Abstract
Wheat straw was subjected to solid-state fermentation (SSF) with lignolytic white-rot fungus (WRF) Crinipellis sp. for 5 days to improve the nutritive value and digestibility. The fungal treatment caused a significant (P < 0.05) decrease in cell wall constituents viz., neutral detergent fiber (NDF), acid detergent fiber (ADF), hemicellulose, lignin, and cellulose to the extent of 10.4, 11.2, 8.7, 8.7, and 12.1 %, respectively, with increase (P < 0.05) in crude protein (CP) (51.6%) and ash (25.8%) contents in fungal treated wheat straw (FT-WS) than untreated wheat straw (UT-WS). Further, in vitro gas production, in vitro true dry matter digestibility and in vitro true organic matter digestibility at 48 h, metabolizable energy (ME) content, microbial biomass production, and short-chain fatty acids synthesis were significantly (P < 0.05) higher in FT-WS. In vivo feeding trial in 10 Sahiwal calves (8–12 months) comprised of (1) control group (T1) fed with ad libitum chopped UT-WS and (2) treatment group (T2) offered with ad libitum chopped FT-WS, in addition to supplementation of groundnut cake and green berseem (Trifolium alexandrium) forage to both groups. Digestibility of nutrients for dry matter (DM), organic matter, CP, NDF, ADF, hemicellulose, cellulose, and total carbohydrates were significantly (P < 0.05) higher in T2 compared to T1. Moreover, daily DM (P < 0.05), digestible crude protein (P < 0.01), and ME intakes were also higher (P < 0.05) in group T2 with higher (P < 0.05) nitrogen (N) retention, which resulted in significantly (P < 0.05) higher average daily gain in body weight (135 vs. 102 g/day). It was concluded that SSF with WRF Crinipellis sp. holds potential in upgrading the nutritional worth of wheat straw for feeding growing calves.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Majority of the ruminants in tropical countries subsist mainly on crop residue-based rations. Improving the livestock productivity by enhancing the utilization of nutrients from these abundantly available poor quality feedstuffs has been the primary focus of research in dairy cattle nutrition in developing countries, including India. Wheat straw is one of such cereal by-products utilized for cattle feeding. Although it represents a potential energy source, ruminal microbial utilization of wheat straw is hindered by the presence of lignin, which forms covalent encrustation with cell wall polysaccharides (cellulose and hemicellulose), thus decreasing its digestibility and overall animal performance (Basu et al. 2002). It is necessary to breakdown lignin either partially or completely from lignocellulose complex and to disrupt the crystallinity of cellulose fraction of wheat straw (Wyman 2007) for its maximum utilization as ruminant feed. Among various treatment methods employed for enhancing the digestibility, biological treatment seems to be most practical and promising alternative (Villas-Bôas et al. 2002; Yu et al. 2009) to physical and chemical methods, as it avoids toxic and corrosive chemicals with the higher product yields, less energy demands, minimal generation of wastes, and enriching the product with microbial proteins (Okano et al. 2009). The potential of biological treatments through solid-state fermentation (SSF) has been explained by the ability of certain microbes, specifically basidiomycetes white-rot fungi (WRF), to disrupt the lignin–carbohydrate complex (Keller et al. 2003), thus improving their utilization in the rumen by increasing the availability of fermentable energy to rumen microbes (Akin et al. 1993; Rahman et al. 2011; Hassim et al. 2012). Different fungi like Ceriporiopsis sp., Cyathus stercoreus, Pleurotus sp., Lentinula edodes, Phlebia sp., Phanerochaete chrysosporium, Ganoderma sp., Trametes versicolor, and Trichoderma reesei have been used successfully to improve the nutritive value of various substrates viz. wheat straw, paddy straw, bamboo, bermuda grass, corn stalks, oil palm fronds, etc. under SSF (Arora and Sharma 2009; Okano et al. 2009; Akinfemi 2010; Shrivastava et al. 2011, 2012; Hassim et al. 2012; Omer et al. 2012).
The objective of the present investigation was to evaluate the fungal treated wheat straw for its compositional changes upon SSF, followed by in vitro and in vivo analysis in growing Sahiwal calves to assess the feeding value.
Materials and methods
SSF of wheat straw
Wheat straw was dried at 60 °C and sieved to attain a uniform particle size (1.5–2.0 cm) before SSF. The unfermented and fermented straws were ground in the laboratory mill (Remi Motors, Delhi, India) and sieved (30 mesh) for analytical purposes.
Crinipellis sp. was isolated from the sugarcane bagasse at the University of Delhi and maintained on malt extract agar. The cultures were stored at 4 °C and subcultured every fortnight. Inoculation of wheat straw with fungal inoculums was carried out as per Shrivastava et al. (2012) with a final substrate to moisture ratio of 1:3 and incubated at 30 °C and 60 % relative humidity for 5 days. The uninoculated trays without fungal inocula served as control. The inoculated wheat straw trays in triplicates were harvested at regular intervals and weight loss of wheat straw was determined by deducting the weight of oven-dried fermented straw (at 60 °C to a constant weight) from the weight of dried control straw (Shrivastava et al. 2011).
In vitro analysis
In vitro gas production test (IVGPT) was performed as per Menke and Steingass (1988). Rumen liquor was collected before morning feeding between 09:00 and 09:30 h from two cannulated Karan–Fries bulls (5–6 years, body weight 450 ± 7 kg) fed with chopped wheat straw (ad libitum), available chopped green forage (about 20 kg), and concentrate mixture (about 3.0 kg). Incubations were carried out using 200 ± 10 mg of air equilibrated samples of either untreated wheat straw (UT-WS) and fungal treated wheat straw (FT-WS) with 30 ml buffered rumen inoculum (10 ml rumen fluid, 5 ml bicarbonate buffer, 5 ml macro-mineral solution, 0.0025 ml micro-mineral solution, and 10 ml distilled water) under continuous flushing with CO2 into 100-ml calibrated syringes (Fortuna Optima, Germany). Syringes were placed in a water bath maintained at 39 °C. Incubations without sample served as blanks (n = 5) with every set. In vitro gas production (GP24 h) was calculated by subtracting blank reading from the syringes with substrates after 24 h. The ME content and short-chain fatty acids (SCFA) production were calculated by the equations suggested by Menke and Steingass (1988) and Getachew et al. (2002), respectively. Microbial biomass production (MBP) was calculated as per Blümmel et al. (1997) considering 2.20 as the stoichiometric factor for wheat straw. A separate set of incubations were carried out through IVGPT for 48 h to determine in vitro true dry matter digestibility (IVTDMD) by treating syringe contents with neutral detergent solution (Goering and Van Soest 1970), and upon ashing the residue at 550 °C for 3 h, in vitro true organic matter digestibility (IVTOMD) was estimated with the standard formula.
Feeding and metabolism study
Ten apparently healthy and dewormed male Sahiwal calves aged between 8 and 12 months were selected from cattle yard of National Dairy Research Institute, Karnal and were divided into two groups based on comparable body weight (T1, 75.3 ± 5.3 kg; T2, 79.5 ± 5.1 kg) in a randomized block design. After an adaptation period of 10 days, animals in both groups were fed with either untreated wheat straw (T1, same composition as that of UT-WS used in SSF) or FT-WS (T2) ad libitum along with 600 g of fresh groundnut cake (GNC) to meet the nitrogen requirement (69.3 g/day) and 1.5 kg of fresh berseem forage to meet the vitamin A requirement (3,000 IU/day) along with mineral mixture (2 % w/w) to meet their all nutrient requirements (Ranjhan 1998). GNC was mixed with chopped (2–4 cm) wheat straw to maximize straw intake, while chopped berseem forage was offered separately at 12:30 h throughout the experimental feeding of 60 days. The animals were individually identified by numbered ear tags, tethered with nylon rope individually in a well-ventilated stall (floor space = 1 m × 1 m per animal) provided with uniform management practices and having facilities for individual feeding. Clean and fresh drinking water was made available ad libitum twice daily at 10:30 h and 14:30 h. Daily DM intake from individual animals was recorded by subtracting the residual DM from the quantity of DM offered. Feed ingredients were sampled weekly to determine their DM content, and diets were adjusted weekly to account for changes in DM content and body weight.
The metabolism study was conducted for 7 days at the end of feeding trial, during which daily intake of feeds and output of feces and urine were recorded. One hundredth, by fresh weight, of daily feces voided by the individual animal was used for DM determination by drying at 60 °C for 48 h to constant weight. For nitrogen determination, feces samples (1/500 of daily voidance) were preserved in 3 ml of 300 ml/l sulfuric acid to make pooled samples of 7 days by individual animal. One hundredth, by weight, of daily urine was pooled on an individual animal basis and preserved in 500-ml wide-mouth polypropylene bottles containing 10 % sulfuric acid and stored at −20 °C until further analysis. ME of the diet was calculated from total digestible nutrients (TDN) using a factor of 0.15.
Oven-dried samples of the feeds offered, residue leftover, and feces were pooled, ground through a 1-mm screen, and preserved for proximate analysis according to the standard methods of AOAC (2005). NDF and ADF were assayed by the procedures of Van Soest et al. (1991) and AOAC (1997), respectively, and expressed without residual ash. Lignin (sa) was determined by solubilization of cellulose with 72 % (w/w) sulfuric acid (Robertson and Van Soest 1981). Hemicellulose was the difference between NDF and ADF, whereas cellulose was calculated by subtracting lignin (sa) from ADF. Total carbohydrates (T-CHO) was calculated as per Sniffen et al. (1992). All the chemical analyses were in triplicates.
Statistical analysis
Data were tabulated as mean ± standard error for all parameters. Degradation of wheat straw constituents by fungal fermentation was analyzed using one-way analysis of variance (ANOVA). Experimental data on feeding trial of two dietary groups were analyzed by simple ANOVA (Snedecor and Cochran 1994) using Sigmastat for Windows version 3.10 (Systat Software Inc. USA) and the means were tested for the significant difference by using Tukey’s b.
Results and discussion
Chemical changes in wheat straw upon SSF
WRF Crinipellis sp. tested in the present study colonized wheat straw in 5 days of incubation and degraded NDF, ADF, and hemicellulose up to 10.4, 11.2, and 8.7 %, respectively, which led to the reduction in cell wall components in the treated straw with an apparent loss of DM up to 5.4 % (Table 1). Further, fungal fermentation brought down lignin content of wheat straw by 8.7 % with subsequent reduction in cellulose content by 12.1 % on the fifth day of incubation. Although there was an apparent OM loss of up to 8.1 %, the fungal fermentation enriched the CP level by 51.6 % as a result of its better colonization in wheat straw. Arora and Sharma (2009) reported a degradation of wheat straw lignin by various Phlebia sp. that was up to an extent of 6–17 % during 10 days of incubation. There was a similar trend of compositional changes in our study in the degradation of NDF, ADF, hemicellulose, cellulose, and lignin to the extent of 18.9, 17.3, 21.7, 22.6, and 24.0 % (on DM basis), respectively, while 11.6 % DM loss was observed by Shrivastava et al. (2012) in wheat straw fermented with Ganoderma sp. rckk02 for 10 days.
In vitro analysis
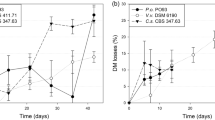
In vitro gas production analysis revealed that GP24 h was elevated (108.3 ± 2.2 ml/g DM) in FT-WS than that in UT-WS (99.2 ± 3 ml/g DM). Furthermore, fungal treatment significantly (P < 0.05) increased ME content, SCFA production as well as MBP with a higher (P < 0.05) ME content (Table 2) on the fifth day. In addition, FT-WS exhibited higher (P < 0.05) IVTDMD and IVTOMD (48 h) as compared to that of UT-WS. Higher gas production and associated parameters observed in FT-WS might be due to a reduction in cell wall constituents (NDF and ADF), along with lignin, upon fungal fermentation. Readily available soluble carbohydrate fractions in fungal fermented substrates for rumen microbiota resulted in more gas production (Chumpawadee et al. 2007), and De Boever et al. (2005) and Sallam et al. (2007) reported a negative correlation between cell wall fractions and in vitro GP parameters, which supports the present findings. Okano et al. (2005) and Akinfemi (2010) reported a higher gas production in Pleurotus sp. fermented wheat straw (13.5–14 ml/100 mg) and peanut husks (20.6 ml/100 mg), respectively. Improved in vitro DM and OM digestibility on fungal treatment implies a better ruminal microbial degradation of wheat straw, as it was already degraded by fungal enzymes during the course of SSF. Furthermore, a supply of soluble sugars and easily digestible fiber can result in a greater number of bacteria, so that new substrates entering the rumen will be invaded more rapidly (Russel 1988; Ørskov 1999) and thus enhancing digestibility as observed in FT-WS. Similar to the present findings, Zadražil and Puniya (1995) reported raised OM digestibility (3–16 %) upon culturing bagasse with Pleurotus eryngii. Hassim et al. (2012) observed an increased in vitro apparently rumen degradable carbohydrates of oil palm fronds inoculated with Ceriporiopsis subvermispora (3 weeks) and Lentinula edodes (9 weeks) up to 13 and 10 %, respectively, while it was up to 20 % with L. edodes and Phlebia brevispora (both at 9 weeks) for the same substrate (Rahman et al. 2011). High SCFA production in FT-WS in the present investigation suggests that a more fermentable energy was available from fungal treated straw in comparison to UT-WS as higher gas production is expected to yield a higher value of SCFA (Blümmel and Ørskov 1993), which are the major indicators of energy availability to the ruminants. Similarly, ME content was increased in FT-WS, which is supported by higher in vitro SCFA production. This is in agreement with earlier reports of Akinfemi (2010) and Shrivastava et al. (2011), who found elevated ME (MJ/kg) values (6.9–9.4 and 4.4–4.9) for fungal treated peanut husk and wheat straw, respectively. As there was an increased IVTOMD with readily available CHO and a higher N content in FT-WS, the MBP was higher compared to UT-WS, which supports the aim of achieving higher microbial efficiency in ruminants by maximizing substrate fixation into microbial cells (Krishnamoorthy and Robinson 2010), in order to increase microbial protein flow to intestine by decreasing carbon flow into fermentative CO2 and CH4. The MBP value obtained for FT-WS lies within the normal range of 160–490 (mg/g TDOM) for forages (Van Soest 1994). Furthermore, Krishnamoorthy et al. (1991) demonstrated that gas production was positively related to in vitro microbial protein synthesis, which substantiates the present findings.
Metabolism trial
Proximate and cell wall components of berseem forage and GNC are presented in Table 3. Two groups of experimental animals differed significantly (P < 0.05) in DM intake which was higher in group T2 (Table 4). Intake of digestible crude protein (DCP) (P < 0.01) and ME (P < 0.05) was also significantly higher in group T2. The nutrient digestibility analysis revealed that animals in group T2 had higher (P < 0.05) digestibility coefficients for DM, OM, CP, and T-CHO than that of group T1. Similarly, digestibility of various cell wall constituents like NDF, ADF, hemicellulose, and cellulose were also higher (P < 0.05) in group T2 (Table 4). Total body weight gain and average daily gain (ADG) in group T2 was higher (P < 0.05) than T1 (Table 4). Higher DM intake in group T2 can be explained by higher acceptability of straw as a result of delignification brought about by fungal enzymes, which caused a reduction in total lignin contents with an increased fiber digestibility leading to reduced rumen fill thereby allowing higher voluntary feed intake (Rodrigues et al. 2008). Similarly, Shrivastava et al. (2012) reported a significantly increased DM intake in goats fed with fermented wheat straw. Higher nutrient digestibility observed in our study is in line with previous reports, which could be due to the improved DM intake of fungal treated straw followed by the loosening of the structural fibers that might have availed more free sugars for rumen microbes (Salman et al. 2008; Okano et al. 2009). Fungal treated straw was palatable to calves and resulted in higher DM, DCP, and ME intakes; higher nutrient digestibility; and increased ADG. Furthermore, the increase in the availability of energy as a result of delignification, and protein as a result of accumulated fungal biomass could be attributed to the increased digestibility which diverted more nutrients towards tissue growth. This is consistent with the earlier feeding experiments involving fungal treated straw diets (Ramirez-Bribiesca et al. 2010; Omer et al. 2012).
Animals in group T2 had significantly (P < 0.05) higher N intake than group T1 (Table 5). Although total N loss was higher (P < 0.05) in group T2, yet the N retention and ADG was significantly (P < 0.05) higher in T2 due to higher N intake. Further, nutritive value of the diets expressed as DCP and ME were higher (P < 0.05) in T2 diet. The present study demonstrated that although N intake was higher, consequent fecal loss was also more in T2 compared to group T1, mainly because mycelial cell wall N is poorly digested (Dias De Silva and Sundstol 1986). Higher N retention resulted in higher DCP value of the T2 diet. Similarly, Walli et al. (1991) reported a higher N intake and retention in cross-bred calves fed fungal treated wheat straw supplemented with GNC. Similar observations were also made by Kakkar et al. (1990), Omer et al. (2012), and Shrivastava et al. (2012) in growing Murrah buffaloes, lambs, and goats, respectively. Owing to the higher ME content of fermented wheat straw and higher digestibility, ME value of the diet T2 was higher than T1, which is similar to the earlier report (Shrivastava et al. 2012). As there was higher intake of ME coupled with improved digestibility of nutrients in group T2, which made nutrients more available in these calves and their assimilation at tissue level, ultimately resulted in higher growth performance.
Conclusion
This study demonstrated that SSF improved the nutritional worth of wheat straw, as indicated by both in vitro followed by in vivo experiments. Hence, optimization of SSF of wheat straw by basidiomycetes fungus Crinipellis sp. for 5 days holds potential in enhancing the feeding value of poor-quality crop residue like wheat straw for ruminants.
References
Akin, D.E., Sethuraman, A., Morrison III, W.H., Martin, S.A. and Erickson, K., 1993. Microbial delignification with white-rot fungi improves forage digestibility. Applied and Environmental Microbiology, 59, 4274–4282
Akinfemi, A., 2010. Bioconversion of peanut husk with white-rot fungi: Pleurotus ostreatus and Pleurotus pulmonarius. Livestock Research for Rural Development 22(3)
AOAC, 1997. Official Methods of Analysis, 16th ed. Association of Official Analytical Chemists, Arlington, VA, USA.
AOAC, 2005. Official Methods of Analysis, 18th ed. Association of Official Analytical Chemists, Washington, DC.
Arora, D.S. and Sharma, R.K., 2009. Enhancement in in vitro digestibility of wheat straw obtained from different geographical regions during solid state fermentation by white-rot fungi. BioResources, 4, 909–920
Basu, S., Gaur, R., Gomes, J., Sreekrishnan, T.R. and Bisaria V.S., 2002. Effect of seed culture on solid state bioconversion of wheat straw by Phanerochaete chrysosporium for animal feed production. Journal of Bioscience and Bioengineering, 1, 25–30
Blümmel, M. and Ørskov, E.R., 1993. Comparison of gas production and nylon bag degradability of roughages in predicting feed intake in cattle. Animal Feed Science and Technology, 40, 109
Blümmel, M., Makkar, H.P.S. and Becker, K., 1997. In vitro gas production: a technique revisited. Journal of Animal Physiology and Animal Nutrition, 77, 24–34
Chumpawadee, S., Chantiratikul, A. and Chantiratikul, P., 2007. Chemical composition and nutritional evaluation of energy feeds for ruminant using in vitro gas production technique. Pakistan Journal of Nutrition, 6, 607–612
De Boever, J.L., Aerts, J.M. and De Brabader, D.L., 2005. Evaluation of the nutritive value of maize silages using gas production technique. Animal Feed Science and Technology, 123–124, 255–265
Dias De Silva, A.A. and Sundstol, F., 1986. Urea as a source of ammonia for improving the nutritive value of wheat straw. Animal Feed Science and Technology, 14, 67–69
Getachew, G., Makkar H.P.S. and Becker, K., 2002. Tropical browse: contents of phenolic compounds, in vitro gas production and stoichiometric relationship between short chain fatty acid and in vitro gas production. Journal of Agricultural Science (Cambridge), 139, 341–352
Goering, H.K. and Van Soest, P.J., 1970. Forage Fiber Analysis (Apparatus, Reagents, Procedures and Some Applications). Agriculture Hand Book 379. ARS, USDA, Washington
Hassim H.A., Lourenc, M., Goh Y.M., Baars J.J.P. and Fievez, V., 2012. Rumen degradation of oil palm fronds is improved through pre digestion with white rot fungi but not through supplementation with yeast or enzymes. Canadian Journal of Animal Sciences, 92, 79–87
Kakkar, V.K., Garcha, H.S., Dhanda, S. and Makkar, G.S., 1990. Mushroom harvested spent straw as feed for buffaloes. Indian Journal of Animal Nutrition, 7, 267–270
Keller, F.A., Hamillton, T.E. and Nguyon, Q.A., 2003. Microbial pretreatment of biomass potential for reducing severity of thermo-chemical biomass pretreatment. Applied Biochemistry and Biotechnology, 105, 27–41
Krishnamoorthy, U. and Robinson, P.H., 2010. Prediction of rumen microbial N supply in bovines from dietary values of partitioning factor (PF), in vitro rate of gas production (k), neutral detergent fiber and crude protein: A brief systematic review of studies completed in Bengaluru (India). Animal Feed Science and Technology, 160, 167–171
Krishnamoorthy, U., Steingass, H. and Menke, K.H., 1991. Preliminary observation on the relationship between gas production and microbial protein synthesis in vitro. Archives of Animal Nutrition, 5, 521–526
Menke, K.H. and Steingass, H., 1988. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Animal Research Development, 28, 7–55
Okano, K., Boonlue, S. and Suzuki, Y., 2005. Effect of ammonium hydroxide treatment on the in vitro dry matter digestibility and gas production of wheat straw, sugarcane bagasse medium and konara oak rotted by edible basidiomycetes. Animal Science Journal, 76, 147–152
Okano, K., Ohkoshi, N., Nishiyama, A., Usagawa, T. and Kitagawa, M., 2009. Improving the nutritive value of madake bamboo, Phyllostachys bambusoides, for ruminants by culturing with the white-rot fungus Ceriporiopsis subvermispora. Animal Feed Science and Technology, 152, 278–285
Omer, H.A.A., Ali, F.A.F. and Gad, S.M., 2012. Replacement of clover hay by biologically treated corn stalks in growing sheep rations. Journal of Agricultural Science, 4, 257–268
Ørskov, E.R., 1999. Supplement strategies for ruminants and management of feeding to maximize utilization of roughages. Preventive Veterinary Medicine, 38, 179–185
Rahman, M.M., Lourenco, M., Hassim, H.A., Baars, J.J.P., Sonnenberg, A.S.M., De Boever, J., Cone, J.W. and Fievez, V., 2011. Improving ruminal degradability of oil palm fronds using white rot fungi. Animal Feed Science and Technology, 169, 157–166
Ramirez-Bribiesca, J.E., Soto-Sanchiez, A., Hernandez-calva, L.M., Salinas-Chavira, J., Galaviz-Rodriguez, J.R., Cruz-Monterrosa, R.G. and Vargas-Lopez, S., 2010. Influence of Pleurotus ostreatus spent corn straw on performance and carcass characteristics of feedlot Pelibuey lambs. Indian Journal of Animal Sciences, 80, 754–757
Ranjhan, S.K., 1998. Nutrient Requirement of Livestock and Poultry, 2nd ed. Indian Council of Agricultural Research, New Delhi, India
Robertson, J.B. and Van Soest, P.J., 1981. The detergent system of analysis. In: James, W.P.T., Theander, O. (Eds.), The Analysis of Dietary Fiber in Food. Marcel Dekker, New York, NY, USA, pp. 123–158
Rodrigues, M.A.M., Pinto, P., Bezerra, R.M.F., Dias, A.A., Guedes, C.E.M., Cardoso, V.M.G., Cone, J.W., Ferreira, L.M.M., Colaco, J. and Sequeira, C.A., 2008. Effects of enzyme extracts isolated from white-rot fungi on chemical composition and in vitro digestibility of wheat straw. Animal Feed Science and Technology, 141, 326–338
Russel, J. B., 1988. Strategies that ruminal bacteria use to handle excess carbohydrates. Journal of Animal Science, 76, 1955–1963
Sallam, S.M.A., Nasser, M.E.A., El-Waziry, A.M., Bueno, I.C.S. and Abdalla, A.L., 2007. Use of an in vitro ruminant gas production technique to evaluate some ruminant feedstuffs. Journal of Applied Science Research, 3, 33–41
Salman, F.M., El-Kadi, R.I., Abdel-Rahman, H., Ahmed, S.M., Mohamed, M.I. and Shoukry, M.M., 2008. Biologically treated sugar beet pulp as a supplement in goat rations. International Journal of Agriculture and Biology, 10, 412–416
Shrivastava, B., Thakur, S., Khasa, Y.P., Gupte, A., Puniya, A.K. and Kuhad, R.C., 2011. White-rot fungal conversion of wheat straw to energy rich cattle feed. Biodegradation, 22, 823–831
Shrivastava, B., Nandal, P., Sharma, A., Jain, K.K., Khasa, Y.P., Das, T.K., Mani, V., Kewalramni, N.J., Kundu, S.S. and Kuhad, R.C., 2012. Solid state bioconversion of wheat straw into digestible and nutritive ruminant feed by Ganoderma sp. rckk02. Bioresource Technology, 107, 347–351
Snedecor, G.W. and Cochran, W.G. 1994. Statistical Methods. 8th edn., Iowa State University Press, USA
Sniffen, C.J., O’ Connor, J.D., Van Soest, P.J., Fox D.G. and Russell, J.B., 1992. A net carbohydrate and protein system for evaluating cattle diets. II. Carbohydrate and protein availability. Journal of Animal Science, 70, 3562–3577
Van Soest, P.J., 1994. Nutritional Ecology of the Ruminant. 2nd ed., Cornell Univ. Press, Ithaca, NY
Van Soest, P.J., Robertson, J.B. and Lewis, B.A., 1991. Methods of dietary fiber, neutral detergent fiber and non-starch polysaccharides in relation to animal nutrition. Journal of Dairy Science, 74, 3583–3597
Villas-Bôas, S.G., Esposito, E. and Mitchell, D.A., 2002. Microbial conversion of lignocellulosic residues for the production of animal feeds. Animal Feed Science and Technology, 98, 1–12
Walli, T.K. Rai, S.N., Gupta, B.N. and Singh, K., 1991. Influence of fungal treated and urea treated wheat straw on nutrient utilization in calves. Indian Journal of Animal Nutrition, 8, 227
Wyman, C.E., 2007. What is (and is not) vital to advancing cellulosic ethanol. Trends in Biotechnology, 25, 153–157
Yu, H., Guo, G., Zhang, X., Yan, K. and Xu, C., 2009. The effect of biological pretreatment with the selective white-rot fungus Echinodontium taxodii on enzymatic hydrolysis of softwoods and hardwoods. Bioresource Technology, 100, 5170–5175
Zadražil, F. and Puniya A.K., 1995. Studies on effect of particle size during solid state fermentation of sugarcane bagasse into animal feed using white-rot fungi. Bioresource Technology, 54, 85–87
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors retracted this article as they had no permission to use the data for publication.
About this article
Cite this article
Mahesh, M.S., Mohini, M., Jha, P. et al. RETRACTED ARTICLE: Nutritional evaluation of wheat straw treated with Crinipellis sp. in Sahiwal calves. Trop Anim Health Prod 45, 1817–1823 (2013). https://doi.org/10.1007/s11250-013-0440-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-013-0440-1