Abstract
Ruminants, as well as other livestock, can synthesize vitamin C (VC) in their liver, and therefore, dietary requirements from exogenous supplementation are often ignored. However, metabolic demand may be exceeded, leading to a decreased endogenous synthetic capacity of VC following exposure to stressful conditions. Such conditions include high thermal load, limited water intake (induced by water scarcity), physiological status and infectious diseases. The obvious consequences are decreased performance, susceptibility to infections and increased mortality. This review discusses the potential role of vitamin C in ruminants’ stress management and summarizes the in vitro and in vivo research to date. The different administration routes, comparative advantages and supplementation outcomes on growth, production parameters and physiological status were also identified. Also, areas where there was a lack of evidence or controversy, including critical literature research gaps, were identified, while the mechanism of VC’s actions on significant outcomes was explained.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Domestic animals have been continuously exposed to various unfavourable physical or psychological stressors, and the welfare of domestic animals during such episodes is of significant interest (Kumar et al. 2012). This continuous exposure to such stressful conditions, including heat, cold, handling, transportation, diseases and introduction to a new flock, disrupt the usual internal milieu, leading to a new adaptation that is perceived to be damaging to the animal (Asres and Amha 2014). Furthermore, it is a common factor responsible for various physiological alterations in the body that indirectly alter the immune system through the generation of metabolic radicals (reactive oxygen species [ROS]), which impair productivity, fertility as well as immunity and cause economic losses to the farmer (Kumar et al. 2012). It is believed that during stress, the body’s natural antioxidants get overwhelmed, and their capacity to cope with the elimination of excess free radicals or ROS reduced, thus exposing the cell to harmful effects of ROS (Kurutas 2016).
Animals get exposed to various kinds of stressors: environmental (extreme heat and cold), physical (handling or transportation), nutritional (feed or water shortages), chemical (toxin, pathogen or disease), psychological (fear or restrain) and physiological (pregnancy or lactation). Heat stress, when combined with limited water intake (water stress) in livestock, appears to be a vital stressor, especially in the tropical, subtropical (Nardone et al. 2010), arid and semiarid (Akinmoladun et al. 2019; Mpendulo et al. 2020) regions of the world. Ruminants, especially those reared in hot tropical environments, are usually exposed to more than one stress at a time. In such cases, the challenges are simultaneous, and the cumulative impact is more severe, unlike when they occur individually (Chaidanya et al. 2017). The coping mechanisms to stressors vary with animal species, nature of stressful stimuli, the genotype in the same species, nutritional status, duration and time of exposure and the animal’s physiological stage (Novais et al. 2017). Increased respiration rate, sweating, vasodilatation, reduced metabolic rate, decreased feed intake and utilization and alteration of water balance are some of the observed responses in livestock under stress (Akinmoladun et al. 2019). Stress compromises an embryo’s ovarian function and development and redistributes body resources, including energy and protein, thus decreasing fertility, performance and animals’ wellbeing (Kandemir et al. 2013). Given the preceding, the most required is an antioxidant therapy that may retard cortisol production (the primary stress hormone), detoxify ROS and enhance the animal’s immunity. Such antioxidants may be incorporated as a reliable management practice option for mitigating the adverse effects of stress in animals (Urban-Chmiel et al. 2009).
L-ascorbic acid and vitamin C (VC) are essential nutrients for only a few species (e.g. humans and guinea pigs) without a vitamin’s synthetic ability due to an enzyme deficiency. This enzyme (L-gulonolactone oxidase) is responsible and necessary for transforming glucose into vitamin C during the last biosynthetic pathway. However, mammals (ruminants, swine, dogs, horses, cats), including marsupials, can synthesize VC from glucose in the liver or the kidney (Combs Jr. 2008). Supplementation of VC was, therefore, not a dietary requirement in animals that can synthesize it. Unfortunately, plasma ascorbic acid concentration reportedly decreases during stress and disease conditions in animals (Kim et al. 2012). In such situations, it is possible that the endogenous synthesis of the vitamin has ended or could be due to increased demand or a combination of both. Nevertheless, the significant improvements usually recorded following supplementations suggested VC deficiency during stress and compromised health status (Ranjan et al. 2012; Khalid et al. 2016).
The ameliorative potentials of ascorbic acid on stressful stimuli are considered non-depressive, safer and more practicable. It is also cheap, readily available, non-toxic, easily administered and absorbed quickly, devoid of the withdrawal period and without consequential effect at very high doses in vivo (Seifi et al. 2010). This stress alleviating potential of VC owes its mechanism of action not only to the scavenging of ROS and other oxidative radicals but also its ability to potentiate α-aminobutyric acid (GABA) (Brikas 1994), which helps to retard the release of prolactin and cortisol usually implicated in splenic contraction and cellular damage (Minka and Ayo 2010). The immunomodulatory and anti-inflammatory attributes of VC, based on cell culture models and animal studies, involve their roles at supporting natural killer cell activity, immune response, production of proteins (interferons) that protect cells against viral attack as well as positive chemotactic and proliferative responses of neutrophils (Wu et al. 2000). VC is also involved in the expression of genes by T cell on activities ranging from signalling, apoptosis, carbohydrate metabolism to transcription (Grant et al. 2007). In addition, cellular functions including delayed-type hypersensitivity responses, synthesis of humoral thymus factor and antibodies of the IgG and IgM classes were also reported to be affected by VC status (Combs Jr. and McClung, 2017).
Some studies on stress-simulated oxidative damage concerning vitamin C have been shrouded with controversy and sometimes inconclusive. However, there is reasonable evidence and body of knowledge supporting the use of VC as an anti-stress (Minka and Ayo 2010; Akinmoladun et al. 2020a; Akinmoladun et al. 2020b). This paper discusses the various physiological and performance indices in stressed ruminants modulated by vitamin C, route of administration differences, its mechanism of action and future research gap.
Antioxidant potentials of vitamin C
Ascorbic acid (VC) is a non-enzymatic and water-soluble antioxidant in plasma and tissues. Ascorbic acid functions as an antioxidant by easily losing electrons in a reversible monovalent biochemical redox system. By undergoing single-electron oxidation, ascorbate reacts with free radicals to yield a relatively poorly reactive intermediate, the ascorbyl radical, which disproportionates to ascorbate and dehydroascorbic acid. In this way, ascorbate can reduce toxic ROS (O2−, OH, RO2) and RNS (NO2). These reactions are of fundamental importance in all aerobic cells (Combs Jr. and McClung, 2017). Coupled with reduced glutathione, tocopherols and other antioxidants, VC protects cells, supports the sparing of vitamin E, recycles α-tocopherol and promotes non-heme iron utilization (Chambial et al. 2013). It also ensures that appropriate oxidation states of enzyme-bound metals are maintained in the enzymatic biosynthesis of carnitine, collagen and non-epinephrine (Tauler et al. 2003). Through its antioxidant ability to donate free electrons (hydrogen molecules), VC ensures membrane integrity stability, limiting its susceptibility to lipid peroxidation (Bernabucci et al. 2002). In heat-stressed animals, VC regulates oxygen consumption by increasing loss through a more efficient thermal exchange between the environment and the body or reducing heat load generated from metabolic activities within the body (Minka and Ayo 2012). Ascorbic acid is thought to improve humoral and cellular immunity and, thus, increases resistance to infection, defence mechanism and antioxidant status of the animal and reduce the detrimental effects of certain eicosanoids (Chambial et al. 2013). Ruminants, especially young ones, are more vulnerable to cold stress and require increased plasma ascorbic acid concentration to confer immunity and protection (Carr and Maggini 2017). Sivakumar et al. (2010) reported that ascorbic acid prevents neonatal calve diarrhoea and scours. In addition to cortisol-inhibition activities, VC actively participates in restricting and preventing free radical propagation, thus protecting blood cells (lymphocytes and monocytes) from oxidative damage (Carr and Maggini 2017). The antioxidant potentials of VC in the management of stressful stimuli in ruminants (Ghanem et al. 2008; Akinmoladun et al. 2020a, 2020b), monogastric (Minka and Ayo 2010) and even humans (Tauler et al. 2003) are well documented.
Biopotency, uptake and tissue distribution
Compounds showing the biological activity of ascorbic acid are also described as vitamin C. It is a six-carbon ketolactone structure whose biological activity depends on this 6-carbon lactone having a 2,3-enediol structure. Thus, it is an effective quencher of free radicals such as singlet oxygen (O2). It reduces ferric (Fe3+) to ferrous (Fe2+) iron (and other metals analogously). As a potent reducing agent, ascorbic acid is oxidized to dehydroascorbic acid via the radical intermediate semi-hydroascorbic acid in a reversible redox system (Tu et al. 2017). Although there are several synthetic analogues of vitamin C, their relative biopotencies and biological activity differ. For example, 6-deoxy-L-ascorbic acid and several esters of ascorbic acid (e.g. 6-deoxy-6-chloro-L-ascorbic acid, ascorbyl-6-palmitate) have good biological activity, whereas others (e.g. L-glucoascorbic acid) have little or no activity (Combs Jr. and McClung 2017). Higher animals can synthesize vitamin C via the glucuronic acid pathway (Fig. 1). In lower animal groups, including egg-laying mammals, reptiles and amphibians, the pathway’s enzyme is found in the kidney. In contrast, the enzyme is located in the liver in higher animal groups (mammals and passerine birds) (Chatterjee et al. 1975). The enzyme (L-gulonolactone oxidase) is absent (evolutionary loss) in primates, humans, bats and some species of fish and birds. It, therefore, requires exogenous VC supplementation to meet dietary needs (Banhegyi et al. 1997). For vitamin C (ascorbic acid, ascorbate) to function as a vitamin, entry into cells is essential (Padayatty and Levine 2016). Simple diffusion across membranes cannot distribute VC across partitions because it is a sizeable polar molecule, charged at physiological pH, requiring transporters for cell entry (Li and Schellhorn 2007). Two distinct transport channels have been characterized: sodium-dependent vitamin C transporters (SVCT1 and SVCT2) and hexose (glucose) transporters (GLUTs) (Corpe et al. 2013). The GLUTs channel first oxidizes ascorbates to dehydroascorbic acid (DHA) and gets reduced back to ascorbate within the cell as a form of ascorbate recycling (May et al. 1995).
Although there is no stable reserve of vitamin C as excesses are quickly excreted, the leukocyte ascorbate concentration provides an accurate measure of the vitamin at the tissue level (Mitmesser et al., 2016). Ruminants, due to their biosynthetic ability, may not require dietary VC supplementation. However, the liver’s synthetic capacity may not accommodate VC’s increased requirements in stressful situations such as road transportation, exposure to high ambient temperature, sub-optimum water intake, diseases and exercise (Sivarkumar et al. 2010). Therefore, supplementation of VC to ruminants under conditions perceived to be stressful may provide a potentially necessary, cheap, non-toxic alternative treatment. The range of plasma ascorbate concentrations in ruminants is shown in Table 1.
Sources, forms and routes of VC administration in ruminants and their comparative advantage
Several means of exogenous supplementation of VC for ruminants have been worked upon in experimental trials. A significant challenge limiting the bioavailability of VC in tissues is the rapid destruction by ruminal microflora and urinary excretion losses (Padilla et al. 2007). However, during low VC status, the amount excreted may be limited (McDowell 2000). VC administration could be routed through the mouth (per os or oral) or parenteral (non-oral). Forms of oral administration could be through drinking water (after dissolving the powder in water) (Akinmoladun et al. 2020a, 2020b), through feed as rumen-protected VC (coating of VC with ethylcellulose or hydrogenated soybean oil) (Padilla et al. 2007) or fed directly (uncoated) as a dietary mixture (Kim et al. 2012). Parenteral administration could either be intravenous (Liu et al. 1994), intramuscular (Sonmez and Demirci 2003) or subcutaneous (Fazeli et al. 2010). Examples of different sources and concentration range of VC used during ruminant feeding trial is shown in Table 2. Assessment of VC’s bioavailability in ruminants is primarily determined by the change in plasma ascorbic acid concentrations after supplementation. Studies have shown plasma ascorbic acid concentration to be higher in ruminants receiving coated VC or administered parenterally than ordinary powdered VC in drinking water or as a dietary mixture (Hidiroglou 1999). Comparing the potency of different VC preparations such as powdered VC, VC coated with ethyl cellulose or silicon and ascorbyl-2-polyphosphate, Hidiroglou et al. (1997) observed a much-increased plasma VC concentration in the VC-silicon-coated preparation compared to others. Also, direct supplementation via the abomasum or intra-duodenum increased plasma VC compared to oral (mouth) (Hidiroglou 1999). For a resource-limited rural farmer, this might be a challenge giving the technical expertise required for intra-duodenum/abomasum VC and other injectable VC administrations. Apart from reduced availability, the high cost of rumen-protected VC might not be sustainable for low-income-based rural farmers. While studies comparing the effectiveness of different routes and forms of vitamin C supplementation in ruminants have been scanty, higher plasma ascorbate with respect to administration routes does not seem to translate into much-improved stress management. In a comparative study on the effectiveness of route of VC administration, Biobaku et al. (2018) observed that, despite higher plasma ascorbic acid concentration, the responses (cortisol, antioxidants and erythrocyte biomarkers) of 2 h transportation-stressed Kalahari goats administered with VC (200 mg/kg) intramuscularly were similar to oral supplementation. There is a need for more studies on the safest and efficient route of administration that will produce the highest level of stress management.
Modulatory role of vitamin C in ruminants
Effect on vital signs and behavioural kinetics
The vital signs, especially rectal temperature (RT) and respiratory rate (RR), provide a quick measure of the degree of stress imposed on animals. Under high thermal load, RR can assess heat stress, and the result is reported to correlate with corticoid concentration. Simultaneously, the pulse rate (PR) indicates the animal body’s homeostasis and general metabolic status (Kassab and Mohammed 2014). Usually, these physiological variables or vitals are raised when ruminants are exposed to unfavourable stressors and may impair the animal’s performance (Akinmoladun et al. 2019). An elevation in the physiological variables (RT and PR) observed during road transportation decreased in goats (Nwunuji et al. 2014) and sheep (Kassab and Mohammed 2014) following the administration of VC. During transportation, stress is usually imposed on the animal during handling, loading and the entire road transportation process, including vehicle, vibration, noise, water and food deprivation, change of environment and confinement (Minka and Ayo 2012). RR improved with VC treatment in water-restricted Awassi ewes (Ghanem et al. 2008) and Xhosa goats (Akinmoladun et al. 2020a). Based on documented reports, it is suggestive that vitamin C lessens stress by decreasing RR and RT (usually elevated during stress) by directly altering thermal set points. These vitamins directly modulate the hypothalamic thermoregulatory activity and explain the observed outcome in stressed ruminants (Sivakumar et al. 2010). However, the adaptive nature and tolerance to stressful stimuli (e.g. heat stress) may sometimes cloud VC supplementation impact in small ruminants. For example, supplementation of VC to Rahmani ewes (Hashem et al. 2016) did not ameliorate the effect of heat stress on the various heat tolerance parameters measured. Following prolonged exposure, small ruminants develop specific heat tolerance mechanisms to reduce metabolic heat production by reducing feed intake and stretching the body to lose heat (Akinmoladun et al. 2019).
Skin thickness, an essential assessor of severe dehydration, especially in calves (Atkinson 1992), could decrease following sustained exposure to stressors. Such a decrease could result from the high thermal load, suboptimal water intake during water scarcity or when animals are deprived of water during transportation from one location to another. Also, the thickness of the skin is affected by cellular substances, collagen fibres and interstitial fluid content. These intrinsic features significantly influence the skin’s biophysical properties including, elasticity, turgor and distensibility (Weller et al. 2008). Normal skin contains high concentrations of vitamin C, and an excessively oxidized environment, either from thermal load or otherwise, can deplete levels of VC below the threshold, thereby affecting its supporting functions like the stimulation of collagen synthesis, free radical scavenging activities and modulation of cell signalling and epigenetic pathways (Pullar et al. 2017). However, there is a possibility of improvement with exogenous VC supplementation. According to Minka and Ayo (2012), the skin thickness of goats undergoing 12-h transportation stress was raised from 1.8 mm in control to 2.1 mm in the vitamin C-treated groups. In an in vitro study, the cell signalling pathway attributes of vitamin C are reported to enhance the differentiation of epidermal keratinocytes cells with the markedly improved ultrastructural organization of the stratum corneum (Marks 2004). This stratum corneum is one of the four major components (other three; stratum spinosum, stratum granulosum and stratum lucidum) of the upper layer of the epidermis responsible for skin thickness (Marks 2004).
Exposure of ruminants to stressful stimuli increases the frequency of urination and the egestion of gut content. This stress-induced bowel movement is attributed to increased intestinal motility and cortisol-induced diuresis (Karl et al. 2018). Nwe et al. (1996) alluded to the increased elimination of urine and faeces from transportation stress to nervousness or excitation. This stressful condition can further be heightened by suboptimal water intake and adverse climatic conditions, leading to dehydration (Popkin et al. 2010). Under elevated temperature, Nejad and Sung (2017) reported increased sitting duration and decreased standing time when sheep were water-deprived for 2 or 3 h after feeding. In the observation of Minka and Ayo (2012), the faecal water content and elimination behaviour were less in transported goats treated with VC. This indicates that VC can reduce the excitation of the nervous system associated with road transportation stressors. However, the behavioural dispositions during vehicular movements of goats, including fall, slip, kick aggression, jump and baulk, were not affected by vitamin C (Minka et al. 2009). The impact of VC on the behavioural kinetics of ruminants undergoing heat or water stress is unavailable when writing this review.
Effect on body weight and feed intake
During stress, anabolic activity decreases while tissue catabolism increases and both combine to affect growth performance. Decreased anabolism responds to decreased voluntary feed intake and essential nutrients, including vitamins and minerals, consequently leading to a loss in the production per unit of feed (Akinmoladun et al. 2019, 2021). In addition to reduced feed intake, attempts to sustain the increased demand for energy result in high induction of free fatty acids and cholesterol from body fat reserves. The mobilized body fats are usually the cause of weight loss due to the loss of body solids. The severity of the stress-induced depression on body weight and feed intake in ruminants is heightened in regions where intense climatic stresses (high ambient temperature and humidity) are combined with feed limitations and insufficient water supply. For example, Osmanabadi goats had their feed intake and body weight reduced when exposed to either restricted feed or heat stress plus restricted feed (Chaidanya et al. 2017). Feed intake depression results when the nerve impulses to the appetite centre in the hypothalamus are suppressed following stimulation by the peripheral thermal receptors. At the centre of this appetite regulation are two protein hormones, leptin and adiponectin, that are upregulated together with their receptors during heat stress (Morera et al. 2012). While adiponectin modulates feeding behaviour, acting as a starvation signal, leptin reduces feed intake by stimulating the hypothalamus axis (Rabe et al. 2008; Hoyda et al. 2012). During cold stress, however, there is increased concentrations of circulating nonesterified fatty acid (NEFA) and plasma corticosteroids (catecholamines and glucocorticoids) as well as in vivo proinflammatory cytokine gene expression (Alvarez and Johnson 1973; Nonnecke et al. 2009), and their combine effect alter metabolism and activate transcription factors (Collier et al. 2017).
Stress, in general, usually results in the excessive generation of free radicals and proinflammatory molecules. Such induced oxidative injury and inflammation are the bedrock of various metabolic disturbances, maladjustment and death (Srivastava and Kumar 2015). A depleted VC level often accompanies increased oxidative stress markers (e.g. lipid hydroperoxide) in ruminants (Kleczkowski et al. 2005), hence the need for exogenous supplementation. In an attempt to simulate water stress conditions traditionally experienced during the period of water scarcity and extreme drought, a 12-d water restriction study was conducted by Ghanem et al. (2008) on Awassi ewes, and they reported a drop in final weight from 70.25 kg (ad libitum control) to 48.75 kg (restricted group). According to the authors, supplementation of VC (2.5 g/d, dissolved in 12.5 mL of water) to each ewe raised the final weight to 55.5 kg. A similar weight loss reduction in a 75-day water restriction study on Xhosa goats was reported following VC (3 g/d) supplementation (Akinmoladun et al., 2020a). Though the difference was not significant, supplementation of VC (125 mg/kg) to Farafra sheep before transportation reduced the weight loss from 1.22 to 0.47 kg (Kassab and Mohammed 2014). Similarly, the final body weight and total feed intake of heat-stressed ram lambs increased by 19.5% and 15.7%, respectively, following a daily dose of vitamin C [45 mg/kg body weight] (Abd-Allah and Zanouny 2014). Deters and Hansen (2020) intramuscularly injected ascorbic acid (6 g sodium ascorbate per steer) to some steers shortly before an 18-h (1675 km) transit drive. According to the authors, after 75-day post-transit, the final body weight, average daily gain and dry matter intake increased from 429 to 436 kg, 1.67 to 1.84 kg and 9.0 to 9.5 kg, respectively. The stress-mediating mechanism of vitamin C in animals, leading to improved performance, is an indirect one. Apart from its antioxidant protective role, VC (ascorbate) functions as a co-substrate in collagen biosynthesis and remodeling (Archile-Contreras and Purslow, 2011), and these are processes that support skeletal muscle hypertrophy by facilitating the migration of satellite cells (Nishimura et al. 2008). Though subject to further research, another potential explanation of growth response to supplemental VC may be due to its role as a cofactor in the enzymes involved in carnitine biosynthesis. During stress, there is increased lipolysis due to feed intake reduction and cortisol elevation. Carnitine, however, is essential for transporting fatty acids into the mitochondria for catabolism. Hence, an increased need for carnitine is possible to utilize the much released fatty acid for growth and energy production (Deters and Hansen 2020).
Physiological response
Blood metabolites and immunomodulatory effect of vitamin C
Empirical observations have suggested an alteration in the host resistance following sustained exposure to stressful conditions. The consequence of prolonged exposure to stress is a decline in reactivity of immune cells as well as a higher incidence of infections, especially during summer (Dahl et al. 2020). According to Brown and Vosloo (2017), the elevated plasma concentrations of cortisol usually induced during heat stress have an immunosuppressant effect followed by a reduced response of lymphocytes to mitogens. The impact of stressors, including physiological status (pregnancy/lactation), thermal load or otherwise, usually results in the production of superoxide radicals.
Interestingly, such low immune status is usually co-related with a depleted plasma VC concentration. Rejeb et al. (2016) reported a compromised immune system in dairy cows alongside a considerable reduction in plasma VC concentration when exposed to a high environmental temperature for a long time. However, supplementation effects of VC on immune response and blood metabolites have not been consistent. Administration of ascorbic acid (2 g/h/d) to heat-stressed ram lambs during the summer season did not affect plasma total protein (TP), albumin (Alb), total cholesterol (TC) and calcium (Ca) concentrations. However, the increased TP in water-restricted Awassi ewes was lowered following VC (2.5 g/d) supplementation (Ghanem et al. 2008).
Similarly, concentrations of the liver enzymes (aspartate aminotransferase, AST and alanine aminotransferase, ALT) were lowered following VC supplementation (Abd-Allah and Zanouny 2014). In addition, elevated serum electrolytes (chloride, sodium, potassium, calcium and magnesium) in goats transported by road for 12 h (Ayo et al. 2009) or subjected to water restrictions (Akinmoladun et al. 2020a) were reduced following administration of VC. Despite these significant outcomes, Kim et al. (2012) reported increased albumin levels, creatinine and glucose in heat-stressed growing calves supplemented with VC. Aside from the elevated plasma cholesterol, VC failed to induce any significant change in blood biochemical indices in the summer heat-stressed Rahmani ewes (Hashem et al. 2016). The role of VC in energy balance, particularly during water stress, is still shrouded in controversy. Due to its role in norepinephrine and carnitine formations, VC increases free fatty acid concentration and transport across the mitochondrial membrane (Mahan et al. 2004). However, other authors have reported ascorbic acid to have a hypocholesterolemic effect when supplemented (Sahin et al. 2002; Yousef, 2004).
Psychological and physiological stress in ruminants constitutes a severe welfare concern. Usually, the released catecholamines during the alarm phase of stress are presumed to be the primary cause of lymphopenia and neutrophilia (Stanger et al. 2005). The combined effects of high relative humidity and ambient temperature, together with the presumed release of elevated corticosteroids, decrease cytokines IL-2 and reduce lymphocytes’ proliferation. Besides, lymphocytes cells become more vulnerable to apoptosis, contributing further to neutrophilia and lymphopenia (Dahl et al. 2020). Dietary supplementation of VC to heat-stressed swampy buffaloes significantly increased the mean concentration values of packed cell volume (PCV), haemoglobin (Hb) and lymphocytes. At the same time, the neutrophil remained the same compared to the control (Konwar et al. 2017). The administration of VC (100 mg/kg body weight) ameliorated the impact of stress from loading and transportation on the neutrophil, lymphocyte counts and neutrophil/lymphocyte ratio in goats (Minka and Ayo 2011). Similar enhancement of hematopoiesis in heat-stressed Rahmani ewes (Hashem et al. 2016) and Brown Swiss bulls (Ecu et al. 2000) with VC has been reported. This significant outcome is attributable to VC’s ability to facilitate increased iron absorption and enhance the immune system. The combined effect of physical and emotional stress can elicit eosinopenia, and it is attributable to an increased surge of plasma adrenaline and cortisol (Minka and Ayo, 2011). The mechanism of decrease (where applicable) is unclear. Still, it is suspected to be caused by decreased release from bone marrow, intravascular lysis (steroid-induced apoptosis of eosinophils), organs (spleen and liver), secludedness and increased tissue migration (Carter 2018). The depression in the eosinophils values in goats undergoing transportation stress remains unaffected in groups treated with VC (Minka and Ayo 2011).
Effect on plasma hormones and oxidative metabolites
Exposure to stress before slaughter may induce an excessive oxidative environment, significantly affecting feedlot performance, thus translating to producers’ losses (Duff and Galyean 2007; Akinmoladun et al. 2020b). The magnitude of the stressors and the oxidizing environment in vivo are a function of the type, intensity and duration of stressors and the animal’s vulnerability to them (Ferguson et al. 2001). The pre-slaughter phase includes all activities and management practices during growth, including the period when the animal is transported to the abattoir. During this period, there is a tendency to expose the animals to a range of challenging stimuli, including transportation stress and changing climatic variables (Ibironke et al. 2010). For example, Chirase et al. (2004) reported decreased serum antioxidant capacity, increased serum malondialdehyde (MOD) and higher susceptibility to bovine respiratory disease as well as mortality in beef calves transported (over 3500 km) to the feedlot. However, the administration of VC (100 mg/kg, i.m) to goats transported for 3.5 h reduced the MDA and superoxide dismutase (SOD) activities (Nwunuji et al. 2014). Also, supplementation of VC (10 g/animal/d) to pregnant and heat-stressed buffaloes during thermal stress reduced the mean superoxide dismutase (SOD) and catalase (CAT) activities (Ganaie et al. 2012). Thus, VC protects the body defence system and stabilizes animals’ health status by scavenging the excessive production of free radicals generated during stress (Sivakumar et al. 2010). According to Belge et al. (2003), VC modulates the decrease in MDA concentration by removing the singlet oxygen, hydroperoxyl, superoxide, lipid peroxyl and lipid-free radicals in animals subjected to stress.
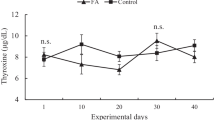
During stress, ascorbic acid exerts its inhibitory effect on cortisol. In addition, it plays a vital role in thermoregulation by its activities as an inhibitory vitaminergic neurotransmitter in the hypothalamus (Karanth et al. 2000). Also, the high induction of serum prolactin concentration during heat stress is attributed to hypothalamic-inhibitory stress response on peptides, which control prolactin secretion (Bernard et al. 2019). According to Civen et al. (1980), an increase of 1% in the rate of ascorbic acid intake would result in a 27% and 22% decrease of both plasma corticosterone and adrenal, respectively. Furthermore, studies indicated that the cortisol levels decreased significantly while the thyroid hormones (thyroxine [T4] and triiodothyronine [T3]) increased in heat-stressed swampy buffaloes (Konwar et al. 2017) and goats (Sivakumar et al. 2010; Akinmoladun et al. 2020b) following supplementation with VC. Contrarily, reported studies in water-stressed animals did not indicate a significant change in cortisol concentrations with VC supplementation (Ghanem et al. 2008; Parker et al. 2003). Also, VC failed to induce any significant difference in the cortisol of summer heat-stressed growing calves (Kim et al. 2012). Therefore, the variations in the response of plasma cortisol content have been attributed to possible differences in VC supplemented (Kim et al. 2012). Free radicals (H2O2) generated during stress inhibit the catalytic synthesis of thyroid hormones through its scavenging role on the enzymes, thyroperoxidase and 5ʹ mono-deiodinase, mediating the synthesis and conversion of T4 to T3 (Mancini et al. 2016). However, VC modulates the stress-induced reduction of these hormones by protecting the above enzymes from free radicals. Table 3 describes studies investigating the effect of vitamin C supplementation on plasma cortisol response in stressed animals.
Effect on reproduction
Due to its presence in ovaries, corpus luteum (CL) and follicular fluid (Das et al. 1993), VC hinders the apoptosis in murine cumulus oocyte and bovine granulosa cells (Murray et al. 2001) and reduces heat shock protein 70 (HSPA1A) and the levels of reactive oxygen species intracellularly (Castillo-Martin et al. 2014). However, plasma concentrations of antioxidants, especially VC, in ruminants are reported to decrease during stressful periods and at the beginning of ovarian cyclic activity and gestation (Salinas et al. 2017). This decrease may not be unconnected to the increased oxidative stress, usually resulting in gestation disorders, embryopathies, abortions, pre-eclampsia and low birth weights (Zhong and Zhou 2013) that are sometimes encountered in ruminants. The induced oxidative stress implicated by the depletion of antioxidant vitamins is explained by the active steroidogenesis and cyclic activities of ovaries. These findings suggest that in vivo production of ascorbic acid alone in ruminants may not be sufficient to sustain and modulate the various physiological processes.
Given the established correlation between VC concentrations in the corpus luteum and plasma progesterone levels as well as weights/diameters of corpora lutea (Serpek et al. 2001), it suffices to say VC affects reproduction functions and the rate of steroid hormone synthesis. Supplementation of VC to summer heat-stressed Rahmani ewes (Hashem et al. 2016) and hypoxic pregnant sheep (Parraguez et al. 2011) resulted in an increased number of ovulatory follicles lambing rate, lamb weight and fecundity. Also, the quality of bovine follicles and their survival in vitro improves with ascorbic acid supplementation (Thomas et al. 2001). Pernes et al. (2016) demonstrated that VC supplementation could enhance nuclear maturation and oocyte development competence in vitro. Supplementation of ascorbic acid (100 µM) to heat-stressed caprine cumulus oocyte complexes (incubated, 41 °C) for 24 h in vitro had an increased cumulus expansion (42.98% to 66.67%) and nuclear maturation (metaphase II stage; 40% to 60%) (Khanday et al. 2019). Similar improvement in nuclear maturation status and expansion of cumulus cells with VC inducing increased meiotic resumption of oocytes and higher proportions of oocytes reached at metaphase I and II stages have been reported in camel (Elsayed et al. 2015) and other caprines (Hammami et al. 2013). Though the oestrous response and duration, embryo retention rate and the number of does kidding were not affected, intramuscular administration of ascorbic acid (50 mg) enhanced conception rate in Red Sokoto goats (Omontese et al. 2014). This improvement in reproductive efficiency with VC, especially in vivo, has been attributed to increased survival and quality of the embryo by mechanisms such as (i) increased production of progesterone during the early stage of gestation; (ii) enhanced function and maturation of the placenta, uterus and oviduct; (iii) improved blastocyte development; and (iv) circumvention of foetal resorption (Hashem et al., 2015). However, the concentration of ascorbic acid that is of physiological significance during supplementation is a crucial research area. Higher concentrations of ascorbic acid in vitro can inhibit physiological processes in the ovary, resulting in follicular degeneration (Murray et al. 2001). In a study conducted by Andrade et al. (2012) on dose-dependent ascorbic acid supplementation to an in vitro culture of bovine preantral follicles, granulosa cell proliferation was stimulated following ascorbic acid supplementation and reached a maximum at 50 µg/ml of ascorbic acid. According to the authors, the maintenance of follicular viability during in vitro culture was not effective at higher (> 100 µg/ml) or lower doses (< 10 µg/ml) of ascorbic acid.
Apart from lacking significant cytoplasmic antioxidants, sperm cells have an abundance of polyunsaturated fatty acids in their membranes and are therefore susceptible to lipid peroxidation from O2− and H2O2 (Bansal and Bilaspuri 2010). Ascorbic acid plays a role in protecting sperm from ROS and maintaining the genetic integrity of sperm cells by protecting sperm DNA from oxidative damage (Eidan 2016). Because of these vital functions, deficient or reduced ascorbate levels in male animals have been correlated with an increased number of abnormal sperm, low sperm counts, agglutination and reduced fertility (Bansal and Bilaspuri 2010). Administration of ascorbic acid (20 mg/kg; i.m) for 30 days reportedly increases sperm concentration, semen volume and motility in rams (Sonmez and Demirci 2003). Also, subcutaneous injection of VC (20 mg and 40 mg/live weight) in Makhoz goats for 90 days linearly increased total sperm ejaculate and sperm volume (Fazeli et al. 2010). Modulation of ascorbic acid in ruminants’ reproductive efficiency is hinged on its ability to maintain lipids, DNA, proteins, enzymes and other antioxidants at the required physiological range and homeostasis sufficient for optimum reproductive interactions (Sonmez and Demirci 2003). However, the level of response and improvements recorded varies with concentrations of ascorbic acid used. The optimum concentration of VC required for maximum improvement is still a research gap.
Conclusion
While ruminants possess the ability to synthesize VC in the liver, the decreased plasma ascorbate concentration during stressful periods may compromise their immune system as well as production and performance indices. Research evaluating vitamin C requirements in ruminants and the attendant responses during stressful periods and the amount that will be supplemented to produce maximum improvement is currently advocated. Differences in ruminant types, breed, stressful stimuli, physiological status, route and form of VC administration may influence and cause variations in response following supplementation. Therefore, there is a need to explore these areas with a view to establishing the most effective supplementation rate of VC and route and form.
Data availability
Not applicable.
Code availability
Not applicable.
References
Abd-Allah M., Zanouny A.I. 2014. Ameliorative effect of ascorbic acid administration and chilled drinking water on ram lambs exposed to heat stress during summer season. Egypt Journal of Sheep and Goat Science, 9(3), 17-28.
Akinmoladun O.F., Fon F.N., Mpendulo C.T., Okoh O. 2021. Intake, nutrient digestibility, nitrogen and mineral balance of water restricted Xhosa goat supplemented with vitamin C. Open Agriculture, 6, 166-177
Akinmoladun O.F., Fon F.N., Mpendulo C.T., Okoh O. 2020a. Performance, heat tolerance response and blood metabolites of water restricted Xhosa goats supplemented with vitamin C. Translational Animal Science, 4(2), 1-15.
Akinmoladun O.F., Fon F.N., Mpendulo C.T. 2020b. Stress indicators, carcass characteristics and meat quality of Xhosa goats subjected to different watering regimen and vitamin C supplementation. Livestock Science, 238, 104083.
Akinmoladun O.F., Muchenje V., Fon F.N., Mpendulo, C.T. 2019. Small ruminants: farmers’ hope in a world threatened by water scarcity. Animals, 9(7), 456.
Alvarez M.B., Johnson H.D. 1973. Environmental heat exposure on cattle plasma catecholamine and glucocorticoids. Journal of Dairy Science, 56, 189-194.
Andrade E.R., van den Hurk R., Lisboa L.A., Hertel M.F., Melo-Sterza F.A., Moreno K. et al. (2012). Effect of ascorbic acid on in vitro culture of bovine preantral follicles. Zygote, 20, 379-388.
Archile-Contreras A.C., Purslow P.P. 2011. Oxidative stress may affect meat quality by interfering with collagen turnover by muscle fibroblasts. Food Research International 44, 582–588
Asres A., Amha, N. 2014. Effect of stress on Animal Health. A review. Journal of. Biology Agriculture and Healthcare, 4, 27.
Atkinson P.J. 1992. Investigation of the effects of transport and lairage on hydration state and resting behaviour of calves for export. The Veterinary Record 130, 413-416.
Ayo J.O., Minka N.S., Sackey A.K.B., Adelaiye A.B. 2009. Responses of serum electrolytes of goats to twelve hours of road transportation during the hot-dry season in Nigeria, and the effect of pretreatment with ascorbic acid. Onderstepoort Journal of Veterinary Research, 76, 409-418.
Banhegyi G., Braun L., Csala M., Puskas F., Mandl J. 1997. Ascorbic acid metabolism and its regulation in animals. Free Radical Biology Medicine, 23(5), 793-803.
Bansal A.K., Bilaspuri G.S. 2010. Impacts of oxidative stress and antioxidants on semen functions. Veterinary Medicine International, 686137.
Belge, F., Cinar, A., Selcuk, M. 2003. Effects of stress produced by adrenocorticotropinon lipid peroxidation and some antioxidants in vitamin C treated and non-treated chickens. South African Journal of Animal Science, 33, 201–205.
Bernabucci U., Ronchi B., Lacetera N., Nardone A. 2002. Markers of oxidative status in plasma and erythrocytes of transition dairy cows during hot season. Journal of Dairy Science, 85, 2173–2179.
Bernard V., Young J. Binart N. 2019. Prolactin-a pleiotropic factor in health and disease. Nature Review Endocrinology, 15, 356-365.
Biobaku K.T., Omobowale T.O., Akeem A.O., Aremu A., Okwelum N., Adah A.S. 2018. Use of goat interleukin-6, cortisol, and some biomarkers to evaluate clinical suitability of two routes of ascorbic acid administration in transportation stress. Veterinary World, 11(5), 674-680.
Brikas P. 1994. The role of GABA receptors in the control of the omVCl myoelectrical activity in sheep. Research in Veterinary Science, 56, 69–74.
Brown E.J., Vosloo A. 2017. The involvement of the hypothalamo-pituitary-adrenocortical axis in stress physiology and its significance in the assessment of animal welfare in cattle. Onderstepoort Journal of Veterinary Research, 84 (1), 1398.
Carr A.C., Maggini S. 2017. Vitamin C and immune function. Nutrients, 9(11), 1211.
Carter C.M. 2018. Alterations in blood components. Comprehensive Toxicology, 249–293.
Castillo-Martin M., Bonet S., Morato R.S.B., Yeste M. 2014. Supplementing culture and vitrification warming media with L-ascorbic acid enhances survival rates and redox status of IVP porcine blastocysts via induction of GPX1 and SOD1 expression. Cryobiology, 68(3), 451-458.
Chaidanya K., Soren N.M., Sejian V., Bagath M., Manjunathareddy G B., Kurien E.K. et al. (2017). Impact of heat stress, nutritional stress and combined (heat and nutritional) stresses on rumen associated fermentation characteristics, histophatology and HSP70 gene expression in goats. Journal of Animal Behaviour and Biometeorology, 5, 36-48
Chambial S., Dwivedi S., Shukla K.K., John P.J., Sharma P. 2013. Vitamin C in disease prevention and cure: An overview. Indian Journal of Clinical Biochemistry, 28(4), 314-328.
Chanda R. 1958. The effect of thyroxine on phosphatize, ascorbic acid and tocopherol content in the blood and milk of the cow and the buffalo. Current Science 3, 102–103.
Chatterjee I.B., Majumder A.K., Nandi B.K., Subramarian N. 1975. Synthesis and some major functions of vitamin C in animals. Annals of the New York Academy of Science, 258, 24-47.
Chirase, N.K., Green, L.W., Purdy, C.W. et al. 2004. Effect of transport stress on respiratory disease, serum antioxidant status, and serum concentrations of lipid peroxidation biomarkers in beef cattle. American Journal of Veterinary Research, 65(6), 860-864.
Civen M., Leeb J.E., Wishnow R.M., Morin R. 1980. Effect of dietary ascorbic acid and vitamin E deficiency on rat adrenal cholesterolester metabolism and corticosteroidogenesis. International Journal of Vitamin Nutrition Research, 50, 70–78.
Collier R.J., Renquist B.J., Xiao Y. 2017. A 100-year review: Stress physiology including heat stress. Journal of Dairy Science, 100, 10367-10380
Combs Jr. G.F., McClung J.P. 2017. Vitamin C. In: The Vitamins. Fifth edition. Elsevier, San Diego, CA, USA.
Combs Jr. G.F. 2008. Vitamin C. In: G.F. Combs (ed.). The Vitamins: Fundamental Aspects in Nutrition and Health. Third edition. Elsevier, San Diego, CA, USA.
Corpe C.P., Eck P., Wang J., Al-Haani H., Levine M. 2013. Intestinal dehydroascorbic acid (DHA) transport mediated by the facilitative sugar transporters, GLUT2 and GLUT8. Journal of Biological Chemistry, 288, 9092–9101.
Dahl G.E., Tao S., Laporta J. 2020. Heat stress impacts immune status in cows across the life cycle. Frontier of Veterinary Science, 7, 116.
Das P.C., Das K.P., Bagchi K., Dey C.D. 1993. Evaluation of tissue ascorbic acid status in different hormonal states of female rat. Life Science, 52, 1493-1498.
Deters E.L., Hansen S.L. 2020. Pre-transit vitamin C injection improves post-transit performance of beef steers. Animal, 14(10), 2083-2090
Duff, G.C., Galyean M.L. 2007. Board-Invited Review: Recent advances in management of highly stressed, newly received feedlot cattle. Journal of Animal Science 85, 823-840.
Ecü T.K.E., Keskün E., Durgun Z. 2000. The effects of environmental temperature and vitamin C on thyroid hormones of blood serum and some haematological values in Brown Swiss bulls. Turkish Journal of Veterinary Animal Science, 24, 353–359.
Eidan S.M. 2016. Effect on post-cryopreserved semen characteristics of Holstein bulls of adding combinations of vitamin C and either catalase or reduced glutathione to Tris extender. Animal Reproduction Science, 167, 1–7.
Elsayed M.A., Taha N.A., Hammam A.M.M., Sawiress F.A.R. 2015. Effect of antioxidant supplementation on in vitro maturation of Camelus dromedaries oocytes. Journal of Nature and Science, 13(2), 17-24.
Fazeli P., Zamiri M.J., Farshad A., Khalili B. 2010. Effects of vitamin C on testicular and seminal characteristics of Markhoz goats. Iranian Journal of Veterinary Research, 11(3), 32.
Ferguson, D.M., Bruce, H.L., Thompson, J.M., Egan, A.F., Perry, D., Shorthose, W.R. 2001. Factors affecting beef palatability–Farmgate to chilled carcass. Australian Journal of Experimental Agriculture, 41, 879–891.
Ganaie A.H., Hooda O.K., Singh S.V., Upadhdyay R.C. 2012. Response of vitamin C supplementation on biochemical, hormonal and physiological parameters of pregnant Murrah buffaloes during hot-humid conditions. Indian Journal of Animal Nutrition, 293, 214-21.
Ghanem A.M., Jaber L.S., Abi Said M., Barbour E.K., Hamadeh S.K. 2008. Physiological and chemical responses in water-deprived Awassi ewes treated with vitamin C. Journal of Arid Environment, 72, 141–149.
Grant M.M., Mistry N., Lunec J., Griffiths H. 2007. Dose-dependent modulation of the T cell proteome by ascorbic acid. British Journal of Nutrition, 97(1), 19-26.
Haiying L., Padilla L., Yoshimatsu K., Matsui T., Kitagawa M., Yano H. 2003. Determination of plasma vitamin C concentration in fattening cattle. Animal Science Journal, 74, 7-10.
Hammami S., Morató R., Romaguera R., Roura M., Catalá M.G., Paramio M.T., Mogas T., Izquierdo D. 2013. Developmental competence and embryo quality of small oocytes from pre-pubertal goats cultured in IVM medium supplemented with low level of hormones, insulin-transferrin-selenium and ascorbic acid. Reproduction in Domestic Animal, 48(2), 339-44.
Hashem N.M., Abd-Elrazek D., Abo-Elezz Z.R., Latiff M.G.A. 2016. Effect of vitamin A or C on physiological and reproductive response of Rahmani ewes during subtropical summer breeding season. Small Ruminant Research, 144, 313-319.
Hashem N.M., El-Azrak K.M., Nour El-Din A.N.M., Taha T.A., Salem M.H. 2015. Effect of GnRH treatment on ovarian activity and reproductive performance flow-prolific Rahmani ewes. Theriogenology, 83, 192–198.
Hidiroglou M. 1999. Technical note: forms and route of vitamin C supplementation for cows. Journal of Dairy Science, 82, 1831-1833.
Hidiroglou M., Batra T.R., Zhao, X. 1997. Comparison of vitamin C bioavailability after multiple or single oral dosing of different formulations in sheep. Reproduction Nutrition Development, 37, 443-448.
Hoyda T.D., Samson W.K., Ferguson A.V. 2012. Central system roles for adiponectin in neuroendocrine and automic function. In: V.R. Preedy R. J. Hunter (eds), Adipokines, Science Publishers, CRC Press, Boca Raton, FL, USA, pp. 167– 184
Ibironke A.A., McCrindle C.M.E., Adejuwon T.A., Cadmus S.I.B. 2010. Losses associated with mortality of cattle and camels during transportation to Oko- Oba abattoir, Lagos, Nigeria. European Journal Translational Myology-Basic Applied Myology, 1, 13-16.
Kandemir C., Kosum N., Taskin T. 2013. The effects of heat stress on physiological traits in sheep. Macedonian Journal of Animal Science, 3, 25–29.
Karanth S., Yu W.H., Walczewska A., Mastronardi C., McCann M. 2000. Ascorbic acid acts as an inhibitory transmitter in the hypothalamus to inhibit stimulated luteining hormone-releasing hormone release by scavenging nitric oxide. PNAS, 97, 1891–1896.
Karl J.P., Hatch A.M., Arcidiacono S.M., Pearce S.C., Pantoja-Feliciano I.G., Doherty L.A., Soares J.W. 2018. Effects of psychological, environmental and physical stressors on the gut microbiota. Frontier Microbiology, 9, 2013.
Kassab A.Y., Mohammed A.A. 2014. Ascorbic acid administration as anti-stress before transportation of sheep. Egyptian Journal Animal Production, 51 (1), 19-25.
Khalid T. B., Jibril M., Ismail A.O., Onifade K.I., Ismaila M.S., Agaie B.M. 2016. Ascorbic acid supplementation improves the quality of meat characteristics in sahel bucks exposed to long distance road transport. Alexandria Journal of Veterinary Science, 5(2), 24-30
Khanday S.B., Ahmed J.A., Nashiruddullah N., Sharma U., Chakraborty D. 2019. Effect of antioxidant ascorbic acid on in vitro maturation of Caprine oocytes under normal and elevated temperatures. Indian Journal Animal Research, 53(8), 1020-1024.
Kim J., Mamuad L.L., Yang C., Chul-Ju K.S., Ha J.K., Lee W., Cho K., Lee S. 2012. Hemato-biochemical and cortisol profile of Holstein growing-calves supplemented with vitamin C during summer season. Asian-Australasian Journal of Animal Science, 25, 361– 68.
Kleczkowski M., Klucinski W., Shaktur A., Sikora J. 2005. Concentration of ascorbic acid in the blood of cows with subclinical mastitis. Polish Journal of Veterinary Science, 8: 121-125.
Konwar D., Amonge T.K., Dutta D.J., Gogoi A.K., Borah R.S., Ch. Das G., Bhuyan R., Roychoudhury R. (2017). Dietary supplementation of ascorbic acid on hemato-biochemical and hormonal parameters in swamp buffaloes. Journal of Animal Research, 7(1), 39-47.
Kumar B., Manuja A., Aich P. 2012. Stress and its impact on farm animals. Frontier in Bioscience, 4, 1759-1767.
Kurutas E.B. 2016. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutrition Journal, 15, 17.
Li Y., Schellhorn H.E. 2007. New developments and novel therapeutic perspectives for vitamin C. Journal of Nutrition, 137, 2171-2184.
Liu L., Scheffer K.K., Shuefea D.M. 1994. Jugular infusion of vitamin C and color stability of beef. Journal of Animal Science, 72(Suppl. 1), 372.
MacLeod D.D., Zhang X., Kennely J.J., Ozimek L. 1996. Ascorbyl-2-polyphosphate as a source of ascorbic acid for dairy cattle. J Dairy Sci 79 (Suppl 1 233 (Abstr)
Mahan D.C., Ching S., Dabrowski K. 2004. Developmental aspects and factors influencing the synthesis and status of ascorbic acid in the pig. Annual Review of Nutrition, 24, 79–103.
Mancini A., Segni C.D., Raimondo S., Olivieri G., Silvestrini A., Meucci E., Curro D. 2016. Thyroid hormones, oxidative stress and inflammation. Mediators of Inflammation, 2016, 6757154.
Marks R. 2004. The stratum corneum barrier: The final frontier. Journal of Nutrition, 134, 2017–2021.
Matsui T. 2012. Vitamin C nutrition in cattle. Asian-Australasian Journal of Animal Science, 25 (5), 597-605
May J.M., Qu Z.C., Whitesell R.R. 1995. Ascorbic acid recycling enhances the antioxidant reserve of human erythrocytes. Biochemistry 34, 12721–12728.
McDowell L.R. 2000. Vitamins in Animal and Human Nutrition. Iowa State University Press, Ames, IA.
Minka N.S., Ayo J.O. 2010. Physiological response of food animals to road transportation stress. African Journal of Biotechnology, 9(40), 6601-6613.
Minka N.S., Ayo J.O. 2011. Modulating effect of ascorbic acid on transport-induced immunosuppression in goats. ISRN Veterinary Science, 749753.
Minka, N.S., Ayo, J.O. 2012. Assessment of thermal load on transported goats administered with ascorbic acid during the hot-dry conditions. International Journal of Biometeorology, 56, 333-341.
Minka N.S., Ayo J.O., Sackey A.K.B., Adelaiye A.B. 2009. Assessment and scoring of stresses imposed on goats during handling, loading, road transportation and unloading, and the effect of pretreatment with ascorbic acid. Livestock Science, 125, 275-282.
Mir N.A., Ashutosh A., Shergojry S.A., Wani S.A., Sheikh F.A. 2019. Effect of induced transportation stress in goats supplemented with vitamin C and jaggery during hot dry season. Biological Rhythm Research, 50(3), 389-399.
Mitmesser S.H., Ye Q., Evans M., Combs M. 2016. Determination of plasma and leukocyte vitamin C concentrations in a randomized, double-blind, placebo-controlled trial with Ester-C. Springer Plus, 5, 1161.
Mohamed H.E., Mousa H.M., Beynen A.C. 2004. Vitamin C status of Sudanese cattle and sheep. Journal of Biological Science, 4, 778–779.
Morera P., Basirico L., Hosoda K., Bernabucci U. 2012: Chronic heat stress up‐regulates leptin and adiponectin secretion and expression and improves leptin, adiponectin and insulin sensitivity in mice. Journal of Molecular Endocrinology, 48, 129– 138.
Mpendulo C.T., Akinmoladun O.F., Ikusika O.O., Chimonyo M. 2020. Effect of hydric stress on intake, growth performance and nutritional status of Nguni goats. Italian Journal of Animal Science, 19(1), 1071-1078
Murray A.A., Molinek M.D., Baker S.J., Kojima F.N., Smith M.F., Hillier S.G., Spears N. 2001. Role of ascorbic acid in promoting follicle integrity and survival in intact mouse ovarian follicles follicles in vitro. Reproduction, 121(1), 89-96.
Nardone A., Ronchi B., Lacetera N., Ranieri M.S., Bernabucci U. 2010. Effects of climate changes on animal production and sustainability of livestock systems. Livestock Science, 130, 57–69.
Nejad J.G., Sung K.I. 2017. Behavioral and physiological changes during heat stress in Corriedale ewes exposed to water deprivation. Journal of Animal Science and Technology, 59, 13.
Nishimura T., Nakamura K., Kishioka Y., Kato-Mori Y., Wakamatsu J.I., Hattori A. 2008. Inhibition of matrix metalloproteinases suppresses the migration of skeletal muscle cells. Journal of Muscle Research and Cell Motility 29, 37–44.
Nonnecke B.J., Foote M.R., Miller B.L., Fowler M., Johnson T.E., Horst R. L. 2009. Effects of chronic environmental cold on growth, health, and select metabolic and immunologic responses of preruminant calves. Journal of Dairy Science, 92, 6134-6143.
Novais A., Monteiro S., Roque S., Correia-Neves M., Sousa N. 2017. How age, sex and genotype shape the stress response. Neurobiology of Stress, 6, 44-56.
Nwe T.M., Hori E., Manda M., Watanabe S. 1996. Significance of catecholamines and cortisol levels in blood during transportation stress in goats. Small Ruminant Research, 20(2), 129–135.
Nwunuji T.P., Mayowa O.O., Yusoff S.M., Bejo S., Salisi S., Mohd E.A. 2014. The ameliorative effect of ascorbic acid on the oxidative status, live weight and recovery rate in road transport stressed goats in a hot humid tropical environment. Animal Science Journal, 85(5), 611-616.
Omontese B.O., Rekwot P.I., Ate I.U., Rwuaan J.S. 2014. Ascorbic acid enhances conception rates of Red Sokoto goats following progestin (FGA-30 ®, FGA-45® and CIDR®) treatment during rainy season. Livestock Research for Rural Development, 26, 130.
Omontese B.O., Adewuyi A.B., Rekwot P.I., Nwannenna A.I., Rwuaan J.S. 2017. Effect of ascorbic acid on the conception rate of Yankasa ewes after estrus synchronization. Revue d’Elevage et de Medecine Veterinaire des Pays Tropicaux, 70 (1): 9-12, https://doi.org/10.19182/remvt.31390
Padayatty S.J., Levine M. 2016. Vitamin C physiology; the unknown and the unknown and Goldilocks. Oral Disease, 22(6), 463-493.
Padilla L., Matsui T., Ikeda S., Kitagawa M., Yano H. 2007. The effect of vitamin C supplementation on plasma concentrations and urinary excretion of vitamin C in cattle. Journal of Animal Science, 85, 3367-3370.
Parker A.J., Hamlin G.P., Coleman C.J., Fitzpatrick L. 2003. Dehydration in stressed ruminants may be the result of a cortisol-induced diuresis. Journal of Animal Science, 81, 512–519.
Parraguez V.H., Atlagich M., Araneda O., García C., Mu˜noz A., De Los Reyes M., Urquieta, B. 2011. Effects of antioxidant vitamins on newborn and placental traits in gestations at high altitude: comparative study in high and low altitude in native sheep. Reproduction Fertility and Development, 23, 285–296.
Pernes A., Miclea I., Zahan M., Miclea V., Orlovschi D., Codea, A.R. 2016. The influence of ascorbic acid on in vitro maturation of canine oocytes. Bulletin UASVM Animal Science and Biotechnology, 73(2), 230-234.
Pliego-Pliego K., Sanchez-Torres T. Salinas-Rios T., Crosby-Galvan M.M., Cuellar C.N., Mora J.L.C. et al. 2019. Reproductive and oxidative status of ewes supplemented with vitamin C during oestrus synchronization and early gestation. South African Journal of Animal Science, 49(4), 664-674.
Popkin B.M., D’Anci K.E., Rosenberg I.H. 2010. Water, hydration and Health. Nutrition. Review, 68(8), 439-458.
Pullar J.M., Carr A.C., Vissers M.C.M. 2017. The roles of vitamin C in skin health. Nutrients 9(8), 866.
Rabe K., Lehrke M., Parhofer K.G., Broedl U.C. 2008. Adipokines and insulin resistance. Molecular Medicine 14, 741– 751.
Ranjan R., Ranjan A., Dhaliwal G.S., Patra R.C. 2012. L-ascorbic acid (vitamin C) supplementation to optimize health and reproduction in cattle. Veterinary Quarterly, 32(3-4); 145-150
Rejeb M., Sadraoui R., Najar T. 2016. Role of Vitamin C on immune function under heat. Proceedings of National Academy of Science, USA, 94, 13816–13819.
Sahin K., Kucuk O., Sahin N., Sari M. 2002. Effects of vitamin C and vitamin E on lipid peroxidation status, serum hormone, metabolite, and mineral concentrations of Japanese quails reared under heat stress (34 1C). International Journal of Vitamin and Nutrition Research, 72, 91–100.
Salinas R.T., Sánchez T.E.M.T., Díaz C.A., Cordero M.J.L., Guinzberg P.R., Rabanales M.J.L., Figueroa V.J.L., Hernández B.J. 2017. Oxidative state of ewes with different number of parity during gestation y lactation. Pesquisa. Veterinaria Brasileira, 37, 1405-1410.
Seifi H.A., Mohri M., Delaramy M., Harati M. 2010. Effect of short-term over-supplementation of ascorbic acid on hematology, serum biochemistry and growth performance of neonatal dairy calves. Food Chemistry and Toxicology, 48, 2059-2052.
Serpek B., Baspinar N., Haliloglu S., Erdem H. 2001. The relationship between ascorbic acid, oestradiol 17 and progesterone in plasma and in ovaries during the sexual cycle in cattle. Revue. Méd. Vét. 152, 253–260.
Sivakumar A.V.N., Singh G., Varshney V.P. 2010. Antioxidants supplementation on acid base balance during heat stress in goats. Asian-Australasian Journal of Animal Science, 23(11), 1462 – 68.
Smith S.B., Kawachi H., Choi C.B., Choi C.W., Wu G., Sawyer J.E. 2009. Cellular regulation of bovine intramuscular adipose tissue development and composition. Journal of Animal Science, 87(E. Suppl.), E72–E82.
Sonmez M., Demirci E. 2003. The effect of intramuscular vitamin C administration on semen quality in rams. Journal of Firat University, Health and Veterinary Science, 17, 195-201.
Srivastava K.K., Kumar R. 2015. Stress, oxidative injury and disease. Indian Journal of Clinical Biochemistry, 30(1), 3-10.
Stanger K.J., Ketheesan N., Parker A.J., Coleman C.J., Lazzaroni S.M., Fitzpatrick L.A. 2005. The effect of transportation on the immune status of Bos indicus steers. Journal of Animal Science, 11, 2632–2636.
Tauler P., Aguilo, A., Gimeno I., Fuentespis E., Tur J.A., Pons A. 2003. Influence of vitamin C supplementation on endogenous antioxidant defense during exhaustive exercise. European Journal of Physiology, 440, 658– 664.
Thomas F.H., Leask R., Sršen V., Riley S.C., Spears N., Telfer E.E. 2001. Effect of ascorbic acid on health and morphology of bovine preantral follicles during long-term culture. Reproduction, 122, 487-495.
Tu Y., Njus D., Schlegel H.B. 2017. A theoretical study of ascorbic oxidation and HOO/O2 radical scavenging. Organic & Biomolecular Chemistry, 15, 4417.
Tyler P.J., Cummins K.A. 2003. Effect of dietary ascorbyl-2-phosphate on immune function after transport to a feeding facility. Journal of Dairy Science, 86, 622–629.
Urban-Chmiel R., Kankofer M., Wernicki A., Albera E., Puchalski A. 2009. The influence of different doses of -tocopherol and ascorbic acid on selected oxidative stress parameters in in vitro culture of leucocytes isolated from transported calves. Livestock Science, 127, 365–370.
Weller R.H., John A., Savin J., Dahl M. 2008. The Function and Structure of Skin. 5th ed. Wiley-Blackwell; Massachusetts, MA, USA.
Wu C.C., Doriarajan T., Lin T.L. 2000. Effect of ascorbic acid supplementation on the immune response of chickens vaccinated and challenged with infectious bursal disease virus. Veterinary Immunology and Immunopathology, 74, 145-152.
Yousef M.I. 2004. Aluminium-induced changes in hemato-biochemical parameters, lipid peroxidation and enzyme activities of male rabbits: protective role of ascorbic acid. Toxicology, 199, 47–57
Zhong R-Z., Zhou D-W. 2013. Oxidative stress and role of natural plant derived antioxidants in animal reproduction. Journal Integrative Agriculture, 12, 1826-1838.
Acknowledgements
The author wants to appreciate the contribution of my main supervisor, late Prof. Voster Muchenje. This review was part of the research funded by the National Research Foundation/The World Academy of Science (Grant number 110851).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The authors confirm that the ethical policies of the journal, as noted on the journal’s author guidelines page, have been adhered to. No ethical approval was required as this is a review article with no original research data.
Conflict of interest
The author declares no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Akinmoladun, O.F. Stress amelioration potential of vitamin C in ruminants: a review. Trop Anim Health Prod 54, 24 (2022). https://doi.org/10.1007/s11250-021-03026-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11250-021-03026-1