Abstract
Mycobacterium avium subsp. paratuberculosis causes paratuberculosis or Johne’s disease (JD) in domestic ruminants and wild species. The aim of the present study was to systematically review the prevalence of paratuberculosis among farmed animals (cattle, sheep, and goats) in Latin America and the Caribbean. The initial search for existing publications reporting systematic reviews and primary studies was carried out by searching the available databases. For the final selection of studies, an initial screen for basic eligibility and a detailed appraisal of quality were performed. After study selection, the relevant data were extracted. The detailed appraisal generated 24 publications that reported 52 studies, of which 73.1, 11.5, and 15.4 % were from cattle, sheep, and goats, respectively. Thirty-three (63.5 %) of the studies were animal level studies, while 19 (36.5 %) were herd-/flock-level studies. No flock-level studies on prevalence in sheep were found. Studies in Latin American and Caribbean countries revealed an overall prevalence of 16.9 (95 % CI (confidence interval) 13.2–20.5) and 75.8 % (95 % CI 50.1–101.5) in cattle at the animal and herd levels, respectively; the prevalence was 16 % (95 % CI 7.9–24.1) in sheep at the animal level and 4.3 % (95 % CI 1.9–6.8) and 3.7 % (95 % CI 0.1–7.4) in goats at the animal and flock levels, respectively. In general, prevalence results reported by the studies were insufficient to accurately determine the prevalence of paratuberculosis in farmed animals in Latin America and the Caribbean. Several flaws in the design of studies limit the quality of evidence regarding the prevalence of paratuberculosis in the region.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mycobacterium avium subsp. paratuberculosis (MAP) causes paratuberculosis or Johne’s disease (JD), which is a slowly developing infectious disease characterized by chronic granulomatous enterocolitis and regional lymphangitis and lymphadenitis, which typically causes progressive weight loss (Clarke 1997). Paratuberculosis is best known as a disease of domestic ruminants, but it also affects a wide range of other domestic and wild (free-living or captive) species (Clarke 1997; Carta et al. 2013). Chronic progressive weight loss and chronic or intermittent diarrhea are the primary clinical indicators of JD in farmed animals (Chiodini et al. 1984; Clarke 1997). However, the MAP strains that infect animals and the characteristics of JD differ among cattle, sheep, and goats.
MAP strains have been classified into two groups or strain types: type I/III (sheep type or S type/intermediate type) and type II (cattle type or C type), which are defined according to their growth characteristics, host preferences or host range, and pathogenicity (Collins et al. 1990; Stevenson et al. 2002; de Juan et al. 2005; Alexander et al. 2009; Stevenson 2010a, b). However, MAP strains can be isolated from a broad range of species, and the species of origin is not necessarily an accurate indicator of strain type (Stevenson 2010a). Type I/III strains have been isolated predominantly but not exclusively from sheep and goats, suggesting a preference for these host species. Type II MAP isolates have a very broad host range and are commonly isolated from both domesticated and wildlife species, including non-ruminants. Type II is by far the most common MAP strain type isolated from cattle (Stevenson 2010a).
In cattle, the cardinal clinical signs of JD are chronic progressive weight loss with chronic or intermittent diarrhea (Clarke 1997). Clinical disease may be precipitated by parturition, lactation, or other stresses (Chiodini et al. 1984). MAP infection can be divided into four stages (“silent” infection, unapparent carrier adults, clinical disease, and advanced clinical disease) depending on the severity of clinical signs, the potential for shedding organisms into the environment, and the ease with which the disease can be detected using current laboratory methods (Fecteau and Whitlock 2010). Worldwide, paratuberculosis is less prevalent in beef cattle than in dairy cattle (Roussel 2011).
In sheep, the clinical signs of JD are limited to chronic weight loss (Begg and Whittington 2010; Robbe-Austerman 2011), which may occur from 2 years of age, with most animals succumbing to disease at 3–5 years of age (Begg and Whittington 2010). Diarrhea is not considered to be a feature of JD in small ruminants, except in the terminal stages of disease (Clarke and Little 1996; Clarke 1997; Begg and Whittington 2010; Robbe-Austerman 2011). Sheep paratuberculosis is widespread and is a serious threat to sheep production because it tends to remain hidden, showing only indirect production effects (Juste and Perez 2011).
In goats, the disease resembles that of sheep in many respect (Djønne 2010). During the subclinical stage of infection, goats can become persistent fecal shedders approximately 1 year after infection without any clinical signs of paratuberculosis (Storset et al. 2001; Djønne 2010). During clinical disease, the only consistent finding is weight loss despite apparently normal food intake (Djønne 2010; Robbe-Austerman 2011). Unlike cattle, diarrhea is rarely seen in goats (Manning and Collins 2001; Robbe-Austerman 2011). During this stage, bacteria are typically found in the feces, and animals usually have antibodies against MAP. Most animals, if not culled, progress to advanced clinical disease, during which animals develop flaky skin and an unhealthy coat. Eventually, progressive emaciation, dehydration, anemia with submandibular edema, and depression are observed. At this stage of infection, diarrhea, or more usually fecal clumping, is observed (Djønne 2010). Paratuberculosis has been a problem in goats, but it has received less attention than in other species, most likely due to its more marginal numbers in countries that have a more developed cattle industry (Juste and Perez 2011).
Antemortem diagnosis of paratuberculosis is challenging because of the nature of the disease and the limitations of diagnostic tests. Because of these limitations, the purpose of diagnosis must be adequately defined so that the most appropriate diagnostic procedure can be applied (Collins et al. 2006; Nielsen and Toft 2008; Nielsen 2010; Stevenson 2010b). In general, the design quality, implementation, and reporting of test results for paratuberculosis have been generally poor (Nielsen and Toft 2008). ELISA, bacteriological cultivation of fecal samples, and PCR are widely used for the antemortem diagnosis of paratuberculosis in cattle herds (Clark et al. 2008; Nielsen and Toft 2008; Stevenson 2010b). Although ELISA is the most widely used test, isolation of MAP from an animal by culture is still considered the gold standard for JD diagnosis (Chiodini et al. 1984; Collins 1996; Whittington 2010). In addition, sampling all or a representative proportion of adult cattle in every herd, environmental sampling, serial testing, and the use of two to three diagnostic tests has been recommended for herd screening and increasing the accuracy of MAP diagnosis (Chacon et al. 2004; Collins et al. 2006; Stevenson 2010b).
The infection prevalence at the herd and animal level is often a key issue when decision or policy makers determine whether the infection should be considered important or not and which measures to apply (Nielsen and Toft 2009). According to Barkema et al. (2010), MAP prevalence should be assessed to determine the disease burden in a particular population and to determine the need for health services for those animals particularly at risk of contracting the disease. Prevalence studies are also carried out to compare disease prevalence in different populations and to follow trends in prevalence or severity over time to better understand the epidemiology of the spread of this disease (Barkema et al. 2010). No country or region has published sufficient information to claim freedom from MAP (Nielsen and Toft 2009; Manning and Collins 2010).
Herd or flock prevalence in regions and countries is loosely associated with their history of animal importation, the level of industrialization, and the degree of economic concentration in animal agriculture (Manning and Collins 2010). Since the first recognition of JD in dairy cattle, a steady dispersion of MAP over geographical space and host species has been observed. The spread of this chronic insidious disease from its first identification in Europe to herds of the New World may have coincided with colonization and the subsequent growth of domestic animal agriculture (Manning and Collins 2010). The only domestic animals in Latin America at the time of the discovery of the Americas were camelids such as the llamas, alpacas, vicuñas, and guanacos, as well as guinea pigs. All other animals were imported, mainly from the Iberian Peninsula and North Africa, and underwent approximately 500 years of natural selection in diverse environments. From the end of the nineteenth century, other cattle were imported from mainland Europe, and Zebu cattle were imported from India (Scholtz et al. 2011). Latin America and the Caribbean region have the second largest population of bovines in the world after East and South Asia and the Pacific regions; in contrast, sheep and goat populations in Latin America and the Caribbean are the smallest in the world (Rosegrant et al. 2009).
Several European countries, USA, Canada, and Australia have JD control programs (Kennedy and Citer 2010; Whitlock 2010; Bakker 2010). In contrast, Latin American and Caribbean countries lack of control programs, and the current prevalences of paratuberculosis in these countries have not been yet calculated. Published information on occurrence, distribution, and prevalence of paratuberculosis often refers to information reported from Europe, USA, Canada, and Australia. Information on paratuberculosis for policy makers, academics, producers, or consumers in Latin American and Caribbean countries is mostly based on information produced outside these countries. The aim of the present study was to systematically review the occurrence/frequency/prevalence of paratuberculosis among farmed animals (cattle, sheep, and goat) in Latin America and the Caribbean.
Materials and methods
The present study was carried out following the procedures for systematic reviews in health care (frequency and rate) suggested by Glasziou et al. (2001).
Identification of relevant studies
The process of identifying relevant studies included searching for existing systematic reviews, published primary studies, and unpublished primary studies. For this purpose, a systematic approach was undertaken. The search criteria were defined by all authors. First, the question for the systematic review was defined as the following: How frequent or prevalent is paratuberculosis in farmed animals (cattle, sheep, and goats) in Latin America and the Caribbean? Regardless of the criteria used in the studies included in this review, the case definition for individual animal infection in the present review was defined as animals reacting to serological or intradermal tests or animals positive by fecal culture or fecal PCR for MAP. Infection in herds or flocks was defined as premises in which infected farmed animals (individual animals reacting to serological or intradermal tests or animals positive by fecal culture or fecal PCR for MAP) were present or premises where MAP was present in the environment or in bulk tank milk detected through culture or PCR without necessarily having/reporting reacting or positive animals.
The question was broken down into following components: prevalence, paratuberculosis, cattle, sheep, goats, Latin America, and the Caribbean. For each component (population, study factor, outcome), synonyms were determined for every question term: bovine, ovine, caprine, for populations; Mycobacterium avium subsp. paratuberculosis, Mycobacterium paratuberculosis, Johne’s disease, for study factor; and occurrence, frequency, and proportion, for outcome. Thus, the search terms used to find relevant studies in databases were (diagnosis OR frequency OR prevalence OR occurrence) AND (paratuberculosis OR Johne’s) AND (bovine OR cattle OR sheep OR ovine OR goat OR caprine).
Publications including studies reporting prevalence, frequency, or proportion of paratuberculosis in at least two herds or flocks located in any of the following countries, independent of the diagnostic technique, were included: Antigua and Barbuda, Aruba, Bahamas, Barbados, Cayman Islands, Cuba, Dominica, Dominican Republic, Grenada and Carriacou, Guadeloupe, Haiti, Jamaica, Martinique, Puerto Rico, Saint Barthélemy, St. Kitts and Nevis, St. Lucia, St. Vincent and the Grenadines, Trinidad and Tobago, Turks and Caicos Islands and Virgin Islands, Belize, Costa Rica, El Salvador, Guatemala, Honduras, Mexico, Nicaragua, Panama, Argentina, Bolivia, Brazil, Chile, Colombia, Ecuador, French Guiana, Guyana, Paraguay, Peru, Suriname, Uruguay, and Venezuela (Anonymous 2014).
The initial search for existing publications reporting systematic reviews and primary studies was carried out by searching the available databases as of January 2014. No time or language restrictions were imposed. Only studies from 1990 to January 2014 were included. Studies conducted on other farmed animal species or wild animals were excluded. The following databases were searched for published primary studies: Scopus (available at http://www.scopus.com), PubMed (http://www.ncbi.nlm.nih.gov/pubmed), Redalyc (http://www.redalyc.org/), and the Virtual Health Library (http://bvsalud.org/en/). The proceedings of the 3rd, 4th, 5th, 6th, 7th, 8th, 9th, 10th, and 11th International Colloquia on Paratuberculosis (ICP) held in 1991, 1994, 1996, 1999, 2002, 2005, 2007, 2009, and 2012, respectively, available at the International Association for Paratuberculosis Web site (http://www.paratuberculosis.info/) were manually searched for existing systematic reviews and published primary studies. Studies from these proceedings were included in the systematic review only if they had not appeared in peer-reviewed journals, which were preferred for eventual inclusion. Database search was performed using criteria determined by one of the authors (J. A. F-S). The reference lists of relevant papers were searched for additional systematic reviews and published primary studies not found through database searching (“snowballing” procedure). The snowballing procedure was continued until no further studies were found. Unpublished but cited primary studies were searched using the same procedure. The term study was defined as research that aimed the determination of the paratuberculosis frequency in a population of a specific animal species tested with one test. In case of publications that include both animal-level and herd-level estimates, each estimate was counted as one study. Reports where two tests were used on the same population also counted as two studies.
Quality appraisal and final selection of studies
An initial screen for basic eligibility and a detailed quality appraisal were performed for the final selection of studies. Both processes were always performed independently by the three authors. The decision for final inclusion of a relevant study in the initial screen and in the final selection was always obtained through consensus. The initial screen for basic eligibility was carried out using titles and abstracts of relevant studies found through searching databases, ICP proceedings, and reference lists through the snowballing procedure for reports that match the general inclusion criteria on population, study factor, and outcome. After the initial screen, detailed quality appraisal was performed. Full-text versions of potentially eligible studies were evaluated for three principal issues: minimization of selection bias of animals or herds/flocks, adequate assertion of final outcomes, and minimization of measurement or misclassification bias.
Data extraction
After the final selection of studies, data on country and region, study period, population, inclusion criteria, selection, diagnostic test, sensitivity and specificity used (if available) for adjusting prevalence estimation, and results in terms of proportion were extracted. Studies from which no crude numbers on frequency or proportion are included were excluded unless the information provided allowed its calculation.
Data analysis
Study results were analyzed according to animal species at individual and the herd/flock level, as well as according to the diagnostic test used to reduce the influence of different test measurements. Microsoft Excel was used for data analysis and the graphical representation of results following the procedures described by Neyeloff et al. (2012). The analysis included the calculation of the outcome (effect size, es) and the standard error (SE), the computation of variance (Var), the individual study weights (w), and each weighted effect size (w*es). Additionally, the analysis included the calculation of the Q test for heterogeneity among studies and the calculation of the I 2 quantity. Finally, the analysis included the calculation of the appropriate effect summary (es) model (fixed effects or random effect model) according to the results obtained in the Q and I 2 tests (Neyeloff et al. 2012).
Results
General characteristics of selected studies
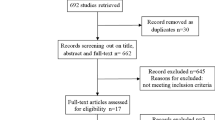
Searching databases and the proceedings of the ICP Colloquia combined with the snowballing procedure generated 252, 28, and 24 publication records matching the search terms, respectively (Fig. 1). Nine of 24 publication records found through the snowballing procedure could not be retrieved. After the exclusion of duplicates, 119 records remained from which titles and abstracts were checked for basic eligibility. The initial screen of abstracts for basic eligibility generated 37 potentially eligible abstracts. Thirty-one of these abstracts were from journals, three were only published as abstracts in any of the ICP Colloquia, and three were only published as an academic degree work: PhD thesis, MSc degree work, and veterinary medicine degree work. The detailed quality appraisal of these 37 full-text potentially eligible publications generated 24 publications that were included in the final analysis.
The 24 selected publications included 52 studies: 38 (73.1 %) from cattle (21 at the animal level and 17 at the herd level), 6 (11.5 %) from sheep (all at the animal level), and 8 (15.4 %) from goats (6 at the animal level and 2 at the flock level, Tables 1, 2, 3, 4 and 5). Thirty-three (63.5 %) were animal-level studies, while 19 (36.5 %) were herd-/flock-level studies. No studies on flock-level prevalence in sheep fulfilled the inclusion criteria of the detailed appraisal. The studies were carried out in Brazil 25 % (13/52), Mexico 21.2 % (11/52), Chile 21.2 % (11/52), Argentina 13.5 % (7/52), Venezuela 7.7 % (4/52), Puerto Rico 3.8 % (2/52), Grenada and Carriacou 3.8 % (2/52), and Costa Rica 3.8 % (2/52). Only half of the studies reported information on study period, and these studies were carried out during the period from 1990 to 2011.
None of the selected studies provided complete and sufficient information on the factors for the quality appraisal. All studies were cross-sectional studies. Case definition was absent from all studies included in the final analysis. Therefore, case definitions for individual animals and for herds/flocks established in the present review (see identification of relevant studies in Materials and methods) were taken into account to select the studies included in the final analysis. Diagnostic tests used in the selected studies to determine whether an animal or a herd/flock were considered a case were very diverse. ELISA (67.3 %, 35/52) was the diagnostic test most commonly used, followed by fecal culture (individual or pooled, 7.7 %, 4/52), skin test (7.7 %, 4/52), culture of environmental samples (5.8 %, 3/52), AGID (3.8 %, 2/52), fecal PCR (3.8 %, 2/52), individual milk culture (1.9 %, 1/52), and bulk tank milk PCR (1.9 %, 1/52).
Only three studies reporting the use of ELISA (Se 0.59, Sp 0.95), culture of environmental samples (Se 0.6, Sp 1), and bulk tank milk qPCR (Se 0.57, Sp 1) in cattle provided information on the sensitivity and specificity used for prevalence adjustment or true prevalence estimation (Moreira et al. 1994; Kruze et al. 2013). The remaining studies provided only apparent prevalence. No attempt to estimate the true prevalence was made due to limitations in the estimation of test accuracies for the populations tested. Only 42.3 % (22/52) of the studies reported information on selection of animals or herds/flocks included for MAP testing in the studies. In the remaining studies, this information was absent. Random selection of animals or herds/flocks was reported only in 23.1 % (12/52) of the studies. However, studies that did not report the sampling method or reported nonrandom sampling or convenience sampling were kept in the study to summarize and synthesize their results if animals or herds/flocks had no previous diagnosis of paratuberculosis.
Prevalence in cattle at the animal level
Prevalence studies in cattle at the animal level (n = 21) are shown in Table 1. Regarding the location of the studies included in the final analysis, Argentina and Brazil each comprised 33.3 % (7/21), Chile and Venezuela each comprised 9.5 % (2/21), and Mexico, Puerto Rico, and Costa Rica each comprised 4.8 % (1/21). Studies from Brazil (n = 7) and Costa Rica (n = 1) were carried out in several regions of these countries, whereas studies from Chile (n = 2) and Argentina (n = 7) were carried out in two regions. The remaining studies were carried out in only one region. Information on study period was provided in only 52.4 % (11/21) of the studies. For the six studies from the publication by Martinis Mercado et al. (2014), the study period was provided, but the publication date was not available. Population and inclusion criteria were very variable among studies. Sample sizes ranged from 123 (Martinis Mercado et al. 2014) to 2,530 (Moreira et al. 1994) animals. The majority of studies reported testing adult animals (i.e., over 24 months of age). One single study reported testing animals younger than 24 months of age (Medeiros et al. 2012).
Seventy-six percent (16/21), 9.5 % (2/21), and 9.5 % (2/21) of the studies were carried out in dairy, beef (Moreira et al. 1994; Silva 2005), and dual-purpose (Alfaro et al. 2006) cattle, respectively. Although no apparent differences between prevalence in dairy cattle and prevalence in beef cattle were seen, prevalence results in dual-purpose cattle in Venezuela (72 %; Alfaro et al. 2006) and in dairy cattle in Brazil (60.2 %; Acypreste et al. 2005) had the highest prevalences in cattle at the animal level found in the present review. Prevalence in beef cattle was moderate (35.4 %; Silva 2005) or low (13.8 %; Moreira et al. 1994). Nevertheless, the effect size (outcome) of the study by Moreira et al. (1994) was the highest using both a fixed effect model and a random effect model (Supplementary file 1). The remaining study (11.9 %; Dolz et al. 1999) did not report the type of cattle tested. Only 9 out of 20 studies (45 %) in cattle at the animal level reported information on the selection of animals tested for MAP. In these studies, random sampling (stratified and nonstratified), nonrandom sampling, and proportional sampling were the selection procedures used.
The type of diagnostic tests used to detect cases (individual animals) in the studies was very diverse. Nevertheless, ELISA was the most common diagnostic test used (16/21; 76.2 %), followed by skin test (avian and bovine purified protein derivative (PPD)) and commercial Johnin (3/21; 14.3 %; Martinis Mercado et al. 2014; Alfaro et al. 2006), fecal culture (1/21; 4.8 %; Martinis Mercado et al. 2014), and milk culture (1/21; 4.8 %; Martinis Mercado et al. 2014). The majority of ELISA-based studies used commercial kits (12/16; 75 %). In three studies (3/16; 18.8), the ELISA test used was prepared using individual reagents that were produced in-house or were commercially available (Dolz et al. 1999; Martinis Mercado et al. 2014; Miranda Bandera 2005). In one case (1/16; 6.3 %), the ELISA test used was not clearly described (Pantoja et al. 2010). Information on fabricant and specific test details of the commercial ELISA test kit (Bratex Laboratories–Campo Grande, MS) used by Acypreste et al. (2005) was not available. Only one study (Moreira et al. 1994) reported information on sensitivity and specificity of the ELISA test used.
Prevalence studies in cattle at the animal level in Latin American and Caribbean countries using a random effect model (Supplementary file 1) revealed an overall prevalence of 16.9 % (95 % CI 13.2–20.5; Fig. 2). Results of the studies that used ELISA and skin tests (random effect model) revealed prevalences of 19.5 % (95 % CI 15.4–23.5) and 4.3 % (95 % CI 2.5–6.1), respectively. Heterogeneity of all studies (I 2 = 80.1 %) and of the ELISA-based studies (I 2 = 81 %) was high. On the contrary, heterogeneity of results of the skin test-based studies (I 2 = 0 %) was low.
According to an arbitrary categorization, the highest prevalences of paratuberculosis in cattle at the animal level using ELISA test were obtained in Monagas (Venezuela) 72.1 % and in Goiás, Sao Paulo, and Pará (Brazil) with prevalences of 60.2, 38, and 35.4 %, respectively (Alfaro et al. 2006; Acypreste et al. 2005; Fonseca et al. 2000; Silva 2005). Intermediate prevalences were obtained in Rio de Janeiro (Brazil) 18.0 %, Corrientes (Argentina) 7.3 % using the Parachek® ELISA test, Buenos Aires (Argentina) 13.8 %, Region X (Chile) 12.8 %, Costa Rica 11.9 %, and Espírito Santo (Brazil) 11.4 % (Ferreira et al. 2001; Martinis Mercado et al. 2014; Moreira et al. 1994; Burgos-Garay 2011; Dolz et al. 1999; Costa et al. 2010). The lowest prevalences were obtained in Paraíba (Brazil, 10.1 %), Corrientes (Argentina, 9.8 %) using the PPA-3 strain 18-ELISA test, Hidalgo (Mexico, 9 %), Region VIII (Chile, 6.4 %), and Pernambuco (Brazil, 2.7 %, Medeiros et al. 2012; Martinis Mercado et al. 2014; Miranda Bandera 2005; Mundaca-Verdugo 2012; Sá et al. 2013).
The highest prevalence as determined by using the skin test was obtained in Corrientes (Argentina, 7.3 %) using skin test avian PPD (DILAB/SENASA®), whereas an intermediate level was obtained in Monagas (Venezuela, 4.2 %) using the skin test MAP-PPD, National Veterinary Services Laboratories® (Alfaro et al. 2006). The lowest level (3.3 %) was also detected in Corrientes (Argentina) and in the same animals using skin test bovine PPD (Instituto de Sanidad Ganadera®, Martinis Mercado et al. 2014). Two studies using fecal and milk culture as a diagnostic test showed no positive results in 123 samples tested (Martinis Mercado et al. 2014). These studies were not analyzed further.
Herd-level prevalence in cattle
Herd-level prevalence studies in cattle (n = 17) are shown in Table 2. Of the studies included in the final analysis, 35.3 % (6/17) were from Brazil, 35.3 % (6/17) were from Chile, 11.8 % (2/17) were from Venezuela, and Mexico, Puerto Rico, and Costa Rica each had 5.9 % (1/17). Information on the study period was provided in only 35.2 % (6/17) of the studies. Population and inclusion criteria were very variable among studies. Sample sizes ranged from 8 (Alfaro et al. 2006) to 364 (Dolz et al. 1999) herds. A total of 82.3 (14/17) and 11.7 % (2/17) of the studies were carried out in dairy and dual-purpose cattle, respectively. The remaining study (Dolz et al. 1999; 5.8 %) did not report the type of herds sampled. Only 6 out of 17 studies (35.2 %) in cattle at the herd level reported information on the selection of animals tested for MAP. In these studies, random sampling (stratified and nonstratified) and proportional sampling were the selection procedures used.
Similar to studies at the animal level, the type of diagnostic test used to detect herd cases was very diverse. ELISA was the diagnostic test used in 64.7 % (11/17) studies, followed by culture of environmental samples (17.6 %; 3/17; Burgos-Garay 2011; Mundaca-Verdugo 2012; Kruze et al. 2013), bulk tank milk qPCR (Kruze et al. 2013), skin test (commercial Johnin; Alfaro et al. 2006), and fecal culture (pooled samples; Salgado et al. 2012), which each had 5.9 % (1/17). Commercial ELISA kits were used in 72.7 % (8/11) of the studies. In two studies (2/11; 18.2 %), the ELISA test used was prepared using individual reagents that were produced in-house or were commercially available (Dolz et al. 1999; Miranda Bandera 2005). In one case (1/11; 9.1 %), the ELISA test used was not clearly described (Pantoja et al. 2010). Information on fabricant and specific test details of the commercial ELISA test kit used by Acypreste et al. (2005) was not available.
Herd-level prevalence studies in cattle in Latin American and Caribbean countries using a random effect model (Supplementary file 2) revealed an overall prevalence of 75.8 % (95 % CI 50.1–101.5; Fig. 3). According to the type of diagnostic test used, study results that used the ELISA and other tests (environmental culture, bulk tank milk qPCR, pooled fecal culture, and skin test) using a random effect model revealed a prevalence of 74.0 % (95 % CI 47.3–100.6) and 37.3 % (95 % CI 25.3–49.4), respectively. Heterogeneity of all studies (I 2 = 0 %), ELISA-based studies (I 2 = 0 %), and environmental culture, bulk tank milk qPCR, pooled fecal culture, and skin test studies (I 2 = 0 %) was low.
The highest paratuberculosis herd-level prevalences in cattle using ELISA were obtained in Goiás (Brazil, 100 %), Monagas (Venezuela, 100 %), Hidalgo (Mexico, 96.6 %), Sao Paulo (Brazil, 95 %), Region X (Chile, 95 %), Espírito Santo (Brazil, 87 %), Rio de Janeiro (Brazil, 82 %), and Puerto Rico (82 %) (Acypreste et al. 2005; Alfaro et al. 2006; Miranda Bandera 2005; Fonseca et al. 2000; Burgos-Garay 2011; Costa et al. 2010; Ferreira et al. 2001; Pantoja et al. 2010). Intermediate prevalences were obtained in Paraíba (Brazil) and Pernambuco (Brazil) with 58.3 and 47.4 % prevalence, respectively (Medeiros et al. 2012; Sá et al. 2013). The lowest prevalence was obtained in Costa Rica with 18.7 % (Dolz et al. 1999).
Regarding other diagnostic tests, the highest prevalence was obtained in Monagas (Venezuela, 87.5 %) using a skin test (MAP-PPD, National Veterinary Services Laboratories®; Alfaro et al. 2006); intermediate prevalences of 50, 45, and 42.9 % were obtained in the regions XIV and X, and VIII in Chile using bulk tank milk qPCR and culture of environmental samples, respectively (Kruze et al. 2013; Burgos-Garay 2011; Mundaca-Verdugo 2012).
The lowest prevalences of 27 and 25 % were found in region XIV and in southern Chile using culture of environmental samples and fecal culture of pooled samples, respectively (Kruze et al. 2013; Salgado et al. 2012).
Prevalence in sheep at animal level
Prevalence studies in sheep at the animal level (n = 6) are shown in Table 3. The studies included in the final analysis were from Grenada and Carriacou and Mexico with 17 % (1/6) and 83.3 % (5/6) of the studies, respectively. Only one study provided information on study period (between December 2009 and January 2011) (Kumthekar et al. 2013). Population and inclusion criteria in Mexican studies were relatively homogeneous in terms of age, subclinical status, and sample size (Morón-Cedillo et al. 2013; Jaimes et al. 2008). Sample sizes ranged from 204 (Jaimes et al. 2008) to 479 (Kumthekar et al. 2013) animals. The studies by Jaimes et al. (2008) and Morón-Cedillo et al. (2013) reported testing adult animals (i.e., sheep older than 2 years), while the study of Kumthekar et al. (2013) also included animals of at least 6 months of age. Only two out of six studies in sheep at the animal level reported information on the selection of animals tested for MAP. In these studies, convenience sampling was used as the selection procedure. The type of diagnostic test used to detect individual cases in the studies was diverse and included ELISA, AGID, fecal culture, and fecal nested PCR. ELISA was used in only one study (Kumthekar et al. 2013). In the studies by Jaimes et al. (2008) and Morón-Cedillo et al. (2013), the AGID test used a commercial reagent and a protoplasmic antigen obtained from a sheep strain (MAP 3065), respectively. None of the studies reported information on sensitivity and specificity of the test used.
Prevalence studies in sheep at the animal level in Latin American and Caribbean countries using a random effect model (Supplementary file 3) showed a prevalence of 16 % (95 % CI 7.9–24.1; Fig. 4). According to the type of diagnostic test used, study results that used serological tests (ELISA and AGID) and direct methods (fecal culture and fecal PCR) using a random effect model revealed prevalences of 17.9 % (95 % CI 2.5–33.3) and 14.7 % (95 % CI 4.3–25.1), respectively. Heterogeneity of all studies (I 2 = 60 %) and of the serological test studies (I 2 = 62.5 %) was moderate to high, while heterogeneity in studies that used direct methods (I 2 = 44.9 %) was moderate.
The highest paratuberculosis prevalence in sheep at the animal level, determined using a serological test (AGID; PPA-M3 Allied), was found in Guanajuato, Jalisco, and State of Mexico (Mexico, 44.6 %, Jaimes et al. 2008). Low prevalences were obtained in San Luis Potosí (Mexico, 9.5 %, Morón-Cedillo et al. 2013) and in St. Andrew and St. David (Grenada) and from Carriacou, 2.3 % using ELISA (Kumthekar et al. 2013). No intermediate prevalences were obtained in sheep at the animal level. Regarding direct tests, an intermediate prevalence was obtained in Guanajuato, Jalisco, and State of Mexico (Mexico, 29.9 %) using fecal nested PCR (Jaimes et al. 2008), whereas low prevalences were detected in Guanajuato, Jalisco, and State of Mexico (Mexico) and San Luis Potosí (Mexico) with prevalences of 8.3 and 7.6 % using fecal culture and fecal nested PCR, respectively (Jaimes et al. 2008; Morón-Cedillo et al. 2013).
Prevalence in goats at the animal level
Prevalence studies in goats at the animal level (n = 6) are shown in Table 4. The studies included in the final analysis were from Chile with 50 % prevalence (3/6), Mexico with 33.3 % prevalence (2/6), and Grenada and Carriacou with 16.7 % prevalence (1/6). In all studies, information on study period was provided. Population and inclusion criteria of studies were variable between countries but were similar within the countries. Sample sizes ranged from 182 (Martínez-Herrera et al. 2012) to 559 (Callejas-García 2013) animals. The studies reported testing adult animals only (i.e., over 24 months of age; Salgado et al. 2007) or testing a broader age group that included younger animals (at least 6 months or older than 3 months; Kumthekar et al. 2013; Callejas-García 2013; Martínez-Herrera et al. 2012). Only two of six of the Mexican studies (33.3 %) reported information on the selection of animals tested for MAP. In these studies, random sampling was the selection procedure used. The type of diagnostic test used to detect cases in these studies was relatively homogeneous. With the exception of the study by Salgado et al. (2007), in which fecal culture was used, all studies used commercial ELISA kits. None of the studies reported information on sensitivity and specificity of the ELISA test used.
Prevalence studies in goats at the animal level in Latin American and Caribbean countries using ELISA for diagnosis and a random effect model (Supplementary file 4) for analysis revealed an overall prevalence of 4.3 % (95 % CI 1.9–6.8; Fig. 5). The study by Salgado et al. (2007), which was based on fecal culture, was not included in the analysis. Heterogeneity of all studies (I 2 = 79 %) was high. Low paratuberculosis prevalence in goats at the animal level using serum and milk ELISA was obtained in the south and central regions of Chile 16.8 and 9.4 % (Salgado et al. 2007). Very low prevalences using serum ELISA were obtained in three studies: two in Veracruz (Mexico) and one in St. Andrew (Grenada) with 0.5, 0.5, and 0.4 % prevalences, respectively (Martínez-Herrera et al. 2012; Callejas-García 2013; Kumthekar et al. 2013). No high or intermediate prevalences were obtained in goats at the animal level.
Flock-level prevalence in goats
Flock-level prevalence studies in goats (n = 2) are shown in Table 5. The studies were both from Mexico. The studies were carried during the period from February 2010 to July 2011. Population and inclusion criteria differed between the two studies. Sample sizes were 81 (Callejas-García 2013) and 26 flocks (Martínez-Herrera et al. 2012). Both studies reported random sampling as the selection procedure used. The type of diagnostic test used was commercial ELISA. The studies did not report information on sensitivity and specificity of the ELISA test used.
Flock-level prevalence studies in goats in Latin American and Caribbean countries using a fixed effect model (Supplementary file 5) revealed a prevalence of 3.7 % (95 % CI 0.1–7.4; Fig. 6). Testing heterogeneity was not taken into account due to the low number of studies, as suggested by Schriger et al. (2010). Both ELISA-based studies showed very low prevalences (3.7 and 3.9 %) for Veracruz (Mexico) using two different commercial ELISA tests (Callejas-García 2013; Martínez-Herrera et al. 2012).
Discussion
Studies on MAP prevalence in Latin America and the Caribbean were reviewed using a systematic methodology for the first time. Our purpose was to recover all available evidence on the frequency or prevalence of paratuberculosis in the Latin American and Caribbean region, assuming greater similarities (production conditions, cattle breeds, historical processes, climates, and idiosyncrasies) among countries of this region compared to countries of other regions of the world (e.g., North America, Europe, Australia, Asia).
The number of publications on paratuberculosis in the Latin American and Caribbean region was higher than expected, which suggests a growing trend in paratuberculosis research and an increasing interest in this disease and its negative effects. However, the number of publications and studies dealing with estimation of prevalence or frequency of paratuberculosis in more than one herd or flock was relatively low but was similar to that of a previous review on the same topic in Europe (Nielsen and Toft 2009).
The type of farmed animals included in the present review was limited to cattle, sheep, and goats. Other relevant species in Latin America and the Caribbean, i.e., deer, camelids, or buffaloes, were excluded due to their relatively low population compared to the farmed animals included. Nevertheless, further studies on the frequency of paratuberculosis in these species and their relationship to paratuberculosis in cattle, sheep, and goats are needed. Unfortunately, several publications could not be retrieved due to lack of availability in databases, which indirectly shows the limitations of wide access to animal health information produced in countries in this region.
As expected, studies on cattle at the animal level were the most common, most likely due to the size of the population in the region and the relative feasibility of this type of study compared to herd-level studies, in which sample sizes require more resources to be representative. In contrast, studies in sheep and goat populations in the region are less common and could be due to their smaller populations (Rosegrant et al. 2009). Surprisingly, no flock-level studies of sheep that fulfilled the inclusion criteria were found without any plausible explanation.
Also, as expected, the majority of studies were carried out in Brazil, because paratuberculosis is present in almost all of its regions (Yamasaki et al. 2013). However, no studies in sheep and goats from this country were included in the present review. Strikingly, studies from other Latin American and Caribbean countries with significant farmed animal populations (e.g., Colombia, Peru, Paraguay, Uruguay, and Cuba) were not included in the review. This fact could be explained by the absence of studies on paratuberculosis, studies carried out in one single herd, or by the lack of inclusion criteria. Paratuberculosis is not a notifiable disease and is not an animal health priority in many countries of the region. Therefore, government and institutional efforts are directed toward other animal diseases (e.g., foot-and-mouth disease, brucellosis, rabies, and tuberculosis). The Caribbean was clearly underrepresented in the review, with only two publications from Puerto Rico and Grenada and Carriacou. Farmed animal production on these islands appeared to be not as significant as in continental Latin America.
Absence of relevant information for study quality assessment was also reported in Europe in a review of prevalence in farmed animals (Nielsen and Toft 2009). In the present review, case definitions as well as variability in diagnostic tests and lack of random sampling for animal and herd/flock selection were the main study flaws included in the final analysis. Rejection of studies that do not completely fulfill the selection criteria would have led to the exclusion of the vast majority of studies. Instead, we decided to retain studies that minimized their selection bias by means of inclusion of animals and herds without clinical cases or previous diagnosis of paratuberculosis. Surprisingly, study period reporting was absent in a significant number of studies (50 %), although describing relevant dates and including data collection has been widely recommended (von Elm et al. 2007). This information is necessary because readers need to know the setting and location to assess the context and generalizability of a study’s results (Vandenbroucke et al. 2007).
Diagnostic results were taken directly as reported in the studies, and further calculation was necessary in only a few cases to make comparisons possible. In contrast to a European review (Nielsen and Toft 2009), calculation of true prevalence was not attempted in the present review due to test variability and a lack of information on sensitivity and specificity in the populations under study. As expected, ELISA was the most common test used to determine prevalence in the studies, which can be easily be explained by the relative low cost of the test, easy adaptability to high-throughput testing (Nielsen 2010), and its availability even in low-income countries of Latin America and the Caribbean. Interestingly, studies using environmental samples, fecal PCR, and bulk tank milk PCR are being carried out more frequently than expected.
We calculated, assessed, and pooled overall and specific prevalence according to type of test. Similarly, we included subpanels by test type for ELISA and skin test in the graphical representation. We determined that this approach was absolutely necessary to give a better reflection of prevalence estimations and to follow current recommendations (Schriger et al. 2010).
Brazilian and Argentinean studies were heavily represented, which was expected because both countries have large cattle populations, are major beef producers, and are large net exporters of beef (over 35 % of the world trade in 2005; Steiger 2006; Scholtz et al. 2011). In the Costa Rican study, serum samples from several regions of the country were included, more accurately reflecting the situation at a national level. The strategy of testing samples from a serum bank obtained previously for studies on other diseases was rarely done in the selected studies. This strategy could be very useful in countries with extensive production, huge territories, and limitations of financial resources for paratuberculosis testing.
As previously reported, studies on paratuberculosis prevalence in beef cattle are much fewer in number, apparently due to a lower awareness of paratuberculosis among beef cattle producers than among dairy cattle producers (Roussel 2011). Similarly, differences in paratuberculosis prevalences between dairy and beef cattle have been previously reported (Barkema et al. 2010; Roussel 2011), but paratuberculosis is a major health and production problem in some North American beef herds (Roussel 2011). The extremely high prevalence reported by the study of Alfaro et al. (2006) is very striking, but to the authors’ knowledge, this article is the first report of paratuberculosis in dual-purpose cattle in the Americas, and it is very difficult to make comparisons or to draw conclusions based on these results. In any case, cattle type in terms of production goal (dairy, beef) could be limited to describe the complex production systems in some Latin American and Caribbean countries, where dairy or beef production is commonly carried out in dual-purpose production systems either with Bos taurus or Bos indicus breeds. In general, beef production in Brazil is based primarily on B. indicus (Zebu) breeds. Uruguay and Argentina base their beef cattle industry on traditional British breeds (Scholtz et al. 2011). In some countries, higher production volumes of dairy and meat are obtained from dual-purpose cattle than from specialized dairy or beef cattle. Therefore, we considered analyzing this aspect based on cattle breed rather than on production purpose or goal to be more appropriate. Unfortunately, information on this feature was frequently omitted in the majority of studies, which limited our analysis.
Overall paratuberculosis prevalence in cattle at the animal level (16.9 %) was slightly lower than the prevalence estimated in Europe (20 %, Nielsen and Toft 2009). Animal-level paratuberculosis prevalence in cattle based on ELISA results (19 %) appeared to be higher than the regional and countrywide prevalence of MAP infection (1.2–9.4 %) determined using serum ELISA in dairy cattle in USA and Europe (Barkema et al. 2010; Wells and Wagner 2000). Animal-level prevalence in beef cattle in the present review based on two studies (Moreira et al. 1994; Silva 2005) also appeared higher than the ELISA-based prevalences obtained previously for beef cattle in USA and Canada (Dargatz et al. 2001; Scott et al. 2007; Waldner et al. 2002; Roussel 2011).
However, the results may not be comparable in all cases because prevalences in the present review are not true prevalences. In our review, estimation and reporting of true prevalences (and the inclusion of Se and Sp of tests used) were absent from all studies with few exceptions. Estimation of true prevalences from the crude data found in the studies was not done due to limited information on Se and Sp determined in the population included in the studies. This detail could explain the fact that (true) prevalences in Europe were at least 3–5 % in several countries, whereas in Latin America and the Caribbean, apparent prevalences reported were rarely below 5 %. In the case of ELISA, multiple antibody ELISAs have been evaluated, and Se and Sp estimates vary greatly within and between tests (Nielsen and Toft 2008). Additionally, tests should preferably be evaluated prior to their use in a specific population (Nielsen 2010), which was not done in any study included in the present review. Therefore, the prevalences reported in these studies are very likely to lead to negative or higher prevalences as reported in Europe (Nielsen and Toft 2008). Importantly, overall prevalence estimation for cattle at the animal level in the European review was done irrespective of the type of test, i.e., no separate analysis of direct methods from indirect methods was performed (Nielsen and Toft 2009).
The skin test is still widely used in Latin America and the Caribbean to test animals for tuberculosis and paratuberculosis. The Johnin skin test for paratuberculosis, which is interpreted as positive when skin thickness increased ≥4 mm, is a specific and low-cost test for the early diagnosis of paratuberculosis in the majority of dairy herds, although the specificity of both tests is influenced by batch of PPD antigen and varies among herds (Kalis et al. 2003). Although very few studies (n = 3) in the present review determined prevalences using this type of tests, previous information from Brazilian studies suggests that the intradermal tuberculin tests can interfere in the reliability of ELISA (Lilenbaun et al. 2007, 2009; Varges et al. 2009) and that serological testing for PTB should be avoided for 90 days after PPD inoculation (Varges et al. 2009). Studies on paratuberculosis in Latin America and the Caribbean rarely reported tuberculosis status or previous tuberculin tests before paratuberculosis testing, which could suggest that results could be higher than reported. However, single intradermal tuberculin has recently been reported to have poor sensitivity to detect positive tuberculosis cases in animals co-infected with advanced stages of paratuberculosis (Seva et al. 2014). This evidence questions the usefulness of the skin test to accurately diagnose both tuberculosis and paratuberculosis.
Strikingly, the interferon gamma assay was absent from the diagnostic tests used in Latin American and Caribbean for paratuberculosis diagnosis. This test has been considered a suitable in vitro alternative to the Johnin skin test or as a method to confirm a diagnosis in skin test-positive animals (Kalis et al. 2003). Nonetheless, this method has been seldom evaluated for paratuberculosis testing in cattle (Nielsen and Toft 2008).
The high heterogeneity detected in overall prevalence estimations could be easily attributed to high diversity in study design or variable quality of measures. In our review, we tried to keep a high standard of study selection. Nevertheless, we chose to include studies with some design flaws to avoid rejection of the majority of studies. Studies that clearly report selection bias toward clinical animals or animals with a history or diagnosis of paratuberculosis were excluded from the review. The diversity of sample diagnostic kits used could explain the high heterogeneity of results from ELISA-based studies. These tests can be very variable in terms of antigen choice, which could not be determined in some cases due to the unavailability of information on test fabricant. Additionally, immunogenicity and cross-reactivity of MAP or MAA antigens have been suggested to be geographically restricted, due to different distributions of bacteria that could cause cross-reactivity, such as MAA, from one area to the other (Nielsen and Toft 2008). This variability could also explain the high variation.
Finally, using ELISA to diagnose paratuberculosis in individual animals has been questioned because individual test results can only be assessed when the true prevalence of the disease in the herd is taken into account, and this characteristic differs from herd to herd and is often unknown (Köhler et al. 2008).
Slightly fewer estimations of herd-level prevalence in cattle were made than at the animal level, and most of these studies were performed at the animal level and extrapolated to the herd level. Apparently, overall prevalence estimations from Latin American and Caribbean studies (75.8 %) were higher than the estimated herd-level prevalence for cattle in other countries (30–50 %) based on ELISA results from Belgium, the Netherlands, Denmark, Canada, and the USA (Barkema et al. 2010). However, a previous European review indicates that herd-level prevalence estimations could be >50 % (Nielsen and Toft 2009), which would make prevalences similar on both continents. Herd-level prevalence based on one or more seropositive animals in beef cattle in the USA ranged from 34 to 76 % (Barkema et al. 2010; Roussel 2011).
In any case, these results may not be comparable due to previously discussed reasons regarding animal-level prevalence estimation in cattle in the present review and critical issues (very few studies providing interpretable estimates and insufficient documentation of herd-level prevalence of MAP in cattle) referred to in the European review (Nielsen and Toft 2009). Nonetheless, a simple comparison of apparent herd-level prevalence results based on antibodies shows that prevalence is higher in Latin American and Caribbean countries compared to those in Europe (74 vs 38–68 %; Nielsen and Toft 2009). Similarly, a study detecting MAP through bulk tank milk qPCR (Kruze et al. 2013) showed a higher apparent herd-level prevalence (50 %) than studies from Europe and Iran using bulk tank milk PCR with prevalences of 8–22 and 3–33 %, respectively (Nielsen and Toft 2009; Barkema et al. 2010). Comparison of apparent and true prevalences on both continents remains unavailable, due to critical issues reported in Europe and lack of relevant information in Latin American and Caribbean studies.
In sheep, paratuberculosis also has a worldwide distribution (Barkema et al. 2010), but MAP prevalence in domestic sheep worldwide is unknown, as the disease is “not notifiable” in many countries (Begg and Whittington 2010). This seems especially true in Latin America and the Caribbean, where paratuberculosis in sheep has been described, but the number of studies reporting prevalences of MAP in sheep is even lower than the number of studies reported for Europe (Nielsen and Toft 2009). In Latin America and the Caribbean, studying MAP in sheep is a relatively low priority compared to MAP in cattle or the more strategically important mycobacterial diseases such as tuberculosis and is most likely due to the high costs involved in identifying the disease compared with the low value of a sheep (Begg and Whittington 2010). According to Begg and Whittington (2010), MAP prevalence in sheep appears to differ markedly between countries and geographical regions within countries, which could be related to the type of MAP strains (sheep or cattle) that infect sheep, which in turn varies with the predominant strain type in the geographical region and whether or not sheep cohabit with other species.
The overall apparent animal-level prevalence in sheep in Latin America and the Caribbean (16 %) was higher than that in previous reviews of farmed animals in Europe (0.3–3.5 %) when considering the results obtained for sheep and goats in mixed sheep and goat production (Nielsen and Toft 2009). In the present review, only one study included mixed sheep and goat productions (Kumthekar et al. 2013), but results for both species were analyzed separately. When only the apparent prevalences in sheep from Europe (0.3–0.6 %, Nielsen and Toft 2009) are considered, Latin American and Caribbean results appeared even higher. Compared to other studies using different diagnostic tests from Canada (3 %; Arsenault et al. 2003), South Africa (0.13 %; Michel and Bastianello 2000), and Australia (0.25 %; Barkema et al. 2010), the prevalence in the Latin American and Caribbean regions also appeared to be higher.
Similarly, apparent prevalences from studies in Latin American and Caribbean using ELISA or AGID (18 %) and fecal culture (14.7 %) were much higher than those in Europe for ELISA (3.5 %) and culture (0.6 %; Nielsen and Toft 2009; Barkema et al. 2010). However, ELISA results were similar (14 %) to a recent small animal seroprevalence estimation at a national scale in Germany (Stau et al. 2012), in which prevalence estimation is not comparable due to the sampling of animals with the poorest body condition, which may overestimate the results. Surprisingly, although AGID has been found to have an even lower sensitivity than the ELISA (Gumber et al. 2006; Robbe-Austerman et al. 2006; Begg and Whittington 2010), this method was used more often for paratuberculosis diagnosis in sheep in several studies used in the present review.
Infection prevalence in goats is difficult to estimate in any region or country because of the uncertainty of diagnosis and the fact that cases are only reported during specific surveys or eradication programs (Djønne 2010). Apparent overall prevalence at the animal level in goats including mixed goat and sheep populations in Latin America and the Caribbean (4.3 %) was higher than apparent prevalences of 0.0–1.7 % from Europe (Nielsen and Toft 2009) but were lower compared to the recent small animal seroprevalence estimation of 21 % at a national scale in Germany (Stau et al. 2012). However, the results of this last study are not comparable as explained previously for sheep. Similar to animal-level prevalence in sheep, Latin American and Caribbean prevalences appeared even higher than those from Europe (0.0–0.7 %, Nielsen and Toft 2009), France (2.9 %; Mercier et al. 2009), and USA (1.9 %; Pithua and Kollias 2012) if only goat apparent prevalences are considered.
In the present review, only two studies reporting flock-level paratuberculosis prevalences were included. Apparently, both studies were carried out by the same group and most likely evaluated the same populations with some variation concerning period of study, populations, inclusion criteria, and commercial ELISA tests. Therefore, flock-level prevalence results in goats provided by these studies may not be representative for Latin America and the Caribbean. Nevertheless, although the results were very homogeneous, they were lower than previous estimations of >20, 36, 71, and 55.2 % in Europe, USA, Germany, and France, respectively (Nielsen and Toft 2009; Mercier et al. 2009; Pithua and Kollias 2012; Stau et al. 2012).
Conclusion
In general, prevalence results reported by the studies included in this systematic review were insufficient to accurately answer the question of how frequent or prevalent paratuberculosis is in farmed animals (cattle, sheep, and goats) in Latin America and the Caribbean. Several flaws in study design limit the quality of evidence on paratuberculosis frequency in Latin American and Caribbean countries. The main weakness of these studies were the absence of clear definition of cases, variability in selected populations and in inclusion criteria for animals and herds, variability or lack of information on type of diagnostic tests, variability or lack of information on sensitivity and specificity of diagnostic tests, and lack of random selection of animals and herds/flocks. Nevertheless, adjustments such as analysis according to diagnostic tests, analysis of heterogeneity, and the use of a random effect model were applied to circumvent the variability of study results. According to this review, the overall apparent prevalence for paratuberculosis in cattle in Latin America and the Caribbean is approximately 17 and 76 % at the animal and the herd level, respectively. The overall apparent prevalence of paratuberculosis in sheep is approximately 16 % at the animal level, whereas flock-level prevalence could not be determined. The overall apparent animal-level prevalence for paratuberculosis in goats is approximately 4.3 %. The overall apparent prevalence for flock-level paratuberculosis in goats is approximately 3.7 %, but the low number of studies included in the review as well as their homogeneity makes this low result unlikely to reflect the prevalence in Latin America and the Caribbean.
References
Acypreste, C.S., Juliano, R.S., Riveira, F.E.B., Silva, L.A.F., Fioravanti, M.C.S. and Dias-Filho, F.C., 2005. Uso da técnica do ELISA indireto na detecção de anticorpos anti-Mycobacterium paratuberculosis em vacas em lactação, Ciencia Animal Brasileira, 6(1), 55–59.
Alexander, D.C., Turenne, C.Y. and Behr, M.A., 2009. Insertion and deletion events that define the pathogen Mycobacterium avium subsp. paratuberculosis, Journal of Bacteriology, 191, 1018–1025.
Alfaro, C., de Rolo, M., Clavijo, A. and Valle, A., 2006. Caracterización de la paratuberculosis bovina en ganado doble propósito de los llanos de Monagas, Venezuela, Revista Científica Zootecnia Tropical, 24(3), 321–332.
Anonymous. Country Directory: Latin American Network Information Center. The University of Texas at Austin http://www.lanic.utexas.edu/subject/countries/index.html. Accessed 18 January 2014.
Arsenault, J., Girard, C., Dubreuil, P., Daignault, D., Galarneau, J.R., Boisclair, J., Simard, C. and Bélanger, D., 2003. Prevalence of and carcass condemnation from maedi-visna, paratuberculosis and caseous lymphadenitis in culled sheep from Quebec, Canada, Preventive Veterinary Medicine, 59, 67–81.
Bakker, D., 2010. Paratuberculosis control measures in Europe. In: Behr, M.A., Collins, D.M. (eds.), Paratuberculosis: Organism, Disease, Control. CAB International, Oxfordshire, pp. 306–318.
Barkema, H.W., Hesselink, J.W., McKenna, S.L., Benedictus, G. and Groenendaal, H., 2010. Global prevalence and economics of Infection with Mycobacterium avium subsp. paratuberculosis in ruminants. In: Behr, M.A., Collins, D.M. (eds.), Paratuberculosis: organism, disease, control. CAB International, Oxfordshire, pp. 10–17.
Begg, D. and Whittington, R., 2010. Paratuberculosis in sheep. In: Behr, M.A., Collins, D.M. (eds.), Paratuberculosis: Organism, Disease, Control. CAB International, Oxfordshire, pp. 157–168.
Burgos-Garay, P.I., 2011. Determinación del estatus de infección por Mycobacterium avium subsp. paratuberculosis mediante cultivo de muestras ambientales en rebaños lecheros de la región de los lagos y su relación con la seroprevalencia predial (memoria de título médico veterinario), Universidad Austral de Chile, Valdivia, 26p.
Callejas-García S.A., 2013. Estudio epidemiológico de la paratuberculosis caprina en la zona centro del estado de Veracruz (tesis médico veterinario zootecnista), Universidad Veracruzana, Veracruz, 62p.
Carta, T., Álvarez, J., Pérez de la Lastra, J.M. and Gortázar, C., 2013. Wildlife and paratuberculosis: a review, Research in Veterinary Science, 94, 191–197.
Chacon, O., Bermudez, L.E. and Barletta, R.G., 2004. Johne’s disease, inflammatory bowel disease, and Mycobacterium paratuberculosis, Annual Review of Microbiology, 58, 329–363.
Chiodini, R.J., Van Kruiningen, H.J. and Merkal, R.S., 1984. Ruminant paratuberculosis (Johne’s disease): the current status and future prospects, Cornell Veterinary, 74, 218–262.
Clark, D.L., Koziczkowski, J.J., Radcliff, R.P., Carlson, R.A. and Ellingson, J.L., 2008. Detection of Mycobacterium avium subspecies paratuberculosis: comparing fecal culture versus serum enzyme-linked immunosorbent assay and direct fecal polymerase chain reaction, Journal of Dairy Science, 91, 2620–2627.
Clarke, C.J., 1997. The pathology and pathogenesis of paratuberculosis in ruminants and other species, Journal of Comparative Pathology, 116, 217–261.
Clarke, C.J. and Little, D., 1996. The pathology of ovine paratuberculosis: gross and histological changes in the intestine and other tissues, Journal of Comparative Pathology, 114, 419–437.
Collins, M.T., 1996. Diagnosis of paratuberculosis, Veterinary Clinics of North America: Food Animal Practice, 12, 357–371.
Collins, D.M., Gabric, D.M. and de Lisle, G.W., 1990. Identification of two groups of Mycobacterium paratuberculosis strains by restriction endonuclease analysis and DNA hybridization, Journal of Clinical Microbiology, 28, 1591–1596.
Collins, M.T., Gardner, I.A., Garry, F.B., Roussel, A.J. and Wells, S.J., 2006. Consensus recommendations on diagnostic testing for the detection of paratuberculosis in cattle in the United States, Journal of American Veterinary Medical Association, 229, 1912–1919.
Costa, J.C.M., Pieri, F.A., Souza, C.F., Espeschit, I.F., Felippe, A.G., Santos, G.M., Tobia, F.L., Silva Junior, A. and Moreira, M.A.S., 2010. Levantamento sorológico de Mycobacterium avium subesp. paratuberculosis em bovinos leiteiros no estado do Espírito Santo [Serological survey of Mycobacterium avium subsp. paratuberculosis in dairy cattle in Espírito Santo state], Arqivo Brasileiro de Medicina Veterinaria y Zootecnia, 62(6), 1491–1494.
Dargatz, D.A., Byrum, B.A., Hennager, S.G., Barber, L.K., Kopral, C.A., Wagner, B.A. and Wells, S.J., 2001. Prevalence of antibodies against Mycobacterium avium among beef cow-calf herds, Journal of American Veterinary Medical Association, 219(4), 497–501.
De Juan, L., Mateos, A., Dominguez, L., Sharp, J.M., Stevenson, K., 2005. Genetic diversity of Mycobacterium avium subspecies paratuberculosis isolates from goats detected by pulsed-field gel electrophoresis, Veterinary Microbiology, 106, 249–257.
Djønne, B., 2010. Paratuberculosis in goats. En: Behr, M.A., Collins, D.M. (eds.), Paratuberculosis: Organism, Disease, Control. CAB International, Oxfordshire, pp. 169–178. 388 p.
Dolz, G., Araya, L.N., Suárez, J. and Jiménez, C., 1999. Prevalence of antibodies to bovine paratuberculosis detected by a LAM-ELISA in Costa Rica, Veterinary Record, 144(12), 322–323.
Fecteau, M.E. and Whitlock, R.H., 2010. Paratuberculosis in Cattle. In: Behr, M.A., Collins, D.M. (eds.), Paratuberculosis: Organism, Disease, Control. CAB International, Oxfordshire, pp. 144–156.
Ferreira, R., Fonseca, L.S. and Lilenbaum, W., 2001. Detecção de anticorpos contra Mycobacterium paratuberculosis em rebanhos bovinos do Estado do Rio de Janeiro, Brasil, Revista Brasileira de Medicina Veterinária, 23(4), 19–24.
Fonseca, L.F.L., Olival, A.A., Pereira, C.C., Heinemann, M.B., Richtzenhain, L.J. and Santos M.V., 2000. Identificação de anticorpos anti-Mycobacterium paratuberculosis em rebanhos bovinos leiteiros do Estado de São Paulo. Arquivos Facultade Veterinaria, Universidades Federal do Rio Grande do Sul (UFRGS), 28(1), 51–60.
Glasziou, P., Irwig, L., Bain, C. and Colditz, G., 2001. Systematic reviews in health care: A practical guide, University of Cambridge, United Kingdom.
Gumber, S., Eamens, G., Whittington, R.J., 2006. Evaluation of a Pourquier ELISA kit in relation to agar gel immunodiffusion (AGID) test for assessment of the humoral immune response in sheep and goats with and without Mycobacterium paratuberculosis infection, Veterinary Microbiology, 115, 91–101.
Jaimes, N.G., Santillán Flores, M.A., Hernández Cruz, O.A., Córdova López, D., Guzmán Ruiz, C.C., Arellano Reynoso, B., Díaz Aparicio, E., Tenorio Gutiérrez, V.R. and Cuéllar Ordaz, A., 2008. Detección de Mycobacterium avium subespecie paratuberculosis, por medio de PCR-anidada a partir de muestras de heces de ovinos [Detection of Mycobacterium avium subspecies paratuberculosis by nested-PCR of ovine fecal samples], Revista Veterinaria México, 39(4), 377–386.
Juste, R.A. and Perez, V., 2011. Control of Paratuberculosis in Sheep and Goats. Veterinary Clinics of North America: Food Animal Practice, 27, 127–138.
Kalis, C.H.J., Collins, M.T., Hesselink, J.W. and Barke, H.W., 2003. Specificity of two tests for the early diagnosis of bovine paratuberculosis based on cell-mediated immunity: the Johnin skin test and the gamma interferon assay. Veterinary Microbiology 97, 73–86.
Kennedy, D. and Citer, L., 2010. Paratuberculosis control measures in Australia. In: Behr, M.A., Collins, D.M. (eds.), Paratuberculosis: Organism, Disease, Control. CAB International, Oxfordshire, pp. 330–343.
Köhler, H., Burkert, B., Pavlik, I., Diller, R., Geue, L., Conraths, F.J. and Martin, G., 2008. Evaluation of five ELISA test kits for the measurement of antibodies against Mycobacterium avium subspecies paratuberculosis in bovine serum. Berl Munch Tierarztl Wochenschr Journal, 121, 203–210.
Kruze, J., Monti, G., Schulze, F., Mella, A. and Leiva, S., 2013. Herd-level prevalence of Map infection in dairy herds of southern Chile determined by culture of environmental fecal samples and bulk-tank milk qPCR, Preventive Veterinary Medicine, 111, 319–324.
Kumthekar, S., Manning, E.J., Ghosh, P., Tiwari, K., Sharma, R.N. and Hariharan, H., 2013. Mycobacterium avium subspecies paratuberculosis confirmed following serological surveillance of small ruminants in Grenada, West Indies, Journal of Veterinary Diagnostic Investigation, 25(4), 527–530.
Lilenbaum, W., Marassi, C.D., Varges, R., Medeiros, L., Oelemann, W.M.R. and Fonseca L.S., 2009. Occurrence of false-positive results in three Paratuberculosis - ELISAs performed in a tuberculous herd, Veterinary Research Communication, 33, 693–699.
Lilenbaun, W., Ferreira, R., Marassi, C.D., Ristow, P; Roland Oelemann, W.M. and de Souza Fonseca, L., 2007. Interference of tuberculosis on the performance of ELISAS used in the diagnosis of paratuberculosis in cattle. Brazilian Journal of Microbiology, 38, 472–477.
Manning, E.J. and Collins, M.T., 2001. Mycobacterium avium subsp. paratuberculosis: pathogen, pathogenesis and diagnosis, Revue Scientifique et Technique, 20, 133–150.
Manning, J.B. and Collins, M.T., 2010. Epidemiology of paratuberculosis. In: Behr, M.A., Collins, D.M. (eds.), Paratuberculosis: Organism, Disease, Control. CAB International, Oxfordshire, pp. 22–27.
Martínez-Herrera, D.I., Sarabia-Bueno, C.C.,, Peniche-Cardeña, A., Villagómez-Cortés, J.A., Magdaleno-Méndez, A., Hernández-Ruíz, S.G., Morales-Alvarez, J.F. and Flores-Castro, R., 2012. Seroepidemiology of goat paratuberculosis in five municipalities of central Veracruz, Mexico, Tropical and Subtropical Agroecosystems, 15(2), 82–88.
Martinis Mercado, D.S., Cicuta, M.E., Boehringer, S.I., Morsella, C., Paolicchi, F., La Paratuberculosis y los bovinos lecheros de la provincia de Corrientes. 2014. http://www.unne.edu.ar/unnevieja/Web/cyt/cyt/2002/04-Veterinarias/V-059.pdf. Accessed 18 January 2014.
Medeiros, J.M.A., Garino Jr, F., Torres Matos, R.A., Torres Matos, R.A., de Medeiros Costa, V.M. and Riet-Correa, F., 2012. Frequência de anticorpos para paratuberculose em bovinos no semiarido paraíbano, Pesquisa Veterinária Brasileira, 32(8), 697–700.
Mercier, P., Beaudeau, F., Laroucau, K., Bertin, C., Boschiroli, M.L., Baudry, C., Seegers, H. and Malher, X., 2009. Comparative age-related responses to serological and faecal tests directed to Mycobacterium avium paratuberculosis (Map) in French dairy goats, Small Ruminant Research, 87, 50–56.
Michel, A.L. and Bastianello, S.S., 2000. Paratuberculosis in sheep: an emerging disease in South Africa, Veterinary Microbiology 77, 299–307.
Miranda Bandera, M.V., 2005. Evaluación del impacto económico de la paratuberculosis en ganado bovino lechero (Sistema intensivo), en el Complejo Agropecuario Industrial de Tizayuca, Hidalgo, México. (Tesis Maestro en Ciencias), Facultad de Medicina Veterinaria y Zootecnia, Universidad Nacional Autónoma de México, México D. F., 2005.p 65.
Moreira, A.R., Spath, E.J.A. and Morsella, C., 1994. Seroprevalence of Johne’s disease in eleven districts of Buenos Aires, Argentina. Abstracts from Oral and Poster presentations at the Fourth International Colloquium on Paratuberculosis, Cambridge, United Kingdom, July 17–21, 1994, International Association for Paratuberculosis, Inc.p. 10.
Morón-Cedillo, F.J., Cortez-Romero, C., Gallegos-Sánchez, J., Figueroa-Sandoval, B., Aquino-Pérez, G. and Amante-Orozco, A., 2013. Prevalencia de la infección por Mycobacterium avium subespecie paratuberculosis en rebaños de ovinos de dos municipios de San Luis Potosí, México, Revista Científica, FCV-LUZ, 23(4), 293–299.
Mundaca-Verdugo, F.J., 2012. Diagnóstico de paratuberculosis bovina mediante cultivo de muestras ambientales y su relación con la seroprevalencia predial en rebaños lecheros de la VIII región, Chile (memoria de título médico veterinario), Universidad Austral de Chile, Valdivia, 23p.
Neyeloff, J.L., Fuchs, S.C. and Moreira, L.B., 2012. Meta-analyses and Forest plots using a Microsoft excel spreadsheet: step-by-step guide focusing on descriptive data analysis, BMC Research Notes, 5, 52.
Nielsen, S.S., 2010. Immune-based diagnosis of paratuberculosis. In: Behr, M.A., Collins, D.M. (eds.), Paratuberculosis: Organism, Disease, Control. CAB International, Oxfordshire, pp. 284–293.
Nielsen, S.S. and Toft, N., 2008. Ante-mortem diagnosis of paratuberculosis: a review of accuracies of ELISA, interferon-gamma assay and faecal culture techniques, Veterinary Microbiology, 129, 217–235.
Nielsen, S.S. and Toft, N., 2009. A review of prevalences of paratuberculosis in farmed animals in Europe, Preventive Veterinary Medicine, 88, 1–14.
Pantoja, J., Vallejo, B. and Acosta, J., 2010. Bovine paratuberculosis in Puerto rican dairy herds and its association with selected performance parameters, Journal of Agriculture of the University of Puerto Rico, 94(3–4), 247–253.
Pithua, P. and Kollias, N.S., 2012. Estimated prevalence of caprine paratuberculosis in Boer goat herds in Missouri, USA, Veterinary Medicine International, Article ID 674085, 5 pages.
Robbe-Austerman, Suelee., 2011. Control of paratuberculosis in small ruminants, Veterinary Clinics of North America: Food Animal Practice, 27, 609–620.
Robbe-Austerman S., Gardner I.A., Thomsen B.V., Morrical D.G., Martin B.M., Palmer M.V., Thoen C.O., Ewing C., 2006, Sensitivity and specificity of the agar-gel-immunodiffusion test, ELISA and the skin test for detection of paratuberculosis in United States Midwest sheep populations, Veterinary research, 37(4) 553–64.
Rosegrant, M.W., Fernandez, M. and Sinha, A., 2009 Looking into the future for agriculture and AKST (Agricultural Knowledge Science and Technology). In: Agriculture at a crossroads. International assessment of agricultural knowledge, science and technology for development (IAASTD): global report. McIntyre B. D., Herren H. R., Wakhungu J., Watson Robert T. (eds), pp. 307–376. Washington, DC: Island Press.
Roussel, A.J., 2011. Control of paratuberculosis in beef cattle, Veterinary Clinics of North America: Food Animal Practice, 27, 593–598.
Sá, L.M., Oliveira, J.M.B., Santos, G.R., Brandespim, D.F., Silva-Júnior, J.L., Mota, R.A. and Pinheiro Júnior, J.W., 2013. Avaliação sorológica e de fatores de risco para a infecção por Mycobacterium avium subsp. paratuberculosis em rebanhos leiteiros da Microrregião de Garanhuns, Pernambuco, Pesquisa Veterinária Brasileira, 33(3), 310–313.
Salgado, M., Kruze, J., Collins, M.T., 2007. Diagnosis of paratuberculosis by fecal culture and ELISA on milk and serum samples in two types of Chilean dairy goat herds, Journal of Veterinary Diagnostic Investigation, 19, 99–102.
Salgado, M., Muñoz, P., Strauch, S. and Zamorano, P., 2012. Association between herd infection level and the detection of Mycobacterium avium subsp. paratuberculosis (map) in bulk tank milk tank using real-time PCR in small holder dairy farms in southern Chile. Proceedings of the11th International Colloquium on Paratuberculosis. Sydney – Australia. 5–10 February 2012 International Association Paratuberculosis, p. 65.
Scholtz, M.M., McManus, C., Okeyo, AM. and Theunissen, A., 2011. Opportunities for beef production in developing countries of the southern hemisphere, Livestock Science, 142, 195–202.
Schriger, D.L., Altman, D.G., Vetter, J.A., Heafner, T. and Moher, D., 2010. Forest plots in reports of systematic reviews: a cross-sectional study reviewing current practice, International Journal of Epidemiology, 39, 421–429.
Scott H. M., Sorensen O., Wu J.T., Chow E.Y., Manninen K., 2007. Seroprevalence of and agroecological risk factors for Mycobacterium avium subspecies paratuberculosis and neospora caninum infection among adult beef cattle in cow-calf herds in Alberta, Canada, The Canadian Veterinary Journal, 48(4), 397–406.
Seva, J., Sanes, J.M., Ramis, G., Mas, A., Quereda, J.J., Villarreal-Ramos, B., Villar, D. and Pallares, F.J., 2014. Evaluation of the single cervical skin test and interferon gamma responses to detect Mycobacterium bovis infected cattle in a herd co-infected with Mycobacterium avium subsp. paratuberculosis, Veterinary Microbiology, 171, 139–146.
Silva, E Bandeira da, 2005. Diagnóstico da Paratuberculose em Bovinos de Corte do Estado do Pará-Brasil. (Dissertação Mestre em Ciência Animal) Universidade Federal do Pará, Empresa Brasileira de Pesquisa Agropecuária, Universidade Federal Rural da Amazônia. Belém, 65 p.
Stau, A., Seelig, B., Walter, D., Schroeder, C. and Ganter, M., 2012. Seroprevalence of Mycobacterium avium subsp. paratuberculosis in small ruminants in Germany, Small Ruminant Research, 105, 361–365.
Steiger, C., 2006. Modern beef production in Brazil and Argentina, CHOICES 2nd Quarter, 21(2).
Stevenson, K., 2010a. Comparative differences between strains of Mycobacterium avium subsp. paratuberculosis. In: Behr, M.A., Collins, D.M. (Eds.), Paratuberculosis: organism, disease, control. CAB International, Oxfordshire, pp. 126–137.
Stevenson, K., 2010b. Diagnosis of Johne’s disease: current limitations and prospects, Cattle Practice, 18, 104–109.
Stevenson, K., Hughes, V.M., de Juan, L., Inglis, N.F., Wright, F. and Sharp, J.M., 2002. Molecular characterization of pigmented and nonpigmented isolates of Mycobacterium avium subsp. paratuberculosis, Journal of Clinical Microbiology, 40, 1798–1804.
Storset, A.K., Hasvold, H.J., Valheim, M., Brun-Hansen, H., Berntsen, G., Whist, S.K., Djønne, B., Press, C.McL., Holstad, G. and Larsen, H.J., 2001. Subclinical paratuberculosis in goats following experimental infection. An immunological and microbiological study, Veterinary Immunology and Immunopathology, 80, 271–287.
Vandenbroucke, J.P., von Elm, E., Altman, D.G., Gøtzsche, P.C., Mulrow, C.D., Pocock, S.J., Poole, C., Schlesselman, J.J. and Egger, M., 2007. Strengthening the reporting of observational studies in epidemiology (STROBE): Explanation and elaboration, PLoS Medicine, 4(10), e297.
Varges, R., Marassi, C.D., Oelemann, W. and Lilenbaum, W., 2009. Interference of intradermal tuberculin tests on the serodiagnosis of paratuberculosis in cattle, Research in Veterinary Science, 86, 371–372.
von Elm, E., Altman, D.G., Egger, M., Pocock, S.J., Gotzsche, P.C. and Vandenbroucke, J.P., 2007. STROBE Initiative: the strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies, Lancet 370, 1453–1457.
Waldner, C.L., Cunningham, G.L., Janzen, E.D. and Campbell, J.R., 2002. Survey of Mycobacterium avium subspecies paratuberculosis serological status in beef herds on community pastures in Saskatchewan, Canadian Veterinary Journal, 43(7), 542–546.
Wells, S.J. and Wagner, B.A., (2000) Herd-level risk factors for infection with Mycobacterium paratuberculosis in US dairies and association between familiarity of the herd manager with the disease or prior diagnosis of the disease in that herd and use of preventive measures, Journal of the American Veterinary Medical Association, 216(9), 1450–1457.
Whitlock, R.H., 2010. Paratuberculosis control measures in the USA. In: Behr, M.A., Collins, D.M. (eds.), Paratuberculosis: Organism, Disease, Control. CAB International, Oxfordshire, pp. 319–329.
Whittington, R., 2010. Cultivation of Mycobacterium avium subsp. paratuberculosis. In: Behr, M.A., Collins, D.M. (eds.), Paratuberculosis: Organism, Disease, Control. CAB International, Oxfordshire, pp. 244–266.
Yamasaki, E.M., Brito, M.F., Mota, R.A., McIntosh, D. and Tokarnia, C.H., 2013. Paratuberculose em ruminantes no Brasil, Pesquisa Veterinária Brasileira, 33(2), 127–140.
Acknowledgments
Estrategia de Sostenibilidad de la Universidad de Antioquia, 2013–2014.
Ethical standards
The manuscript does not contain clinical studies or patient data.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fernández-Silva, J.A., Correa-Valencia, N.M. & Ramírez, N.F. Systematic review of the prevalence of paratuberculosis in cattle, sheep, and goats in Latin America and the Caribbean. Trop Anim Health Prod 46, 1321–1340 (2014). https://doi.org/10.1007/s11250-014-0656-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-014-0656-8