Abstract
Bovine tuberculosis (bTB) is a chronic granulomatous disease that primarily affects lung tissue and lymph nodes (LN) in cattle, with economic impact on their productivity. Furthermore, it is potential zoonoses that may cause public health hazard. In this study, we evaluated the presence of bTB in two abattoirs: Cayambe and Pelileo countries located in the Ecuadorian provinces of Pichincha and Tungurahua, respectively. In total, 578 cattle were sampled (Cayambe 271 and Pelileo 307): 1,156 LN and 578 lung tissue samples were collected to apply in vitro culture and nested-PCR, respectively. The results determined a total apparent prevalence of 4.33 %, with 4.06 % at Cayambe’s abattoir and 4.56 % at Pelileo’s abattoir. Additionally, the Bayesian analysis showed a total true prevalence of 2.51 %, with 89.7 % of sensitivity and 97.6 % of specificity. The risk factors were evaluated by the use of simple logistic regressions with and without the random effect of places of origin. Associations of the origin of cattle in the selected slaughterhouses were found. The results showed an efficient method for the detection of bTB, which could identify a large number of infected animals, and the usefulness of lung tissue samples for early diagnosis of the disease was demonstrated in this study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Veterinary inspection (VI) at slaughterhouses is an important tool used in the control and eradication of bovine tuberculosis (bTB). It is based on detailed observation of the carcasses, which include sampling of compatible lesions with the disease to apply culture and finally confirm the presence of Mycobacterium bovis by bacterial growth; a process which can take several weeks. However, PCR used directly in biological samples, i.e., lung tissue and lymph node (LN), from suspected cattle offers another alternative of diagnostic with more efficiency compared to in vitro culture (Zumárraga et al. 2005). In general, DNA extraction from tissue samples is crucial to improve the success of the test, as well as employing magnetic beads for getting better DNA from clinical tissues samples of people and animals (Shiyang et al. 2013). PCR advantages include speed, high specificity, moderate confirmation of the presence of the bacillus in samples with no visible lesions negative to culture (Parra et al. 2008), and discard of the susceptibility due to the elimination of inhibitors reaction (Taylor et al. 2001). Nevertheless, it is not a perfect test because it has difficulty obtaining DNA in samples with low number of microorganisms (Zarden et al. 2013).
In Ecuador, the total national prevalence of bTB is still unknown. The cases of the disease are not well documented, published, or quantified for several reasons, i.e., limited animal carrier records, scarce diagnostic testing, and lack of reporting of the disease when suitable (Proaño-Pérez et al. 2011b).
The aim of this study was to estimate the apparent prevalence (AP) and true prevalence (TP) of bTB in the two slaughterhouses from two dairy areas of Ecuador, and evaluate the direct use of nested-PCR (n-PCR) in lung tissue from cattle modifying the protocol described by Mangiapan et al. (1996).
Materials and methods
Study design
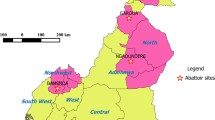
This was an exploratory cross-sectional study, applied in two local slaughterhouses: Cayambe (0.04 N–78.14 W) and Pelileo (1.33S–78.54 W), located in Pichincha and Tungurahua provinces, respectively (Fig. 1). These areas were chosen due to a high density of dairy herds and movement of cattle. The methodology comprised two parts: (a) veterinary inspection carried out in 578 cattle randomly selected from farms of nearby abattoirs, and (b) laboratory analysis of the biopsy samples taken from slaughtered cattle during two to three interventions per week. In total, 1,734 biopsy samples were analyzed: in Cayambe from 271 cattle, 813 samples (542 LN and 271 lung); and in Pelileo from 307 cattle, 921 samples (614 LN and 307 lung). In both places, it was considered the sampling method for a homogeneous population, and previously a priori prevalence of 0.15 was used, the confidence level of 95 % and the maximum error was set at 0.04 for each place. Information of each slaughtered animal was recorded by the use of a questionnaire to obtain specific information, i.e., age (≥6 and <6 years), sex, place of origin (province, canton, and parish), owner, and whether or not visible injuries were present during the VI. Finally, to diagnose bTB, the LN samples were process in the laboratory of microbiology, and the lung samples in the molecular biology laboratory of the International Centre for Zoonosis (CIZ).
Map of Ecuador showing the zones of the study (INEC 2012)
Postmortem examination
Postmortem examination was applied by inspection and palpation of several organs, i.e., lungs, liver, kidneys, and spleen, with each organ internally and externally evaluated. Then a cross section of the mandibular, tracheobronchial, retropharyngeal, mediastinal, mesenteric, and hepatic LN were inspected carefully.
Samples
Lung tissue samples (578/1,734) and tracheobronchial and mediastinal LN (1,156/1,734) of each animal slaughtered with and without visible lesions (VL) were collected for further examination, using disinfected (chlorine 10 %) dissecting equipment, then placed in individual sterile tubes, stored in a cool box, and transported the same day to the laboratory. All samples were reduced by homogenizing into fine pieces. Subsequently, these samples were placed into 1.5 ml of sterile water and stored at −20 °C. All LNs were cultured and lung tissues were amplified by n-PCR. Pulmonary samples were taken for the lower part of the lobe because the pathogen can be deposited in the peripheral respiratory alveoli in the first stages (Kritski and Fiuza 2007; Müller et al. 2008). In Ecuador, Proaño-Pérez et al. (2011a) tested the diagnostic methods used at this study and demonstrated that the respiratory route is the most important route in the transmission of the disease; however, many VL were not found, and most of the cases were identified by culture.
Polymerase chain reaction
The DNA extraction protocol for lung tissues was performed according to Mangiapan et al. (1996), with some modifications, i.e., initially, digestion was conducted by placing 275 μl of sample into 275 μl of lysis buffer (200 mM Tris–HCl pH 7.4, 300 mM NaCl, 100 mM EDTA, Ultra-Pure water) and 65 μl proteinase K (20 mg/ml in PK buffer–50 mM Tris–HCl pH 7.4, 10 mM CaCl2, Ultra-Pure water), incubated at 50 °C with stirring for 18 h to improve the mechanical and chemical lysis of the tissue; then to prepare the sample for the hybridization, 520 μl was centrifuged and the supernatant was heated at 100 °C for 10 min and quickly cooled down at 0 °C. Then, 200 μl of 3.75 M NaCl-3.3 ρmol was added with each biotinylated primer and incubated at 60 °C with stirring for 2.5 h. The solution was placed with 10 μl of M-280 Streptavidin Dynalbeads and incubated for 2 h at 20 °C. Finally, magnetic beads captured the target DNA; after washing three times, they were resuspended in 25 μl of TE buffer (10 mM Tris–HCl, 1 mM EDTA pH 8, Ultra-Pure water). Biotinylated primers and primers used in the first run of the n-PCR are specific for the genus Mycobacterium and have been described by Portaels et al. (1996), which focused on the 16S rRNA gene: P1 (5′-TGCTTAACACATGCAAGTCG-3′) and P2 (5′-TGAGATTTCACGAACAACGC-3′). For the second run, there were used primers designed at the Institute for Tropical Medicine in Antwerp, Belgium, specific for Mycobacterium tuberculosis complex, i.e., P3 (5′-AACCCGGACCTTCGTCGATG-3′) and P9 (5′-CATGTCTTGTGGTGGAAAGCGC-3′) described by Proaño-Pérez et al. (2006, 2011a, b) and Durnez et al. (2008)The parameters used for amplification have been described by Proaño-Pérez et al. (2006). Finally, visualized bands 962 and 271 bp were seen in agarose gel 2 % (w/v) (Fig. 2).
Microscopy and in vitro culture
All LN biopsies were cultured with the method described by Proaño-Pérez et al. (2011a). In addition, Ziehl–Neelsen staining was carried out; one drop of the processed tissue was observed by microscopy to detect the acid alcohol-resistant bacilli.
Statistical analysis
The analysis was made of the two areas together and separately, the AP and CI95 % were obtained in the R version 2.12.1. The TP was calculated using the rule of Bayesian modeling with Rogan–Gladen equation [AP = TP Se + (1 − TP) (1 − Sp)] that estimates the TP starting from the AP, sensitivity (Se) and specificity (Sp) of the diagnostic test, and the total number of samples used. Uncertainty about the test characteristics is required under a prior distributions. The Bayesian approach uses information from previous studies of the methodology used (Enøe et al. 2000) and allows approximating the Se and Sp with 95 % probability interval (95 % PI).
Beta prior distributions of the parameters were calculated based on the mode and one percentile for Se and Sp with BetaBuster software (Branscum et al. 2005). Then, the data were incorporated in WinBUGS to make Bayesian estimation and build a binomial model (Lunn et al. 2009). A burn-in phase of 1,000 iterations was used and the model was run for another 10,000 iterations to obtain estimates. The outcomes were mean and percentiles sampled from the posterior distributions of TP, Se, and Sp. Three chains starting in different values were set and their convergence was analyzed graphically in order to evaluate how prior beliefs could affect the posterior estimates of TP, Se, and Sp. Three scenarios were built to analyze the best distribution of the parameters: scenario 1—the Se of the n-PCR was between 85 and 91 % (Parra et al. 2008; Shah et al. 2006) and Sp between 93 and 98 % (Shim et al. 1998); scenario 2—security level was of 90 %, the Se was greater than 85 with a mode of 87 % and a higher Sp to 95 with a mode of 99 %; scenario 3—security level was of 90 %, the Se was greater than 85 with a mode of 91 % and a Sp over 95 with a mode of 98 % (Table 1). Those parameter values were used due to the fact that more uncertainty was needed to reach prevalence estimates and test characteristics according to literature and last experiences; also, plots of posterior distributions were generated to verify an appropriate convergence.
The agreement of diagnostic tests was assessed using Cohen's Kappa analysis with different samples (i.e., lymph nodes) and diagnostic test. The results were classified according to the Altman scale (Table 2). Risk factor analysis using n-PCR results was additionally studied. Mixed models of logistic regression were calculated, taking into account the place of animal origin, parish of origin was the random factor, due to the impossibility for identifying the farm of origin. Relationships were evaluated using the odds ratios obtained from logit link function (Table 4). To test differences among places, a simple logistic regression was generated.
Results
The AP of bTB in slaughtered cattle from two abattoirs was 4.33 % (25/578) [CI95 % = 2.87–6.41]: 11/25 belonged to the slaughterhouse of Cayambe (4.06 %) [CI95 % = 2.14–7.35] while 14/25 corresponded to the slaughterhouse of Pelileo (4.56 %) [CI95 % = 2.61–7.7] (Table 3). The estimation of TP was 2.51 % [PI95 % = 0.19–5.32], and the Se and Sp for this study was 89.7 % [PI95 % = 81.5–95.6] and 97.6 % [PI95 % = 95.4–99.4], respectively (Table 1).
The kappa analysis for diagnostic tests showed a slight agreement (κ = 0.02) among the n-PCR/VI held in Pelileo, and poor agreement (κ = 0) among the n-PCR/other techniques used for the analysis of bTB (Table 2).
The risk factors analysis obtained with the data from both slaughterhouses did not show any significant association (P > 0.05), as well in Cayambe; nevertheless, at Pelileo it found associations with positive results, province and canton of origin of animals sampled (n = 203) showed highly significant difference (P < 0.01) (Table 4).
Discussion
VI is routinely used to diagnose bTB with several advantages such as low cost, provide useful information, and can be considered as an important tool to identify the disease during control programs (Biffa and Bogale 2010). However, the main disadvantage is its low sensitivity due to minor injuries in some cattle or being an early stage of disease (Biffa and Bogale 2010). The difference in appearance of VL can cause the detection to be very difficult, especially for inexperienced personnel (Cousins et al. 2004). In addition, not all infected cattle have VLs because the pathogen has been isolated from samples apparently healthy (Katale et al. 2012). The presence of lesions caused by non-tuberculosis mycobacteria (NTM) (Müller et al. 2009), or other conditions such as leukosis (i.e., disease with high prevalence in the studied area), might make the identification difficult because the lesions are restricted in LN which can be confused with granulomas at early states.
In Ecuador, most reports have been based on tuberculin testing and VI. Proaño-Pérez et al. (2011a) analyzed different laboratory tests (microscopy, in vitro culture, histopathology, and n-PCR) for confirmation of macroscopic lesions found in slaughtered cattle (2.3 %, 33/1390); they obtained M. bovis in 1.2 % of the samples. In each studied areas, two unpublished reports were performed applying tuberculin simple and comparative skin tests: in Cayambe, a prevalence of 0.47 % (14/3006) was found and in nearby areas of Pelileo 2.43 % (49/4012) (Proaño-Pérez et al. 2011b). The high AP (4.32 %) value indicated a large number of sick animals in both zones. In Cayambe, the high AP (4.06 %) could be due to a high density of animals engaged to milk production; although VLs were not found during VI, 11 cattle were found n-PCR-positive. Complementarily, the prevalence by bacteriological culture of LNs in the same cattle estimating an AP of 0.72 % (2/279) was determined, which do not present amplification in the lung biopsy analysis; it may have occurred because of an early extra-pulmonary dissemination or an alternative pathogenic route (Murphy et al. 2010). The results showed that Cayambe had a large number of livestock with more cases of 4.97 % (8/161) because the slaughterhouse is located in this area and also presented a high density of livestock and production systems.
Despite of the existence of smallholder dairy breeders in Pelileo country, a high AP (4.56 %) was found through molecular identification. However, VI showed an AP of 17.92 % (55/307) that could not be confirmed as M. bovis by culture of LN with VLs. Only 2 of 14 lung tissue samples with VLs were also positive by n-PCR.
Analyzing the provinces of origin of infected cattle, Imbabura and Tungurahua had 22.22 % (6/27) and 5.26 % (5/95), respectively. Nevertheless, 104 cattle did not have its data source complete, due to the deficient records of the movement of animals.
The modeling of the Se, Sp, and TP revealed that the scenario 3 is the most fitting, showing the advantages of the methodology but also presenting problems, especially when positive cases were found. The estimated TP showed false positives that was a reduction of the AP, probably because of external factors to the test (Berkvens et al. 2006). Investigations using different modifications of the technique have succeeded in increments of Se (n-PCR and SC) that have been reported to be 91.0 % (Taylor et al. 2001). The calculated data were comparable with the parameters raised by other researchers.
Kappa analysis showed that the use of n-PCR and SC in the extraction detected bTB in lung samples of animals that were negative to VI, in vitro culture, and smear of lymph node (Table 2). Therefore, this method showed better pathogen detection. In addition, the changes in the extraction method improved technical results and increased the Se and Sp values in the study. Previously using the same protocols in samples collected at the slaughterhouse from the Mejia canton, a significant concordance (κ = 0.61) was found between the culture and the n-PCR (Proaño-Pérez et al. 2011a). While the poor correlation that occurred in this study has to do with the different samples collected because it has been determined that the lung is the first locus to M. bovis infection (Müller et al. 2008). The respiratory tract is recognized as the major route of transmission of infection in most species (Doran et al. 2009). In Ecuador, it was observed that 75 % of the lesions were located in the thoracic area in cattle slaughtered at the Mejia Canton (Proaño-Pérez et al. 2011a).
n-PCR can detect DNA from live or dead mycobacteria (Cardoso et al. 2009), which were destroyed by the immune system of the animal, during transport, storage, or decontamination of specimens. The results allow us to determine that the disease was in the early stages; thus, VLs were not found in some cases. Although several cattle in Pelileo showed VLs, DNA of M. bovis was not confirmed, possibly caused by nontuberculous mycobacterial. Proaño-Pérez et al. (2006) found Mycobacterium avium-intracellulare-scrofulaceum, Mycobacterium gordanae, Mycobacterium szulgai, and Mycobacterium celatum in dairy cows slaughtered in a nearby area, which were previously identified as causing injuries, along with Mycobacterium terrae, Mycobacterium fortuitum, Mycobacterium smegmatis, and Mycobacterium chelonae, which can be confused with those caused by the tubercle bacillus (Cleaveland et al. 2007).
Although the overall analysis of risk factors was not different, 80 % (20/25) of the positive samples were females because they stay longer in the herd and are used in milk production. As to the place of origin, at the Imbabura, Pichincha, and Tungurahua provinces, there is a chance to find the disease of 2.3, 1.67, and 1.57 times, respectively, more than the province of Carchi. In Cayambe, differences in the associations were not found, although 90.91 % (10/11) of the positive animals were <6 years. In Pelileo, the place of origin was a factor, which may be related to the history of the disease in each province and canton (Humblet et al. 2009) or to the endemism (Carslake et al. 2011). In the UK, bTB outbreaks recur in the same areas, possibly because the disease could not be controlled and/or factors of the environment may facilitate recurrence of infection (White and Benhin 2004).
In conclusion, the results demonstrated that n-PCR is efficient for the detection bTB from lung tissue samples, and this method can identify the disease in the early stages.
References
Berkvens, D., Speybroeck, N., Praet, N., Adel, A. and Lesaffre, E., 2006. Estimating disease prevalence in a Bayesian framework using probabilistic constraints. Epidemiology. 17, 145-53.
Biffa, D., Bogale, A. and Skjerve, E., 2010. Diagnostic efficiency of abattoir meat inspection service in Ethiopia to detect carcasses infected with Mycobacterium bovis: implications for public health. BMC Public Health.10, 462.
Branscum, A., Gardner, I. and Johnson, W., 2005. Estimation of diagnostic-test sensitivity and specificity through Bayesian modeling. Prev Vet Med. 10, 68, 145-63.
Cardoso, M., Cardoso, R., Hirata, R., Hirata, M., Leite, C., Santos, A., Siqueira, V., Okano, W, Rocha, N. and Lonardoni, M., 2009. Direct detection of Mycobacterium bovis in bovine lymph nodes by PCR. Zoonoses Public Health.56, 8, 465-70.
Carslake, D., Grant, W., Green,L., Cave, J., Greaves, J., Keeling, M., McEldowney, J., Weldegebriel, H. and Medley, G., 2011. Endemic cattle diseases: comparative epidemiology and governance. Philos Trans R Soc Lond B Biol Sci. 12; 366(1573), 1975–1986.
Cleaveland, S., Shaw, D., Mfinanga, S., Shirima, G., Kazwala, R., Eblate, E. and Sharp, M., 2007. Mycobacterium bovis in rural Tanzania: risk factors for infection in human and cattle populations. Tuberculosis (Edinb). 87, 1, 30-43.
Cousins, D., Huchzermeyer, H., Griffin, J., Bruckner, G., Van rensburg, I. and Kriek, N., 2004. Tuberculosis. In: Coetzer, J., Tustin, R., (eds), Infectious Diseases of Livestock, 3 ed. Oxford, Oxford University Press. pp 1973-1993.
Doran, P., Carson, J., Costello, E. and More, S., 2009. An outbreak of tuberculosis affecting cattle and people on an Irish dairy farm, following the consumption of raw milk. Irish Veterinary Journal. 62, 6, pp 390–397.
Durnez, L, Eddyani, M., Mgode, G., Katakweba, A., Katholi, C., Machang'u, R., Kazwala, R., Portaels, F. and Leirs, H., 2008. First detection of mycobacteria in African rodents and insectivores, using stratified pool screening. Appl Environ Microbiol.74, 768-73.
Enøe, C., Georgiadis, M. and Johnson, W., 2000. Estimation of sensitivity and specificity of diagnostic tests and disease prevalence when the true disease state is unknown. Prev Vet Med. 45, 61-81.
Humblet, M., Boschiroli, M. and Saegerman, C., 2009.Classification of worldwide bovine tuberculosis risk factors in cattle: a stratified approach. Vet Res. 40, 5, 50.
INEC (Instituto Ecuatoriano de Estadísticas y Censos), 2012. Plan de desarrollo cantonal para Pelileo y Cayambe. (unpublished data, Gobierno Provincial de Pichincha y Tungurahua).
Katale, B., Mbugi E., Kendal, S., Fyumagwa, R., Kibiki, G., Godfrey-Faussett, P., Keyyu, J., Van Helden P., Matee, M., 2012. Bovine tuberculosis at the human–livestock–wildlife interface: is it a public health problem in Tanzania? A review. Onderstepoort J Vet Res. 20, 79(2), 463.
Kritski A. and Fiuza F., 2007. Tuberculosis in Adults. In: Tuberculosis: From Basic Science to patient care. Palomino, J.C., Leão, S., Ritacco, V. (Eds).(First edition). Antwerp: Emma Raderschadt.
Lunn, D., Spiegelhalter, D., Thomas, A., and Best, N., 2009. The BUGS project: Evolution, critique and future directions. Stat Med. 10, 28(25), 3049-67.
Mangiapan, G., Vokurka, M., Schouls, L., Cadranel, J., Lecossier, D., van Embden, J. and Hance, A., 1996. Sequence capture-PCR improves detection of mycobacterial DNA in clinical specimens. J ClinMicrobiol. 34, 1209-1215.
Müller, B., Steiner, B., Bonfoh, B., Fané, A., Smith, N. and Zinsstag, J., 2008. Molecular characterisation of Mycobacterium bovis isolated from cattle slaughtered at the Bamako abattoir in Mali. BMC Veterinary Research. 4, 26.
Müller, B., Vounatsou, P., Ngandolo, B., Diguimbaye-Djaïbe, C., Schiller, I., Marg-Haufe, B., Oesch, B., Schelling, E. and Zinsstag, J., 2009. Bayesian receiver operating characteristic estimation of multiple tests for diagnosis of bovine tuberculosis in Chadian cattle.PLoS One.12, e8215.
Murphy, D., Gormley, E., Costello, E., O'Meara, D. and Corner, L., 2010.The prevalence and distribution of Mycobacterium bovis infection in European badgers (Melesmeles) as determined by enhanced post mortem examination and bacteriological culture. Res VetSci. 88, 1, 1-5.
Parra, A., García, N., García, A., Lacombe, A., Moreno, F., Freire, F., Moran, J. and Hermoso de Mendoza, J., 2008. Development of a molecular diagnostic test applied to experimental abattoir surveillance on bovine tuberculosis. Vet Microbiol. 127, 315-324.
Portaels, F., Realini, L., Bauwens, L., Hirschel, B., Meyers, W.M. and de Meurichy, W., 1996. Mycobacteriosis caused by Mycobacterium genavense in birds kept in a zoo: 11-year survey. J ClinMicrobiol. 34, 319-323.
Proaño-Pérez, F., Rigouts, L., Brandt, J., Dorny, P., Ron, J., Chávez, M., Rodríguez, R., Fissette, K., Van Aerede, A., Portaels, F. And Benitez, W., 2006. Preliminary Observations on Mycobacterium spp. in Dairy Cattle in Ecuador. Am. J. Trop. Med. Hyg. 75, 318-323.
Proaño-Pérez, F., Benitez-Ortiz, W., Desmecht, D., Coral, M., Ortiz, J., Ron, L., Portaels, F., Rigouts, L. and Linden, A., 2011a. Post-mortem examination and laboratory-based analysis for the diagnosis of bovine tuberculosis among dairy cattle in Ecuador. Prev Vet Med. 101, 1-2, 65-72.
Proaño-Pérez, F., Benitez-Ortiz, W., Portaels, F., Rigouts, L. and Linden, A., 2011b. Situation of bovine tuberculosis in Ecuador. Rev Panam Salud Publica. 30, 279-286.
Shah, N., Singhal, A., Jain, A., Kumar, P., Uppal, S., Srivatsava, M. and Prasad, H., 2006. Occurrence of overlooked zoonotic tuberculosis: detection of Mycobacterium bovis in human cerebrospinal fluid. J Clin Microbiol. 44, 1352-1358.
Shim, J., Cheong, H., Kang, E., In, K., Yoo, S. and Kang, K., 1998. Nested polymerase chain reaction for detection of Mycobacterium tuberculosis in solitary pulmonary nodules. Chest. 113, 20-4.
Shiyang, P., Bing, G., Hong, W., Zihe, Y., Peng, W., Hao, P., Weiping, X., Dan, C., and Genyan, L., 2013.Comparison of four DNA extraction methods for detecting Mycobacterium tuberculosis by real-time PCR and its clinical application in pulmonary tuberculosis.J ThoracDis. 5(3), 251–257.
Taylor, M., Hughes, M., Skuce, R. and Neill, S., 2001. Detection of Mycobacterium bovis in bovine clinical specimens using real-time fluorescence and fluorescence resonance energy transfer probe rapid-cycle PCR. J Clin Microbiol. 39, 1272-1278.
Thrusfield, M., 2005. Veterinary Epidemiology. Wiley-Blackwell, Sydney, Australia.
White, P. and Benhin, J., 2004. Factors influencing the incidence and scale of bovine tuberculosis in cattle in southwest England. Prev. Vet. Med. 63, 1–7.
Zarden, C., Marassi, C., Figueiredo, E. and Lilenbaum W., 2013. Mycobacterium bovis detection from milk of negative skin test cows. Vet Rec. 172: 130.
Zumárraga, M., Meikle, V., Bernardelli, A., Abdala, A., Tarabla, H., Romano, M. and Cataldi, A., 2005. Use of touch-down polymerase chain reaction to enhance the sensitivity of Mycobacterium bovis detection. J Vet Diagn Invest. 17, 232-238.
Acknowledgments
Special thanks to the “Commission Universitaire pour le Développement” (CUD), University of Liege—Project PIC, for their financial support in this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Echeverría, G., Ron, L., León, A.M. et al. Prevalence of bovine tuberculosis in slaughtered cattle identified by nested-PCR in abattoirs from two dairy areas of Ecuador. Trop Anim Health Prod 46, 1015–1022 (2014). https://doi.org/10.1007/s11250-014-0610-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-014-0610-9