tallamy (2004) pointed out that the capacity of native insects to discriminate between hosts becomes reduced if exotic plants are found in greater abundance. Consequently, the use of exotic plants by native herbivores would become more frequent. In this study, the availability of exotic flower heads to the endophagous insects was considerably higher than that of native flower heads, but only a small subset of the flower head feeding insects were found on the exotic Asteraceae. Although our results did not provide support for this hypothesis, we found that the majority of generalist herbivores (three agromyzids from a total of five species of this family and a single pyralid species) were capable of using the exotic Asteraceae. Thus, Tallamy’s (2004) discrimination hypothesis probably applies for generalist insect fauna and studies focusing on insect guilds that are mostly composed of typically specialist herbivores rarely will support this discriminatory effect. If this is a widespread pattern, then the importance of host-plant abundance as one of the main determinants of local insect richness (Frenzel and Brandl 2003; Marques et al. 2000; Marquis 1991) may not apply for urban environments.
Ectophagous (external feeders) insects are supposed to be less specialized than endophages because they are free of intimate adaptations to internal plant environment (Cornell and Kahn 1989; Gaston et al. 1992; Lewinsohn et al. 2005). Most species of flower head endophages, for instance, are specialists, consuming plant species from the same genus or subtribe (Gagné 1994; Prado and Lewinsohn 2004). This is especially the case for Cecidomyiidae and Tephritidae, the most representative insect families on flower heads of Asteraceae in the Neotropics (Almeida-Neto et al. 2011; Lewinsohn 1991). Microlepidopterans and agromyzid flies seem to be less specialized, being frequently found on different tribes of Asteraceae (Braun et al. 2008; Lewinsohn 1991). Not by coincidence, the only flower head endophages that we found on exotic Asteraceae were three agromyzids and one pyralid moth (Table 2). In a comparison of the flower head fauna associated with 13 species of Asteraceae, Fenner and Lee (2001) found only one herbivorous species on a single host species in New Zealand (exotic distribution) and ten herbivores on the native distribution of these plant species (Britain). On the other hand, Zuefle et al. (2007) did not include internal feeders (gall-makers, stem borers or leaf-miners) and found no difference in the ratio of specialist to generalist insects found in native, non-native and alien plants species.
Time since introduction has been shown to be another important determinant of the number of native herbivore species associated with exotic plants (Andow and Imura 1994; Brändle et al. 2008; Carpenter and Cappuccino 2005; Frenzel and Brandl 2003). We hypothesize that time since introduction is particularly important for more generalist herbivorous insects, because monophages or highly specialist herbivores will rarely use plants that are not closely related to their original hosts. Thus, for most flower head endophagous species, time since introduction would be of minor importance compared to phylogenetic proximity.
Here we found that native plants have, on average, twice as many herbivore species than the exotic ones. If our findings remain qualitatively unchanged in other urban plant herbivore systems, then we surmise that urban ecosystems are characterized by low biomass transfer efficiency from plants to herbivores. This supposition is based on two assumptions: (1) the more diverse the consumers (i.e. more species), the larger the range of consumed resources, and (2) the more specialized a consumer, the more efficiently it converts food into growth or production of offspring. A more diversified, and more specialized, herbivore fauna on native plants has the potential for higher complementarity in appropriating plant biomass, as well as stabilizing the aggregate effect of herbivory on plants (Hooper et al. 2005). Note also the high abundance of generalist flower-head feeders on exotic hosts, contrary to what has been found for leaf-feeders in Canada (Hill and Kotanen 2010). This can increase their population density and bring more pressure on native hosts. Potentially, urban communities may thus experience destabilization of their interaction structure and degradation of biodiversity-related ecosystem processes, akin to the loss of rare non-target native hosts as a consequence of biological weed control (Louda et al. 2003).
In conclusion, as long as studies in diverse geographic regions and with different taxonomic and ecological groups are not carried out, the effects of the urbanization of natural landscapes on the structure of the interactions between plants and insects cannot be generalized. The present study adds important information to the understanding of insect-plant interactions in human-modified landscapes by comparing herbivorous insect assemblages on native and exotic host plants in an urban environment. Specifically, our findings indicate that native host plants in urban areas play a critical role in supporting the native fauna of herbivorous insects.
References
Almeida AM, Fonseca CR, Prado PI, Almeida-Neto M, Diniz S, Kubota U, Braun MR, Raimundo RLG, Anjos LA, Mendonça TG, Futada SM, Lewinsohn TM (2005) Diversidade e ocorrência de Asteraceae em cerrados de São Paulo. Biota Neotrop 5:27–43. doi:10.1590/S1676-06032005000300003
Almeida AM, Fonseca CR, Prado PI, Almeida-Neto M, Diniz S, Kubota U, Braun MR, Raimundo RLG, Anjos LA, Mendonça TG, Futada SM, Lewinsohn TM (2006) Assemblages of endophagous insects on Asteraceae in São Paulo cerrados. Neotrop Entomol 35:458–468. doi:10.1590/S1519-566X2006000400006
Almeida-Neto M, Prado PI, Lewinsohn TM (2011) Phytophagous insect fauna tracks host plant responses to exotic grass invasion. Oecologia 165:1051–1062. doi:10.1007/s00442-010-1783-1
Andow DA, Imura O (1994) Specialization of phytophagous arthropod communities on introduced plants. Ecology 75:296–300. doi:10.2307/1939535
Batalha MA, Mantovani W (2000) Reproductive phenological patterns of cerrado plant species at the Pé-de-Gigante reserve (Santa Rita do Passa Quatro, SP, Brazil): a comparison between the herbaceous and woody floras. Rev Bras Biol 60:129–145. doi:10.1590/S0034-71082000000100016
Batalha MA, Martins FR (2004) Reproductive phenology of the cerrado plant community in Emas National Park (central Brazil). Aust J Bot 52:149–161. doi:10.1071/BT03098
Blank S (2010) Resampling stats for Excel v 4.00.statistics.com
Brändle M, Kühn I, Klotz S, Belle C, Brandl R (2008) Species richness of herbivores on exotic host plants increases with time since introduction of the host. Divers Distrib 14:905–912. doi:10.1111/j.1472-4642.2008.00511.x
Braun MR, Almeida-Neto M, Loyola RD, Prado AP, Lewinsohn TM (2008) New host-plant records for neotropical agromyzids (Diptera: Agromyzidae) from Asteraceae flower heads. Neotrop Entomol 31:97–99. doi:10.1590/S1519-566X2008000100016
Carpenter D, Cappuccino N (2005) Herbivory, time since introduction and the invasiveness of exotic plants. J Ecol 93:315–321. doi:10.1111/j.1365-2745.2005.00973.x
Cornell HV, Kahn DM (1989) Guild structure in the British arboreal arthropods: is it stable and predictable? J Anim Ecol 58:1003–1020
Fenner M, Lee WG (2001) Lack of pre-dispersal seed predators in introduced Asteraceae in New Zealand. New Zeal J Ecol 25:95–99
Fonseca CR, Prado PIK, Almeida-Neto M et al (2005) Flowerheads and their insects: food web structure along a fertility gradient of Cerrado. Ecol Entomol 30:36–46. doi:10.1111/j.0307-6946.2005.00664.x
Frenzel M, Brandl R (2003) Diversity and abundance pattens of phytophagous insect communities on alien and native host plants in Brassicaceae. Ecography 26:723–730. doi:10.1111/j.0906-7590.2003.03649.x
Futuyma DJ, Mitter C (1996) Insect–plant interactions: the evolution of component communities. Philos Trans R Soc Lond B 351:1361–1366. doi:10.1098/rstb.1996.0119
Gagné RJ (1994) The Gall Midges of the Neotropical Region. Cornell University Press, Ithaca
Gandhi KJK, Herms DA (2010) Direct and indirect effects of alien insect herbivores on ecological processes and interactions in forests of eastern North America. Biol Invasions 12:389–405. doi:10.1007/s10530-009-9627-9
Gaston KJ, Reavey D, Valladares GR (1992) Intimacy and fidelity: internal and external feeding by the British microlepidoptera. Ecol Entomol 17:86–88. doi:10.1111/j.1365-2311.1992.tb01044.x
Gotelli NJ, Colwell RK (2001) Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol Lett 4:379–391. doi:10.1046/j.1461-0248.2001.00230.x
Grimm NB, Faeth SH, Golubiewski NE, Redman CL, Wu J, Bai X, Briggs JM (2008) Global change and the ecology of cities. Science 319:756–760. doi:10.1126/science.1150195
Headrick DH, Goeden RD (1998) The biology of nonfrugivorous tephritid fruit flies. Annu Rev Entomol 43:217–241. doi:10.1146/annurev.ento.43.1.217
Hill SB, Kotanen PM (2010) Phylogenetically structured damage to Asteraceae: susceptibility of native and exotic species to foliar herbivores. Biol Invas 12:3333–3342. doi:10.1007/s10530-010-9726-7
Hooper DU, Chapin FS, Ewel JJ, Hector A, Inchausti P, Lavorel S et al (2005) Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol Monogr 75:3–35. doi:10.1890/04-0922
Jaenike J (1990) Host specialization in phytophagous insects. Annu Rev Ecol Evol Syst 21:243–273. doi:10.1146/annurev.es.21.110190.001331
Keane RM, Crawley MJ (2002) Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol 17:164–170. doi:10.1016/S0169-5347(02)02499-0
Lewinsohn TM (1991) Insects in flower heads of Asteraceae in Southeast Brazil: a tropical case study on species richness. In: Price PW, Lewinsohn TM, Fernandes GW, Benson WW (eds) Plant-animal interactions: evolutionary ecology in tropical and temperate regions. Wiley-Interscience, New York, pp 525–559
Lewinsohn TM, Novotny V, Basset Y (2005) Insects on plants: diversity of herbivore assemblages revisited. Annu Rev Ecol Evol Syst 36:597–620. doi:10.1146/annurev.ecolsys.36.091704.175520
Louda SM, Pemberton RW, Johnson MT, Follett PA (2003) Nontarget effects-the Achilles’ heel of biological control? Retrospective analyses to reduce risk associated with biocontrol introductions. Annu Rev Entomol 48:365–396. doi:10.1146/annurev.ento.48.060402.102800
Marchini LC, Moreti ACCC, Teixeira EW, Silva ECA, Rodrigues RR, Souza VC (2001) Plantas visitadas por abelhas africanizadas em duas localidades do estado de São Paulo. Sci Agricola 58:413–420
Marques ESA, Price PW, Cobb NS (2000) Resource abundance and insect herbivore on woody fabaceous desert plants. Environ Entomol 29:696–703. doi:10.1603/0046-225X-29.4.696
Marquis RJ (1991) Herbivore fauna of Piper (Piperaceae) in a Costa Rican wet forest: diversity, specifity, and impact. In: Price PW, Lewinsohn TM, Fernandes GW, Benson WW (eds) Plant-animal interactions: evolutionary ecology in tropical and temperate regions. Wiley/Interscience, New York, pp 179–208
McDonnell MJ, Pickett STA (1990) Ecosystem structure and function along urban-rural gradients: an unexploited opportunity for ecology. Ecology 71:1232–1237. doi:10.2307/1938259
McIntyre NE (2000) Ecology of urban arthropods: a review and a call to action. Ann Entomol Soc Am 93:825–835. doi:10.1603/0013-8746(2000)093[0825:EOUAAR]2.0.CO;2
McKinney ML (2010) Effects of urbanization on species richness: a review of plants and animals. Urban Ecosyst 11:161–176. doi:10.1007/s11252-007-0045-4
Novotny V, Drozd P, Miller SE, Kulfan M, Janda M, Basset Y, Weiblen GD (2006) Why are there so many species of herbivorous insects in tropical rainforests? Science 313:1115–1118. doi:10.1126/science.1129237
Prado PI, Lewinsohn TM (2004) Compartments in insect-plant associations and their consequences for community structure. J Anim Ecol 73:1168–1178. doi:10.1111/j.0021-8790.2004.00891.x
Prado PI, Lewinsohn TM, Almeida AM, Norrbom AL, Buys BD, Macedo AC, Lopes MB (2002) The fauna of Tephritidae (Diptera) from capitula of Asteraceae in Brazil. Proc Entomol Soc Wash 104:1007–1028
Pysek P (1998) Alien and native species in central European urban floras: a quantitative comparison. J Biogeogr 25:155–163. doi:10.1046/j.1365-2699.1998.251177.x
Raupp MJ, Shrewsbury PM, Herms DA (2010) Ecology of herbivorous arthropods in urban landscapes. Ann Rev Entomol 55:19–38. doi:10.1146/annurev-ento-112408-085351
Santos AR, Rocha CFD, Bergallo HG (2010) Native and exotic species in the urban landscape of the city of Rio de Janeiro, Brazil: density, richness, and arboreal deficit. Urban Ecosyst 13:209–222. doi:10.1007/s11252-009-0113-z
Strong DR, Lawton JH, Southwood TRE (1984) Insects on plants. Blackwell, Oxford
Tallamy DW (2004) Do aliens plants reduce insect biomass? Conserv Biol 18:1689–1692. doi:10.1007/s10530-009-9639-5
Zuefle ME, Brown WP, Tallamy DW (2007) Effects of non-native plants on the native insect community of Delaware. Biol Invas 10:1159–1169. doi:10.1007/s10530-007-9193-y
Zwölfer H, Romstöck-Völkl M (1991) Biotypes and evolution of niches in phytophagous insects on Carduae hosts. In: Price PW, Lewinsohn TM, Fernandes GW, Benson WW (eds) Plant-animal interactions: evolutionary ecology in tropical and temperate regions. Wiley/Interscience, New York, pp 487–507
Acknowledgements
We are grateful to Rosane Picon, Marina Braun and Ricardo Fabiano for helping us with field work. This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) grants # 04/15482-1 to TML, # 03/02541-0 and # 06/56889-2 to MAN, and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) grant # 306049/2004 to TML. RDL’s research is supported by CNPq.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Perre, P., Loyola, R.D., Lewinsohn, T.M. et al. Insects on urban plants: contrasting the flower head feeding assemblages on native and exotic hosts. Urban Ecosyst 14, 711–722 (2011). https://doi.org/10.1007/s11252-011-0179-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-011-0179-2