Abstract
Panting syndrome and respiratory infection have been recorded in complicated cases of foot and mouth disease (FMD) in cattle. However, investigations on the causative agents of respiratory disease in such cases are scarce. In this study, 30 animals (13 buffalo and 17 cattle) suffering from respiratory distress associated with signs of FMD were examined. Serum samples were collected and FMD infection was confirmed. Bacteriological examination of lungs from eight necropitized cases revealed the presence of C. perfringens. Multiplex polymerase chain reaction (mPCR) was performed on the positive samples followed by sequencing analysis. The alpha toxin gene (plc) of C. perfringens was identified in six cases. The present investigation highlights the role of clostridial infection as a complication of FMD in cattle and buffalo. This is the first report identifying the C. perfringens toxins from lung of animals with respiratory distress associated with FMD infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
FMD has a great endemic importance in Egypt and various African countries (Knowles et al. 2007; Rweyemamu et al. 2008). The disease is expressed by a high morbidity with low mortality rates and is characterized by the development of vesicles and erosions in the mucosa of the mouth and on the skin between and above the hoofs (Alexandersen et al. 2003; Lee et al. 2013). Importantly, foot and mouth disease virus (FMDV) produces a transient immunosuppression in the affected animals that provides an opportunity for a secondary bacterial infection resulting in severe clinical diseases and sometimes death of the affected animal (Chhabra et al. 2004; Díaz-San Segundo et al. 2009). Indeed, a recent study had demonstrated the complications of FMD-infected Indian gaurs (Bosgaurus) with Pasteurellamultocida causing respiratory manifestations (Chandranaik et al. 2015). Moreover, Pasteurellamultocida has been proposed to be the most common secondary bacterial pathogen responsible for deaths during FMD outbreaks (Venkataramanan et al. 2005). FMD has been also found to be a potential risk factor hemorrhagic septicemia in buffalo and cattle in Karachi, Pakistan (Moustafa et al. 2017). Moreover, there was an evidence of increased rate of mastitis after one month of outbreaks of FMD in the dairy cattle (Lyons et al. 2015).
Although Clostridia are uncommon causes of pleuropulmonary infection, many researchers reported that Clostridium perfringens is a causative agent of pneumonia in animals (Fernandez-Miyakawa et al. 2007; Uzal et al. 2002).
Alpha (α), beta (β), epsilon (μ), and iota represent the four main C. perfringens toxins, forming the basis of categorizing this bacterium into five different types, from A to E (Gurjar et al. 2008). Therefore, recognition of bacteria is not only sufficient, but determination of exotoxin type is also crucial (Sasani et al. 2013). Alpha toxin is produced by all five types and is a phospholipase C (PLC) that can hydrolyze lecithin into phosphorylcholine and diglyceride and is proposed to be a major factor responsible for the organism’s tissue pathology (Hatheway 1990; Katayama et al. 1993). Consequently, diagnosis of Clostridium infection can be achieved by molecular detection of C. perfringens alpha toxin gene (plc gene). In the present study, we identified and characterized the plc gene of C. perfringens from cattle and buffalo suffering from respiratory syndrome–associated complicated cases of FMD disease.
Materials and methods
Animals and clinical examination
A total of 30 animals (13 buffalo and 17 cattle) at age range between 4 months and 2 years were studied. The animals were related to 12 farms located at Qalyubia governorate, Egypt. Initially, selection of the animals under study was based on the presence of clinical signs of FMD infection (Constable et al. 2016), associated with severe panting and nasal discharge.
Blood samples
Venous blood samples were collected via jugular vein-puncture from 30 animals under investigation. The separated serum samples were subsequently stored at − 20 °C for further ELISA screening.
ELISA
The commercial FMD 3ABC ELISA kit (IZSLER: Brescia, Italy) was used for detection of FMDV infection via detection of antibodies against non-structural proteins of FMDV (De Diego et al. 1997).
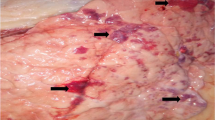
A postmortem examination was carried out on eight dead animals. Lung, spleen, heart, and liver samples were collected from eight dead animals and kept on ice until further use for identification of bacterial infection.
Bacteriological examination
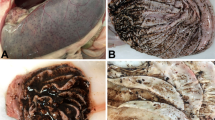
Tissue samples were homogenized and serially diluted. The aforementioned dilutions were inoculated into blood base agar with 7% sterile blood under aerobic. Inoculation was also carried out on C. perfringens base agar enriched with supplements (Oxiod, UK). The inoculated plates were placed in modified atmosphere jars and were incubated under anaerobic conditions at 37 °C for 20 ± 2 h. Identification of C. perfringens was accomplished initially by classical microbiological techniques described previously (Brett 1994), microscopical appearance (Beveridge 2001), and by conventional biochemical tests (Koneman et al. 1992).
DNA extraction
DNA extraction from recovered isolated was performed using the QIAamp DNA Mini kit (Qiagen, Germany, GmbH). Briefly, a total of 200 μl of the sample suspension was incubated with 10 μl of proteinase K and 200 μl of lysis buffer at 56 °C for 10 min. After incubation, 200 μl of 100% ethanol was added to the lysate. The sample was then washed and centrifuged following the manufacturer’s recommendations. DNA was eluted with 100 μl of elution buffer provided in the kit. DNA concentration was measured by using a Nano-Drop TMND-1000 Spectrophotometer (PeqlabBiotechnolo-gie GmbH, Erlangen, Germany).
Molecular detection and sequencing of C. perfringens toxin gene
Multiplex polymerase chain reaction assay was used for the detection of C. perfringens toxin (alpha, beta, iota, and epsilon) genes (Yoo et al. 1997). All protocols, primer sequences, amplification, and sequencing conditions were employed as previously described (Lorenz 2012).
The forward and reverse primer sequences of C. perfringens toxins, respectively, were as follows: alpha toxin, GTTGATAGCGCAGGACATGTTAAG and CATGTAGTCATCTGTTCCAGCATC; beta toxin, ACTATACAGACAGATCATTCAACC and TTAGGAGCAGTTAGAACTACAGAC. Epsilon toxin, ACTGCAACTACTACTCATACTGTG and CTGGTGCCTTAATAGAAAGACTCC. However, for iota toxin, the sequences were GCGATGAAAAGCCTACACCACTAC and GGTATATCCTCCACGCATATAGTC, respectively.
Primers were utilized in a 25-μl final reaction volume containing 12.5 μl of EmeraldAmp Max PCR Master Mix (Takara, Japan), 1 μl of each primer (20 pmol concentration), 4.5 μl of water, and 6 μl of DNA template. The reaction was performed in an applied biosystem 2720 thermal cycler. The amplification condition for all surveyed toxins was done with initial denaturation at 94 °C for 5 min, and 35 cycles of denaturation at 94 °C for 30 s, primer annealing at 55 °C for 45 s. The primer annealing was followed by amplification at 72 °C for 1 min, followed by a final extension at 72 °C for 5 min. Subsequently, 1.5% agarose gel was used for gel electrophoresis of the PCR products. Amplified products were stained with ethidium bromide (Sigma-Aldrich, St. Louis, MO, U.S.A.) and visualized under UV-transilluminator (Hybaid, UK). The detected product size that was similar to the size of positive control for a particular species was considered positive. For detection of C. perfringens alpha toxin gene, ATCC® 13124 was used as positive control. For other toxins, samples previously confirmed in reference lab for veterinary quality control on poultry production to be positive were used. Negative control was confirmed; samples in the same lab to be negative were also used. Sequencing of the plc gene was performed as previously described (Tsutsui et al. 1995).
Phylogenetic analysis of the plc gene
The data were analyzed by the National Center for Biotechnology Information (NCBI, http://www.ncbi.nlm.nih.gov/) using BLAST analysis. The previously deposited sequences of phospholipases C gene in GenBank were used to perform phylogenetic analysis. This study was conducted on the plc alleles of 24 C. perfringens strains. The used accession number in phylogenetic analysis was as follows: MF803086 (current study), KP143660 (C. perfringens-saigas 2012), KP143659 (C. perfringens-saigas 2011), KP143658 (C. perfringens-saigas 2010), JQ071566 (C. perfringens-LF-4A), JQ071542 (C. perfringens-AI-3A), JF298802 (C. perfringens-CPB228), FR688009 (C. perfringens-H2-3), FR687999 (C. perfringens-AG07-1), FR687998 (C. perfringens-AG04-3), FJ655569 (C. perfringens-ELA03), EU839836 (C. perfringens-S03), DQ184137 (C. perfringens-NRRL B-23782), D32128 (C. perfringens-NCBI19681), L43547 (C. perfringens-alpha toxin complete cds), M24904 (C. perfringens-alpha toxin complete cds), X13608 (C. perfringens-alpha toxin complete cds), JQ071570 (C. perfringens-RAN-21b), FR688011 (C. perfringens-AL05-12), KJ729015 (C. perfringens-Zon-CU-ah16), AM888383 (C. perfringens-JS8), DQ184056 (C. perfringens-NRRL B-23658), KX793136 (C. perfringens-Zon-CU012), KY386862 (C. perfringens-CP3), KY038859, C. perfringens-CDDD). All the given sequences were trimmed and aligned with our obtained sequence using Clustal omega software (Sievers and Higgins 2014). Phylogenetic analysis was performed using MEGA 6 software (Tamura et al. 2013). Phylogeny was executed using both neighbor-joining and maximum-parsimony methods, where bootstrap statistical test was adjusted on 100 replicates. The deduced amino acid sequences were also analyzed using MEGA 6 software for confirmation of the phylogenetic relationship between nucleotide sequences.
Results
Using ELISA screening, FMD viral infection was serologically detected in all suspected cases (100%). Respiratory manifestations including respiratory distress, nasal discharge, and panting were observed (13 buffalo and 17cattle).
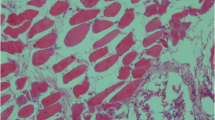
Postmortem findings on 8 out of 30 infected animals showed different PM lesions (Table 1). C. perfringens was recovered from lung samples of only six cases. Briefly, on blood base agar with 7% sterile blood, C. perfringens colonies were 2–3 mm in diameter, rounded, raised, glistened, and showed double zone of hemolysis. On TSC agar, C. perfringens colonies were black in color. Microscopically, C. perfringens isolates appeared to be Gram-positive short plumb bacilli rarely having central oval non-bulging endospores. Biochemical tests were confirmative to C. perfringens, while multiplex PCR revealed presence of C. perfringens alpha toxin gene (Fig. 1).
Multiplex PCR of the C. perfringens enterotoxin (CPE) plc gene in the lung tissue of C. perfringens-infected cattle and buffalo. PC, positive control; NC, negative control; Lg, lung; a, alpha; b, beta; e, epsilon; and I, iota toxins of C. perfringens; M indicates a 100-bp DNA ladder. The expected size of the PCR product was 402 bp, 236 bp, 541 bp, and 317 bp for alpha, beta, epsilon, and iota toxins of C. perfringens, respectively
In the current study, the neighbor-joining and maximum parsimonies were performed on the obtained nucleotide sequences of phospholipase C of C. perfringens, which was further confirmed by performing phylogenetic analysis of deduced amino acid sequences of our isolated strain and revealed a close relationship between the obtained sequence from the current study and the phospholipase C isolated from Sharkia governorate, Egypt, among other closely related species (Fig. 2). The computed pairwise genetic difference was executed using Poisson model and showed the similar genetic difference between our isolated species and other species except JQ071570 (Brazil) and AM888383 (India); the highest genetic divergent strain was in KY038859 (China). Surprisingly, an Egyptian strain isolated from the Giza governorate (KJ729015) revealed a significant genetic divergent in comparison with the isolate recovered from the current study, although it was also recovered from Egypt.
Discussion
FMD is a highly contagious, vesicular disease affecting primarily cloven-hoofed animals with severe economic consequences worldwide (Sutmoller et al. 2003). Indeed, there is scarcity in detecting the bacterial agent associated with FMD clinically infected cases.
To the best of the author’s knowledge, this is the first study that reports Clostridium infection as a complication of FMD in buffalo and cattle worldwide. Although pneumonia related to C. perfringens has rarely been described (Chen et al. 2011), the respiratory distress by clinical examination and pneumonia by postmortem examination in FMD-infected cattle and buffalo complicated with C. perfringens infection observed in this study is coincided with previous report that documented C. perfringens to affect the respiratory system as it causes severe acute pulmonary edema, which was particularly marked in the interlobular septa in calves (Constable et al. 2016), lung edema in mouse (Fernandez-Miyakawa et al. 2007), interstitial pneumonia, purulent fibrinous bronchopneumonia, abscess, and interlobular fibrosis sick calves and cow cattle (Sasani et al. 2013). Moreover, the presence of necrotizing pneumonia in a woman due to C. perfringens was previously recorded (Palmacci et al. 2009). The complications with bacterial infections in such cases might be attributed to the suppression in the body immunity that is associated with viral infection as previously proposed (Francoz et al. 2015; Grubman and Baxt 2004; Howard 2007). Moreover, outbreaks of respiratory form of hemorrhagic septicemia (HS) followed FMD in the same animals have been reported (Verma et al. 2004). Preceding FMD infection probably lowers the resistance to diseases or causes stress on the animals, leading to precipitation of HS. Several predisposing factors for systemic clostridial dissemination include intraoral pathology (carious teeth or gingival disease) and intrabdominal pathology such as malignancy and enteric vascular malformation (Craven 1989; Tanabe et al. 1989).
To investigate the genetic diversity of the plc gene among recovered isolates, nucleotide sequences were compared with the other corresponding strain of C. perfringens. The results proposed a close proximity between the isolated strain and the plc gene isolated from C. perfringens in Sharkia governorate. Interestingly, the isolated strain of C. perfringens type A showed a divergent relationship in comparison with the currently studied isolate, which was also observed that the plc gene showed a minimum correlation with the source of infection (Siqueira et al. 2012).
The high genetic diversity observed among the plc gene isolated from different geographical regions creates a roadblock in constructing a solid phylogenetic relationship among the same species of C. perfringens, but the close genetic relationship between some Egyptian strain of clostridial alpha toxin was also existed. It has been found that most toxin plasmids carry a transfer of clostridial plasmid locus–mediating conjugation, which likely explains the presence of similar toxin plasmids in otherwise unrelated C. perfringens strains (Freedman et al. 2015). Moreover, certain similarities were also detected between different species of Clostridia toxins as recorded between C. heamolyticum and C. Botulinum (Nakamura et al. 1983). Another similarity was also observed between C. sordellii and C. bifermentans regarding the plc gene (Karasawa et al. 2003).
Although this is the first study addressing the Clostridium infection associated with FMD in cattle and in buffalo in Egypt, some limitations in this study including the small sample size used in this study may not allow obtaining a concrete conclusion. Therefore, future studies should consider non-examined provinces and other animal species subjected to the infection by FMDV.
In conclusion, the present investigation draws the attention to the FMD-infected cases associated with C. perfringens infection in cattle and buffalo in Egypt. Cattle raisers are recommended to undertake the proper management practice and preventive measures to avoid co-infection with bacterial agents, especially C. perfringens.
References
Alexandersen, S., Zhang, Z., Donaldson, A., Garland, A., 2003. The pathogenesis and diagnosis of foot-and-mouth disease. Journal of Comparative Cathology, 129, 1–36.
Beveridge, T.J., 2001. Use of the Gram stain in microbiology. Biotechnic and Histochemistry, 76, 111–118.
Brett, M., 1994. Outbreaks of food-poisoning associated with lecithinase-negative Clostridium perfringens. Journal of Medical Microbiology, 41, 405–407.
Chandranaik, B.M., Hegde, R., Shivashankar, B.P., Giridhar, P., Muniyellappa, H.K., Kalge, R., Sumathi, B.R., Nithinprabhu, K., Chandrashekara, N., Manjunatha, V., 2015. Serotyping of foot and mouth disease virus and Pasteurella multocida from Indian gaurs (Bos gaurus), concurrently infected with foot and mouth disease and haemorrhagic septicaemia. Tropical Animal Health and Production, 47, 933–937.
Chen, C.-H., Ho, S.-Y., Lin, K.-H., 2011. Necrotizing Pneumonia Associated with Septicemia Caused by Clostridium perfringens: A Case Report. Internal Medicine, 22, 287–291.
Chhabra, R., Sharma, R., Kakker, N.K., 2004. Comparative immunogenecity of foot and mouth disease virus antigens in FMD-haemorrhagic septicaemia combined vaccine and FMD vaccine alone in buffalo calves. Indian Journal of Experimental Biology, 42, 259–264.
Constable, P.D., Hinchcliff, K.W., Done, S.H., Grünberg, W., 2016. Veterinary medicine-e-book: a textbook of the diseases of cattle, horses, sheep, pigs and goats. Elsevier Health Sciences.
Craven, C., 1989. Fatal Clostridium perfringens septicemia associated with gastrointestinal arteriovenous malformations (vascular ectasias). Archives of pathology and laboratory Medicine, 113, 534–535.
De Diego, M., Brocchi, E., Mackay, D., De Simone, F., 1997. The non-structural polyprotein 3ABC of foot-and-mouth disease virus as a diagnostic antigen in ELISA to differentiate infected from vaccinated cattle. Archives of Virology, 142, 2021–2033.
Díaz-San Segundo, F., Rodríguez-Calvo, T., de Avila, A., Sevilla, N., 2009. Immunosuppression during acute infection with foot-and-mouth disease virus in swine is mediated by IL-10. PLoS One, 4, e5659.
Fernandez-Miyakawa, M.E., Sayeed, S., Fisher, D.J., Poon, R., Adams, V., Rood, J.I., McClane, B.A., Saputo, J., Uzal, F.A., 2007. Development and application of an oral challenge mouse model for studying Clostridium perfringens type D infection. Infection and Immunity, 75, 4282–4288.
Francoz, D., Buczinski, S., Bélanger, A., Forté, G., Labrecque, O., Tremblay, D., Wellemans, V., Dubuc, J., 2015. Respiratory pathogens in Quebec dairy calves and their relationship with clinical status, lung consolidation, and average daily gain. Journal of Veterinary Internal Medicine, 29, 381–387.
Freedman, J.C., Theoret, J.R., Wisniewski, J.A., Uzal, F.A., Rood, J.I., McClane, B.A., 2015. Clostridium perfringens type A-E toxin plasmids. Research in Microbiology, 166, 264–279.
Grubman, M.J., Baxt, B., 2004. Foot-and-mouth disease. Clinical Microbiology Reviews 17, 465–493.
Gurjar, A., Hegde, N., Love, B., Jayarao, B., 2008. Real-time multiplex PCR assay for rapid detection and toxintyping of Clostridium perfringens toxin producing strains in feces of dairy cattle. Molecular and Cellular Probes, 22, 90–95.
Hatheway, C.L., 1990. Toxigenic clostridia. Clinical microbiology reviews 3, 66–98.
Howard, B.G., 2007. Alimentary system. In: Pathologic basis of veterinary medicine. McGavin MD, Zachary JF, (ed.). (4thedn), Mosby Elsevier, USA 30.
Karasawa, T., Wang, X., Maegawa, T., Michiwa, Y., Kita, H., Miwa, K., Nakamura, S., 2003. Clostridium sordellii phospholipase C: gene cloning and comparison of enzymatic and biological activities with those of Clostridium perfringens and Clostridium bifermentans phospholipase C. Infection and Immunity, 71, 641–646.
Katayama, S., Matsushita, O., Minami, J., Mizobuchi, S., Okabe, A., 1993. Comparison of the alpha-toxin genes of Clostridium perfringens type A and C strains: evidence for extragenic regulation of transcription. Infection and Immunity 61, 457–463.
Knowles, N.J., Wadsworth, J., Reid, S.M., Swabey, K.G., El-Kholy, A.A., El-Rahman, A.O.A., Soliman, H.M., Ebert, K., Ferris, N.P., Hutchings, G.H., 2007. Foot-and-mouth disease virus serotype A in Egypt. Emerging Infectious Diseases 13, 1593.
Koneman, E., Allen, S., Janda, W., Schreckenberger, P., Winn, W., 1992. Diagnostic Microbiology, 4. baski. J В Lippincott Co, Philadelphia, USA.
Lee, H.-S., Lee, N.-H., Seo, M.-G., Ko, Y.-J., Kim, B., Lee, J.-B., Kim, J.-S., Park, S., Shin, Y.-K., 2013. Serological responses after vaccination of growing pigs with foot-and-mouth disease trivalent (type O, A and Asia1) vaccine. Veterinary Microbiology, 164, 239–245.
Lorenz, T.C., 2012. Polymerase chain reaction: basic protocol plus troubleshooting and optimization strategies. Journal of Visualized Experiments, 22, e3998.
Lyons, N.A., Alexander, N., Strk, K.D., Dulu, T.D., Rushton, J., Fine, P.E., 2015. Impact of foot-and-mouth disease on mastitis and culling on a large-scale dairy farm in Kenya. Veterinary Research, 46, 015–0173.
Moustafa, A.M., Ali, S.N., Bennett, M.D., Hyndman, T.H., Robertson, I.D., Edwards, J., 2017. A Case-control Study of Haemorrhagic Septicaemia in Buffaloes and Cattle in Karachi, Pakistan, in 2012. Transboundary and Emerging Diseases, 64, 520–527.
Nakamura, s., Kimura, i., Kamakawa, k., Nishida, S., 1983. Taxonomic relationships among Clostridium novyi types A and B, Clostridium haemolyticum and Clostridium botulinum type C. Microbiology, 129, 1473–1479.
Palmacci, C., Antocicco, M., Bonomo, L., Maggi, F., Cocchi, A., Onder, G., 2009. Necrotizing pneumonia and sepsis due to Clostridium perfringens: a case report. Cases Journal, 2, 50.
Rweyemamu, M., Roeder, P., Mackay, D., Sumption, K., Brownlie, J., Leforban, Y., Valarcher, J.F., Knowles, N., Saraiva, V., 2008. Epidemiological patterns of foot-and-mouth disease worldwide. Transboundary and Emerging Diseases, 55, 57–72.
Sasani, F., Marzban, H., Javanbakht, J., Moosakhani, F., Imanparast, M., 2013. The Relationship between Microscopic Lesions and Different Types of Clostridium perfringens and their Related Toxins by Sandwich ELISA in Cattle. Journal of Microbial and Biochemical Technology, 5, 034–038.
Sievers, F., Higgins, D.G. 2014. Clustal Omega, accurate alignment of very large numbers of sequences, In: Multiple Sequence Alignment Methods,. Springer, 105–116.
Siqueira, F.F., Almeida, M.O., Barroca, T.M., Horta, C.C., Carmo, A.O., Silva, R.O., Pires, P.S., Lobato, F.C., Kalapothakis, E., 2012. Characterization of polymorphisms and isoforms of the Clostridium perfringens phospholipase C gene (plc) reveals high genetic diversity. Veterinary Microbiology, 159, 397–405.
Sutmoller, P., Barteling, S.S., Olascoaga, R.C., Sumption, K.J., 2003. Control and eradication of foot-and-mouth disease. VirusResearch, 91, 101–144.
Tamura, K., Stecher, G., Peterson, D., Filipski, A., Kumar, S., 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30, 2725–2729.
Tanabe, K., Jones, W., Barie, P., 1989. Clostridial sepsis and malignant disease. Surgery, Gynecology and Obstetrics, 169, 423–428.
Tsutsui, K., Minami, J., Matsushita, O., Katayama, S., Taniguchi, Y., Nakamura, S., Nishioka, M., Okabe, A., 1995. Phylogenetic analysis of phospholipase C genes from Clostridium perfringens types A to E and Clostridium novyi. Journal of Bacteriology, 177, 7164–7170.
Uzal, F., Kelly, W., Morris, W., Assis, R., 2002. Effects of intravenous injection of Clostridium perfringens type D epsilon toxin in calves. Journal of Comparative pathology, 126, 71–75.
Venkataramanan, R., Bandyopadhyay, S., Oberoi, M., 2005. Present status and strategies for the control of transboundary and other economically important animal diseases in India: a review. The Indian Journal of Animal Sciences, 75.
Verma, S.C., Mahajan, N.K., Malik, G., Dahiya, J.P., 2004. An Epidemiological Study On Bovine Haemorrhagic Septicaemia In Haryana. Indian Journal of Animal Research, 38, 14–19.
Yoo, H.S., Lee, S.U., Park, K.Y., Park, Y.H., 1997. Molecular typing and epidemiological survey of prevalence of Clostridium perfringens types by multiplex PCR. Journal of Clinical Microbiology, 35, 228–232.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethical disclosure
Farm owners approved and signed the informed consent forms for the study. All animal procedures implemented in this study complied with institutional guidlines obtained from ethics committee in Mansoura University, Egypt.
Rights and permissions
About this article
Cite this article
Elgioushy, M., Rizk, M.A., El-Adl, M. et al. The first molecular detection of Clostridium perfringens from pneumonic cases associated with foot and mouth disease in cattle and buffalo in Egypt. Trop Anim Health Prod 51, 847–852 (2019). https://doi.org/10.1007/s11250-018-1763-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-018-1763-8