Abstract
I examined tree recruitment and mortality over a ten-year period at permanent plots in an urban forest, Forest Park, in Portland, Oregon. The density and diameter at breast height (dbh) for all trees living and dead were measured in 1993 and again in 2003. Data were analyzed using paired Student t-tests. I found significantly fewer live and significantly more dead trees in 2003 than in 1993. The increase in mortality was significant for all species of trees and for all sizes except large diameter trees. Mortality rates ranged from 0% to 67% at my sites. Recruitment was lower at all sites in 2003 with significantly fewer seedlings and saplings. The high mortality and low recruitment resulted in a net loss of trees at all sites. Loss of trees was not offset by increasing tree diameter, which suggests self-thinning is not the cause. No strong correlation with an urban to rural land use gradient was observed. The results may be related to global climate change or pollution. The high mortality of trees of all species in many diameter classes without a concomitant increase in recruitment could lead to dramatic changes in forest structure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urbanization has been shown to affect natural areas in a variety of manners. Many urban forest ecosystems exhibit reduced species diversity (Airola and Buchholz 1984, Sharpe et al. 1986, Pysèk and Pysèk 1990, Tait et al. 2005, Broshot 2007). Tree stand characteristics affected by urbanization include changes in density, basal area and/or importance value of trees in urban forests (Airola and Buchholz 1984, Rudnicky and McDonnell 1989, Broshot 2007). Tree growth rates have been shown both to increase (Iakovoglou et al. 2001) and decrease (Quigley 2002; Carreiro and Tripler 2005). Tree regeneration has been shown to decrease for some species, yet increase for others, especially earlier successional or non-native species (Lehvävirta and Hannu 2002, Zipperer 2002, Komuro and Koike 2005).
Several studies show urbanization may be interfering with normal succession processes in forests (Kowarik 1990, Matlack 1993, Leak and Smith 1996, Porter et al. 2001, Quigley 2002, Komuro and Koike 2005, Broshot 2007). Tree mortality and recruitment are important parameters in determining how succession will proceed, but limited studies have been done in urban areas. Most of the urban studies have assessed street trees (Gilbertson and Bradshaw 1985, Nowak et al. 2004), which do not necessarily respond similar to trees in natural forested urban areas. Several workers have reported reduced recruitment in urban forests (Greller 1975, Airola and Buchholz 1984, Fahey 1998, Lehvävirta and Hannu 2002, Zipperer 2002, Bertin et al. 2006, Broshot 2007), and some have reported increased tree mortality in urban areas (Davis 2004, Nowak et al. 2004, Carreiro and Tripler 2005, Broshot 2007). Nowak et al. (2004) reported that tree mortality in urban, forested areas was moderate as compared to that in more urban areas, but made no comparison with more rural forests.
Tree recruitment and mortality have been shown to be patchy in nature (Franklin et al. 1987; Peet and Christensen 1987; Busing 2005) making it important to determine whether changes seen in urban forests are different from those in more rural forest systems. In order for succession to proceed, mortality of canopy trees must be offset by recruitment of later successional species. A combination of high mortality and low recruitment could lead to dramatic changes in forest structure (Franklin et al. 1987, Lorimer et al. 2001, Bertin et al. 2006, Lutz and Halpern 2006).
Recent studies have reported increased tree mortality over broad geographical areas (van Mantgem and Stephenson 2007, Adams et al. 2009, van Mantgem et al. 2009). High worldwide tree mortality has been positively correlated with increased water deficits caused by higher temperatures related to global climate change (Guarín and Taylor 2005, Mueller et al. 2005, van Mantgem and Stephenson 2007, Adams et al. 2009, van Mantgem et al. 2009). Because urban forests often experience warmer temperatures due to an urban heat sink effect (Bornstein 1968, Kim 1992), tree mortality could provide early indication of the impact a changing climate may have in the future (Carreiro and Tripler 2005). The issue is also confounded by the fact that urban forests are often exposed to higher levels of air pollutants, particularly ozone that has been shown to increase mortality of younger trees (Lehvävirta and Hannu 2002, Gregg et al. 2003). In addition, the high recreational use of urban forests may increase mortality by trampling and/or soil compaction (Bagnell 1979, James et al. 1979, Bhuju and Ohsawa 1998, Littlemore and Barker 2001, Lehvävirta and Hannu 2002, Zipperer 2002, Moffatt and McLachlen 2004).
In 1993, I found reductions in tree species richness and diversity, as well as in the density and diameter of shade-tolerant (later successional) species of trees such as western red cedar and western hemlock with urbanization. I also found a dearth of young trees, especially shade tolerant species, in the more urban areas of the park (Broshot, 2007). Since 1993, development of the land surrounding Forest Park has continued and recreational use has intensified (Broshot, unpublished data). My initial study showed changes along an urban to rural land use gradient, however, long-term studies on urban forests are needed (Nowak et al. 2004). In this study, I returned to permanent plots in 2003 and repeated earlier measurements in Forest Park.
This paper presents the results of a 10-year study of tree recruitment and mortality in an urban forest. I had several objectives: (1) to determine whether levels of tree recruitment and mortality have changed over the 10 years of the study and if so, whether those rates vary by tree size or species; (2) to determine whether tree recruitment or mortality correlated with level of urbanization; and (3) to propose potential reasons for the findings, which could lead to further research.
Methods
Site description
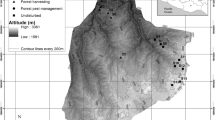
Twenty-four permanent study sites were established in Forest Park in Portland, Oregon in 1993. Forest Park is located northwest of downtown Portland and covers an area of over 2,000 ha (Fig. 1). It is approximately 11 km in length and ranges from 0.8 to 2.4 km wide, with an irregular perimeter. I divided Forest Park approximately into thirds longitudinally and randomly assigned eight sites to each third, hereby distributing the sites along the perceived urban-rural land use gradient (Fig. 1). This process resulted in approximately the same number of sites on the northeast (13 sites) and the southwest (11 sites) sides of the park. One additional site (called the old-growth in this study) was located in a 12-hectare stand of mature Douglas-fir approximately 4 km northwest of Forest Park. At the beginning of the study, contiguous forest stretched between the old-growth stand and Forest Park, however, after a series of large clear cuts, this is no longer the case. The old-growth today is almost entirely surrounded by a Douglas-fir plantation that was planted in 1991, but some land with older second growth remains adjacent to the stand. Although the old-growth stand has some very large Douglas-firs (some dated to approximately 435 years old, Broshot 1999), it is not technically classified as old-growth because there are too few very large trees, too few large snags, too few large downed logs, and the stand is too small (Franklin and Dyrness 1988; Franklin and Spies 1991; Spies 1991).
Map showing location of sites in Forest Park and the old-growth stand (gray area) relative to downtown Portland, Oregon (Broshot 2007)
The areas surrounding my sites have various land uses that create an urbanization gradient running from crowded urban Portland residential neighborhoods and industrial sites on the southeast to rural lands on the northwest and surrounding the old-growth (Broshot 2007). Over the ten-year period between data collections, land surrounding the park has continued to be developed, mostly for single-family residential homes.
Forest Park extends in a northwesterly direction along the Willamette River floodplain on the east side of the Tualatin Mountains. Elevations within the park range from 20 m near the base of the Tualatin Mountains to 335 m above sea level at the crest. Soils at my sites are fairly uniform, being composed of Cascade and Goble types, both of which have an underlying fragipan between 51 and 114 cm deep that prevents water penetration. Much of the area includes slopes that exceed 30% grade, making erosion, slumping, and landslide hazards high. The climate for the area is maritime with mild temperatures, prolonged cloudy periods, warm dry summers and wet cool winters (Green 1983).
The study area lies in the Douglas fir (Pseudotsuga menziesii Mirbel Franco)—western hemlock (Tsuga heterophylla Raf. Sarg.) vegetation zone. Western hemlock and western red cedar (Thuja plicata Donn.) are considered climax species for the area. Although Douglas-fir is an early seral species, many forests are dominated by it because it routinely lives for more than 500 years (Franklin and Dyrness 1988, Agee 1991, Franklin and Spies 1991). In addition, Douglas-fir is a fire-adapted species able to survive fires that kill other tree species, including western hemlock and western red cedar. In western Oregon, the return time for fire is estimated to have been 230 years before Europeans began a policy of fire suppression in the mid 1800s (Agee 1991). Frequent disturbances in more urban areas favor invasion by bigleaf maple (Acer macrophyllum Pursh) and red alder (Alnus rubra Bong) (Franklin and Dyrness 1988). As a result of continual disturbance, many sites in the Portland area are dominated by those two species of shade-intolerant deciduous trees (Houle 1996).
Data collection
I collected data from the 25 randomly located field sites during the summer of 1993; I returned to the same sites and recollected data in the same manner in the summer of 2003. Three, 30 m long, parallel transects were established at each study site. Transects were laid so that they ran up slope at each location. The 30 m transect length limited the variability in slope, aspect and elevation, each of which was measured at midpoint on each transect and found to not be correlated with any measure of urbanization. All members of tree species, living or dead, within a 250 square meter quadrat (4.17 m on either side of the 30 m transect) were identified to species and the diameter at breast height (dbh) of each was measured. Each quadrat was 20 m away from the adjacent quadrat. In this study, I defined trees as members of arboreal species with a dbh greater than 10 cm. Saplings were defined as members of tree species that were greater than 1.37 m tall with a dbh less than 10 cm. All tree seedlings (members of tree species less than 1.37 m tall) that were present on each transect were identified to species and the basal diameter and height were measured. I noted whether dead trees were standing or blown down.
Statistical analysis
Data were compared between 1993 and 2003 using two-tailed, paired Student t-tests. Data were also analyzed using analysis of variance (ANOVA), where each section of Forest Park (e.g., city, middle, far), as well as the old-growth stand was designated as a treatment. Tukey-Kramer HSC values were used to determine significance among the categorical variables in each ANOVA. This particular test was selected because it is a conservative test and because I did not have equal numbers of samples in each group (Johnson and Wicher 1982, Sokal and Rohlf 1995; JMP 2005). The statistical program JMP 6.0 (SAS Institute 2005) was used for all statistical analyses. To meet the assumptions of normality required for t-test and ANOVA, some variables were transformed (Sokal and Rohlf 1995). Tree variables were reported only for those species that were present in at least three sections and whose density was large enough to examine statistically.
Results
The total number of live trees measured declined from 1,956 stems in 1993 to 1,555 stems in 2003. Although 17 tree species were measured, only six had high enough densities to be analyzed; five of the tree species were non-native. Over the same ten-year period, the total number of dead trees increased from 554 to 1124. The total number of seedlings (trees <1.37 m tall) decreased from 76 to only 37 stems.
I found significantly fewer live trees and significantly more dead trees in 2003 than in 1993 (Table 1). I found significantly fewer live seedlings, saplings and trees in all size classes except for trees greater than 40 cm dbh (Fig. 2). I found significantly more dead trees in all size classes except for those greater than 60 cm dbh (Fig. 3). The average percent of dead trees at my sites significantly increased from 20.5% in 1993 to 40.7% in 2003 (Table 1). The mean total tree diameter was significantly lower in 2003 than in 1993.
When results were examined by species, I found significantly fewer live trees in all species plentiful enough to be examined except Cascara and significantly more dead trees in all examined species (Figs. 4 and 5). I found significantly fewer live and more dead shade tolerant trees in 2003 than in 1993 (Table 1).
When the results were analyzed by section of the park, I found the decrease in live tree density and live shade tolerant tree density in 2003 were true in all sections except the old growth (Table 2). Because the old-growth had only three transects versus the 24 in each of the sections of Forest Park, this is not too surprising. Although there was a tendency for fewer live seedlings in each section, the results were not significant. There were, however, significantly fewer live saplings and trees in Forest Park in all size classes smaller than 40 cm dbh. There were significantly fewer live shade tolerant trees in all sections except the old growth. In 2003, the rate of recruitment (trees counted in 2003 that were not present in 1993) ranged from 0 new trees per site in the old growth to 2.1 new trees in the middle section, but the results did not significantly vary among the sections. I found significantly fewer live trees, live seedlings, and trees 10–20 cm dbh in the city section than the far section.
The number and percent of dead trees was significantly greater in all sections of the park in 2003 than in 1993 (Table 3). There were significantly more dead trees in all size classes less than 40 cm dbh in most sections of Forest Park in 2003. I found significantly more dead shade tolerant trees in every section except the middle in 2003. Mean annual mortality rate (change in the density of dead trees divided by the number of years) ranged from 1.7% in the middle section to 2.7% in the old growth. All sections experienced a decline in live trees ranging from an average loss of 3.9 trees in the middle section to a loss of 10.7 trees in the old-growth. The old growth had significantly more trees lost than either the city or the middle sections of Forest Park.
Discussion
On average, mortality doubled at sites from 1993 to 2003. Forest Park appears to be experiencing high levels of tree mortality, with significantly higher mortality in all tree species and in all but the largest trees. A study of old growth forests in Yosemite Valley, California showed similar findings (Guarín and Taylor 2005). Studies in other locations have shown high mortality in very small and very large trees (Busing 2005). Frequently mortality rate varies by tree species (DeBell and Franklin 1987, Lorimer et al. 2001, Smith et al. 2005), but I found high mortality in all tree species. I also found reduced rates of recruitment as indicated by significantly fewer seedlings and saplings. This finding is similar to other studies (Lorimer et al. 2001, Bertin et al. 2006), but van Mantgem and Stephenson (2007) found no significant change in recruitment rates in the Sierra Nevada.
The average annual mortality rate at my sites was 2.2%. This rate is greater than that found by some (Franklin et al. 1987, Busing 2005, Lutz et al. 2009) (Table 4), lower than that found by others (Nowak et al. 2004, van Mantgem and Stephenson 2007) and about the same as that found by Acker et al. (2006). Rates in my study are greater than those observed in mature forests, but less than old-growth forests (Lorimer et al. 2001). My old-growth stand (really mature forest) had a rate of 2.7%, which is high compared to the mortality rate of 0.7% observed in mature and old-growth forest areas in Andrews experimental forest in Corvallis, Oregon (Franklin et al. 1987). Annual mortality rates very widely by forest age and tree species (Franklin et al. 1987, Lorimer et al. 2001, Guarín and Taylor 2005, Smith et al. 2005).
The average rate of recruitment as examined by the number of new trees (not just seedlings and saplings) present on sites in 2003 ranged from 0 per ha in the old-growth to 84 per ha in the middle section. Given that some of these trees may have been up to a decade old, this number is very low compared to data from other studies (Lehvävirta and Hannu 2002, van Mantgem et al. 2009). The combination of low recruitment and high mortality resulted in a net loss of trees ranging from 428 trees per ha in the old growth to 156 trees per ha in the middle section. The number of trees lost exceeded the number of new trees in all sections and at every site. I also found that the total diameter of trees in the park declined significantly. This suggests that the high tree mortality was not the result of self-thinning. During the thinning stage of stand development, a loss in tree density should be balanced by an increase in tree diameter (Peet and Christensen 1987, van Mantgem et al. 2009). DeBell and Franklin (1987) observed slight increase in tree biomass that balanced the loss of biomass to mortality in old-growth forests. I also found significantly fewer shade tolerant trees in every section of the park compared to what was found in 1993. The loss of canopy trees should allow the release of shade tolerant trees in the understory under normal circumstances (DeBell and Franklin 1987, Franklin and Dyrness 1988), but the dearth of shade tolerant trees in the understory could lead to a major disruption in the successional processes in Forest Park. DeBell and Franklin (1987) found mortality in Douglas-fir in old growth forests in Washington was compensated for by growth of western hemlock. This was not observed in this study where I found high mortality and reduced diameter of all species relative to 1993.
If the process of self-thinning is not responsible for the observed increase in mortality, is there an effect due to urbanization, which could show up along an urban to rural land use gradient. In both 1993 and 2003, I found significantly more dead trees in the far than either in the city or middle sections of Forest Park, however the annual mortality rate was not significantly different among the different sections. In both years there were significantly more live seedlings in the far section than the city or middle sections and although there was a tendency for fewer seedlings in all sections in 2003, the findings were not significant. There were significantly fewer saplings (trees less than 10 cm dbh) in 2003. In 1993 there were significantly more saplings and shade tolerant trees in the far section than in the city and middle sections of the park, however there were no significant differences among the sections in 2003. A large proportion of the trees in Forest Park are dying without shade tolerant saplings in the understory to grow into the consequential gaps. In 1993, it appeared that succession was being impeded by urbanization in the section of the park closest to downtown Portland (Broshot 2007). The 2003 findings suggest that the park is becoming more homogeneous. It seems unlikely that disturbances associated with surrounding land usage have expanded to the extent that all sections face the same pressures. This leads to the conclusion that something else is responsible for the observed changes.
Tree mortality is a complex process. Mortality of young trees is often caused by suppression or self-thinning, whereas deaths of older trees are usually brought about by a combination of factors. Biotic factors include competition, herbivory, insects and disease. Abiotic factors include environmental stresses such as drought, wind and pollution (Franklin et al. 1987, Tkacz et al. 2008). Competition and self-thinning can be ruled out as the major cause for my findings because the diameter of trees and the density of shade tolerant trees also declined. Tree mortality can also be episodic due to major storms or diseases (Franklin et al. 1987), but there have been no major insect or disease outbreaks. Herbivory by ungulates does not appear to be causing widespread death of young trees in Forest Park (Broshot, unpublished data). Only 5.8% of tree mortality resulted from blow down, whereas 39% of the dead trees were still standing. Thus some other reason must account for the findings.
The increase in mortality in this study was observed in all species and sizes except larger trees (>60 cm dbh). Potential causes for the mortality could be the effects of air pollution or global climate change. Increased dust deposition of particulate pollution has been reported in urban areas (Pouyat et al. 1995, Lovett et al. 2000). Such pollutants could alter the soil ecosystem in such a manner as to reduce growth rate and vigor of trees (Ashby and Fritts 1972, McLaughlin et al. 1983, Pouyat et al. 1995, Zipperer et al. 2000, Tkacz et al. 2008). Gregg et al. (2003) reported decreased growth in rural trees due high ozone levels. Urban air pollutants interact with biogenic volatile organic compounds produced by forests and agricultural lands to raise ozone levels in areas downwind from cities (Campbell et al. 2000). The resultant ozone can accumulate to levels above a threshold (40 ppb—the AOT 40) that has been documented to cause foliar damage (Kinnee et al. 1997, Vollenweider et al. 2003, Goldstein et al. 2004). High levels of ozone cause visible damage to foliage, but reductions in growth without visible damage have also been documented (Gregg et al. 2003, Gregg et al. 2006). Plants exposed to ozone in controlled growth chambers lost control of stoma, resulting in reduced photosynthetic rates, which led to a significant reduction in biomass accumulation (Gregg et al. 2006). The Oregon Department of Environmental Quality reported that in 2008 the average level of ozone measured in areas surrounding Portland ranged between 75 and 85 ppb (Oregon DEQ 2010), levels sufficient to cause damage to vegetation (Vollenweider et al. 2003). This points out that measuring levels of ozone in Forest Park will be an important step.
Another possible explanation for the observed higher mortality could be related to global warming with concomitant increases in water stress. Several studies have recently reported higher tree mortality in many areas of the world (Mueller et al. 2005, van Mantgem and Stephenson 2007, Adams et al. 2009, van Mantgem et al. 2009). The increase in tree mortality has been observed in both tropical and temperate (both Europe and North America) forests (van Mantgem and Stephenson 2007, Adams et al. 2009, van Mantgem et al. 2009). A correlation has been observed between the observed increase in mortality and water stress resulting from the higher temperatures of global climate change (Guarín and Taylor 2005, Mueller et al. 2005, van Mantgem and Stephenson 2007, Adams et al. 2009, Lutz et al. 2009, van Mantgem et al. 2009). The high mortality in observed in my study may be the result of climate change, perhaps intensified by the urban heat sink effect (Bornstein 1968, Von Stulphagel et al. 1990, Carreiro and Tripler, 2005).
In this 10-year study, I found increased mortality in all species of trees in all sizes except very large trees. I also found a reduction in recruitment as exhibited by a reduction in seedlings and saplings in 2003. My data suggest that Forest Park may undergo a dramatic change in forest structure in the future. Although a forest in normal successional development could be expected to have increased mortality during the thinning stage, the loss of shade tolerant trees, low levels of recruitment and reduction in tree diameter all suggest that this is not the cause of my findings. The changes in tree mortality and recruitment did not seem to correlate with measures of urbanization using the urban to rural land use gradient. It is possible that the findings may be the consequences of increased ozone pollution or global climate change or a combination of the two. Higher temperatures result in increased ozone development (Goldstein et al. 2004). Warming temperatures could lead to higher ozone levels. The combination of water stress and higher ozone levels could be impacting the trees in Forest Park. Further study into the levels of ozone and the temperatures to which trees at the sites are exposed are necessary to help understand the observations.
References
Acker SA, Franklin JF, Greene SE, Thomas TB, Van Pelt R, Bible KJ (2006) Two decades of stability and change in old-growth forest at Mount Rainier National Park. NW Sci 80:65–72.
Adams HD, Guardiola-Claramonte M, Barron-Gafford GA, Villegas JC, Breshears DD, Zou CB, Trouch PA, Huxman TE (2009) Temperature sensitivity of drought-induced tree mortality portends increased regional die-off under global-change-type drought. Proc Natl Acad Sci USA 106(17):7063–7066
Agee J (1991) Fire history of Douglas-fir forests in the Pacific Northwest. pages 25–33. In: Ruggiero LF, Aubry KB, Carey AB, Huff MH (eds). Wildlife and vegetation of unmanaged Douglas-fir forests. U.S. Dept. of Agric. For. Serv. Pac. NW. Res. Sta. Gen. Tech. Rep. PNW-GTR-285. May 1981. p. 533
Airola T, Buchholz K (1984) Species structure and soil characteristics of five urban forest sites along the New Jersey Palisades. Urban Ecol 8:149–164
Ashby WC, Fritts HC (1972) Tree growth, air pollution, and climate near LaPorte, Indiana. Bull of Amer Meteor Soc 53:246–251
Bagnell RG (1979) A study of human impact on an urban forest remnant: Redwood Bush, Tarva, near Wellington, New Zealand. N Z J Bot 17:117–126
Bertin RI, DeGasperis BG, Sabloff JM (2006) Land use and forest history in an urban sanctuary in Central Massachusetts. Rhodora 108:119–141
Bhuju DR, Ohsawa M (1998) Effects of nature trails on ground vegetation and understory colonization of a patchy remnant forest in an urban domain. Biol Conserv 85:123–135
Bornstein RD (1968) Observations of the urban heat island effect in New York City. J Appl Meteorol 7:575–582
Broshot N (1999) The effects of urbanization and human disturbance upon plant community structure and bird species richness, diversity, and abundance in a natural forested area (Forest Park) in Portland, Oregon. Doctoral Dissertation. Portland State University. Portland, Oregon, p 244
Broshot NE (2007) The influence of urbanization upon forest stand dynamics in Northwestern Oregon. Urban Ecosyst 10:285–298
Busing RT (2005) Tree mortality, canopy turnover, and woody detritus in old cove forests of the Southern Appalachians. Ecol 86:73–84
Campbell S, Smith G, Temple P, Pronos J, Rochefort R, Anderson C (2000) Monitoring for ozone injury in west coast (Oregon, Washington, California) forests in 1998. USDA For Serv Pac NW Res Sta Gen Tech Rep PNW-GTR-495
Carreiro MM, Tripler CE (2005) Forest remnants along urban-rural gradients: examining their potential for global chance research. Ecosyst 8:568–582
Davis L (2004) Urban forestry: engineering cities into natural systems. Page 6 of Virginia Tech Research Magazine. Virginia Tech
DeBell DS, Franklin JF (1987) Old-growth Douglas-fir and western hemlock: a 36-year record of growth and mortality. West J For Res 2(4):111–114
Fahey TJ (1998) Recent changes in an upland forest in south-central New York. J Torrey Bot Soc 125:51–59
Franklin J, Dyrness CT (1988) Natural vegetation of Oregon and Washington. Oregon State University Press, Corvallis, p 452
Franklin J, Spies T (1991) Composition, function, and structure of old-growth Douglas-fir forests. In: Ruggiero LF, Aubry KB, Carey AB, Huff MH (Eds). Wildlife and vegetation of unmanaged Douglas-fir forests. USDA For Ser Pac NW Res Sta, Gen Tech Rep PNW-GTR-285. May 1991, pp 71–80
Franklin J, Shugart HH, Harmon ME (1987) Tree death as an ecological process. Biosci 37:550–556
Gilbertson P, Bradshaw AD (1985) Tree survival in cities: the extent and nature of the problem. Arboricul J 9:131–142
Goldstein AH, McKay M, Kurpius MR, Schade GW, Lee A, Holzinger R, Rasmussen RA (2004) Forest thinning experiment confirms ozone deposition to forest canopy is dominated by reaction with biogenic volatile organic compounds. Geophys Res Lett 31:1–4
Green G (1983) Soil Survey of Multnomah County, Oregon. U. S. Department of Agriculture. Soil Conservation Service and Forest Service. pp 225
Gregg JW, Jones CG, Dawson TE (2003) Urbanization effects on tree growth in the vicinity of New York City. Nature 424:183–187
Gregg JW, Jones CG, Dawson TE (2006) Physiological and developmental effects of O3 on cottonwood growth in urban and rural sites. Ecol Appl 16(6):2368–2381
Greller AM (1975) Persisting natural vegetation in Northern Queens County, New York, with proposals for its conservation. Env Cons 2:61–69
Guarín A, Taylor AH (2005) Drought triggered tree mortality in mixed conifer forests in Yosemite National Park, California, USA. For Ecol Manage 218:229–244
Houle M (1996) One city’s wilderness: Portland’s forest park. The Oregon Historical Society Press, Portland, p 151
Iakovoglou V, Thompson J, Burras L, Kepper R (2001) Factors related to tree growth across urban-rural gradients in the Midwest, USA. Urban Ecosyst 5:71–85
James TDW, Smith DW, Mackintosh EE, Hoffman MK, Mont P (1979) Effects of camping recreation on soil, Jack pine, and understory vegetation in a Northwest Ontario park. For Sci 25:333–349
JMP. (2005) Statistics and graphics guide for jmp. SAS Institute
Johnson R, Wicher D (1982) Applied multivariate statistical analysis. Prentice-Hall, Inc, Englewood Cliffs, p 594
Kim HH (1992) Urban heat island. Intern J Remote Sens 13:2319–2336
Kinnee E, Geron C, Pierce T (1997) United States land use inventory for estimating biogenic ozone precursor emissions. Ecol Appl 7(1):46–58
Komuro T, Koike F (2005) Colonization by woody plants in fragmented habitats of a suburban landscape. Ecol Appl 15:662–673
Kowarik I (1990) Some responses of flora and vegetation to urbanization in Central Europe. pages 45–74. In: Sukopp H, Hejny S (eds) Urban ecology. Plants and plant communities in urban environments. SPB Academic Publ., The Hague, p 282
Leak W, Smith M (1996) Sixty years of management and disturbance in a New England forested landscape. For Ecol Manag 81:63–73
Lehvävirta S, Hannu R (2002) Natural regeneration of trees in urban woodlands. J Veg Sci 13:57–66
Littlemore J, Barker S (2001) The ecological response of forest ground flora and soils to experimental trampling in British urban woodlands. Urban Ecosyst 5:257–276
Lorimer CG, Dahia SE, Nordheim EL (2001) Tree mortality rates and longevity in mature and old-growth hemlock hardwood forests. J Ecol 89:960–971
Lovett GM, Traynor MM, Pouyat RV, Carreiro MM, Zhu W, Baxter JW (2000) Atmospheric deposition to oak forests along an urban-rural gradient. Environ Sci Tech 34:4294–4300
Lutz JA, Halpern CB (2006) Tree mortality during early forest development: a long-term study of rates, causes, and consequences. Ecol Monogr 76:257–275
Lutz JA, van Wagendonk JW, Franklin JF (2009) Twentieth century decline of large diameter trees in Yosemite National Park, California, USA. For Ecol Manag 257:2296–2307
Matlack G (1993) Sociological edge effects: spatial distribution of human impact in suburban forest fragments. Environ Manag 17:829–835
McLaughlin SB, Blasing TJ, Mann LK, Duvick DN (1983) Effects of acid rain and gaseous pollutants on forest productivity. A regional scale approach. J of Air Poll Control Assoc 33:1042–1049
Moffatt SF, McLachlen SM (2004) Understory indicators of disturbance for riparian forests along an urban-rural gradient in Manitoba. Ecol Indic 4:1–16
Mueller RC, Scudder CM, Porter ME, Trotter RT III, Gehring CA, Whitham TG (2005) Differential tree mortality in response to severe drought: evidence for long-term vegetation shifts. J Ecol 93:1085–1093
Nowak DJ, Kuroda M, Crane DE (2004) Tree mortality rates and tree population projections in Baltimore, Maryland, USA. Urban For Urban Green 2:139–147
Oregon Department of Environmental Quality. (2010) Ground Level Ozone web site. 3 pages. http://www.deq.state.or.us/aq/planning/ozone.htm
Peet RK, Christensen NL (1987) Competition and tree death. Bioscience 37:586–595
Porter EE, Forschner BR, Blair RB (2001) Woody vegetation and canopy fragmentation along a forest-to-urban gradient. Urban Ecosyst 5:131–151
Pouyat RV, McDonnell MJ, Pickett STA (1995) Soil characteristics of oak stands along an urban-rural land-use gradient. J Environ Qual 24:516–526
Pysèk P, Pysèk A (1990) Comparison of the vegetation and flora of the West Bohemia villages and towns. In: Sukopp H, Hejny S (eds) Urban ecology. Plants and plant communities in urban environments. SPB Academic Publ., The Hague
Quigley MF (2002) Franklin Park: 150 years of changing design, disturbance and impact on tree growth. Urban Ecosyst 6:223–235
Rudnicky J, McDonnell M (1989) Forty-eight years of canopy change in a hardwood-hemlock forest in New York City. Bull Torrey Bot Club 116:52–64
SAS Institute Incorporated (2005) JMP 6.0
Sharpe D, Stearns F, Leitner L, Dorney J (1986) Fate of natural vegetation during urban development of rural landscapes in Southeastern Wisconsin. Urban Ecol 9:267–287
Smith TF, Rizzo DM, North M (2005) Patterns of mortality in an old-growth mixed-conifer forest of the southern Sierra Nevada, California. For Sci 51:266–275
Sokal RR, Rohlf FJ (1995) Biometry, 3 rd Edition. W.H. Freeman and Co
Spies TA (1991) Plant species diversity and occurrence in young, mature, and old-growth Douglas-fir stands in western Oregon and Washington. In: Ruggiero LF, Aubry KB, Carey AB, Huff MH (Eds). Wildlife and vegetation of unmanaged Douglas-fir forests. USDA, For Ser Pac NW Res Sta, Gen Tech Rep PNW-GTR-285. May 1991, pp 111–121
Tait CJ, Daniels CB, Hill RS (2005) Changes in species assemblages within the Adelaide metropolitan area, Australia, 1836–2002. Ecol Appl 15:346–359
Tkacz R, Moody B, Castillo JV, Fenn ME (2008) Forest health conditions of North America. Environ Pollut 155:409–475
van Mantgem PJ, Stephenson NL (2007) Apparent climatically induced increase of tree mortality rates in a temperate forest. Ecol Lett 10:909–916
van Mantgem P, Stephenson NL, Byrne JC, Daniels LD, Franklin JF, Fulé PZ, Harmon ME, Larson AJ, Smith JM, Taylor AH, Veblen TT (2009) Widespread increase of tree mortality rate in the western U.S. Science 323:521–524
Vollenweider P, Ottigert M, Günthardt-Goerg MS (2003) Validation of leaf ozone symptoms in natural vegetation using microscopic methods. Environ Pollut 124:101–118
von Stulphagel A, Horbert, M, Sukopp H (1990) The importance of vegetation for the urban climate. pages 175–193. In: Sukopp H, Hejny S (eds) Urban Ecology. Plants and Plant Communities in Urban Environments. SPB Academic Publ., The Hague, p 282
Zipperer WC, Wu J, Pouyat RV, Pickett STA (2000) The application of ecological principles to urban and urbanizing landscapes. Ecol Appl 10:685–688
Zipperer WC (2002) Species composition and structure of regenerated and remnant forest patches within an urban landscape. Urban Ecosyst 6:271–290
Acknowledgements
This project was funded in part by Portland Parks and Recreation, Portland State University, and Linfield College. I wish to thank Jack Keyes and Todd Rosenthiel for helpful comments and reviews that improved the document. I also wish to thank Peggy Sedlak and Michelle Heater for assistance in the field.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Broshot, N.E. Mortality and recruitment in an urban forest (Forest Park in Portland, Oregon) between 1993 and 2003. Urban Ecosyst 14, 553–567 (2011). https://doi.org/10.1007/s11252-011-0170-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-011-0170-y