Abstract
In this study, the hard ticks, whole blood and serum samples collected from small ruminants (sheep and goat) in middle Black Sea region of Turkey where Crimean–Congo hemorrhagic fever (CCHF) human cases were observed in the past years were surveyed for the presence of RNA and specific IgG antibodies from CCFH virus (CCHFV). CCHFV RNA was found in 30 of 255 tick pools (11.76%) and nine of 105 (8.57%) leucocyte samples. No CCHFV genomic RNA was detected from animals in Yildizeli and Vezirkopru. However, CCHFV RNA was found from animals in Gerze and Resadiye. Seventy-eight of 105 goat and sheep blood serum samples tested were antibody-positive for CCHFV by enzyme-linked immunosorbent assay (ELISA) (goat: 42/63; sheep: 36/42). Viral RNA was detected from tick samples in all of four provinces. Positivity rates for the provinces varied and were as follows: Gerze 13.04%, Resadiye 35.41%, Vezirkopru 1.61% and Yildizeli 6.06%. CCHFV genomic RNA was detected in four of seven tick species tested. These results suggest that these hard ticks may act as a reservoir for CCHFV in northern Turkey.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Crimean–Congo hemorrhagic fever virus (CCHFV) belongs to the Nairovirus genus in the Bunyaviridae family. It is transmitted to humans either by bites of Ixodid ticks (mostly of the Hyalomma genus) or by contact with blood or tissues from CCHF patients or viremic livestock (Ergunay et al. 2010). The life cycle of the virus in nature includes transovarial and transstadial transmission among ticks and a tick–vertebrate host cycle involving wild and domestic animals. Smaller wildlife species such as hares and hedgehogs, as well as ground-feeding birds, act as hosts for the immature stages of the tick vectors, while adult ticks prefer large mammals such as livestock and wild boar. Although CCHFV causes a severe disease in humans, there is no evidence of disease in its natural hosts. However, seroepidemiological surveys in endemic areas revealed high prevalence of anti-CCHFV antibodies in domestic animals, particularly in cattle, goat and sheep. Like all other vector-borne diseases, the presence and persistence of zoonotic foci of infection depend on biological and ecological relationships between three very different kinds of organisms: virus, ticks, and vertebrates (Randolph and Rogers 2007).
The disease was first recognized in the Crimean region of Russia in the 1940s and is now reported in many regions of the world: Africa, Europe and Asia (Ergunay et al. 2010). Turkey, a country with a population of 72 million, is located in southeastern Europe. Since the disease was first recognized in 2002, cases have been reported here almost every year. There have been outbreaks, mostly among family members, and nosocomial transmission has also been reported (Gunes et al. 2009). The most endemic CCHF regions in Turkey are two neighboring districts in the northern part of the country, Kelkit Valley and middle Black Sea region (40°19′N, 36°43′E).
Since 2002, a total of 6,545 human cases have been documented in Turkey, 320 of them being fatal. Most of these patients had a history of a tick bite or handling livestock (Gunes et al. 2009; Kubar et al. 2011; MOH 2012). The aim of the present study was to estimate the prevalence of CCHFV among ticks and animals in the endemic regions of Turkey, as they are the main organisms involved in the virus life cycle, and also this study was carried out to investigate the seroprevalence of anti-CCHFV antibody in small ruminants of four provinces of Turkey which had the highest number of human clinical cases of CCHF in the last 8 years.
Materials and methods
Tick processing, whole blood and serum samples
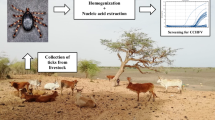
A total of 564 ticks were collected between March and July of 2009 from 42 sheep (123 pools, 235 ticks in total) and 63 goat (132 pools, 329 ticks in total) grazing in middle Black Sea region and Kelkit Valley. The ticks were collected directly from the animals. After identification using standard keys, ticks were stored at −80°C until testing for the presence of viral RNA. They were pooled according to size and pools ranged from one to 20 ticks. They were placed in 2 ml PBS diluent with MagNA Lyser Green Beads (Roche, Mannheim, Germany). Pools were homogenized at 3,000×g for 3 min by MagNa Lyser (Roche, Mannheim, Germany). Homogenates were centrifuged in Eppendorf tubes at 12,000×g for 3 min to remove the suspended solids, without removing the beads. The supernatants were stored at −80°C until further analysis. A total of 105 whole blood and serum samples were collected from same animals.
RNA extraction, nested RT-PCR assays and enzyme-linked immunosorbent assay (ELISA)
Viral RNA was extracted from 350 μl of tick pool supernatant by using the MagNA Pure LC RNA Isolation Kit III (Roche, Mannheim, Germany) and stored at −80°C. All samples were tested by nested reverse transcriptase-polymerase chain reaction (RT-PCR) using two sets of primers. F2-R3 and F3-R2 primers were described previously (Albayrak et al. 2010a). These primers allowed the amplification of two regions of the nucleocapsid (N) protein gene encoded by the S segment of the CCHFV genome. Second-round PCR products were analysed on agarose gel (1.5%) electrophoresis at 80 V for 30 min (Albayrak et al. 2010a).
All serum samples were tested for the presence of specific IgG antibodies against CCHFV by commercial ELISA kit (Vectorbest, Novosibirsk, Russia) with minor modification. The commercial rabbit anti-goat and rabbit anti-sheep immunoglobulin G peroxidase (Antibodies-online GmbH, Aachen, Germany) were used as a conjugate.
Results
Tick species and distribution
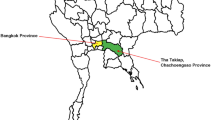
A total of 564 adult ticks were collected from sheep (123 pools, 235 ticks in total) and goat (132 pools, 329 ticks in total) in the middle Black Sea region and Kelkit valley of Turkey (Yildizeli, Gerze, Resadiye, V.kopru) where Crimean–Congo hemorrhagic fever (CCHF) human cases were observed in the past years. The numbers and distribution of tick species according to the collection points on farms are documented in Tables 1, 2, and 3. Seven tick species were identified and the most abundant were Rhipicephalus bursa 45.03% (254/564), Hyalomma marginatum marginatum 25.88% (146/564), Dermacentor marginatus 13.12% (74/564) and Rhipicephalus turanicus 7.26% (41/564). Hyalomma detritum represented 5.14% (29/564) of the total number of ticks. Haemaphysalis punctata and Rhipicephalus annulatus were less common and represented 3.01% (17/564) and 0.53% (3/564) of the tick population, respectively. R. bursa was found in all provinces in the surveyed region. H. punctata was found in only Yildizeli province, while R. annulatus and R. turanicus were found in only Gerze province. D. marginatus was encountered in three localities (Gerze, Resadiye and Yildizeli). H. marginatum marginatum was found in all provinces in the region, except for Gerze province, which lies in the coastal area of the surveyed region. H. detritium was found in only two localities (Yildizeli and Resadiye).
CCHFV nucleic acid and antibody detection
A total of 255 tick pools (564 adult ticks) and 105 whole blood were tested by nested RT-PCR for CCHFV. The presence of CCHFV was confirmed by nested RT-PCR in 30 of 255 (11.76%) tick pools and nine of 105 (8.57) whole blood. Positivity rates ranged from 0% to 50% and from 1.61% to 35.41% among tick species and provinces, respectively (Tables 1, 2, and 3). No CCHFV genomic RNA was detected from animals in Yildizeli and Vezirkopru. However, CCHFV RNA was found in animals from Gerze and Resadiye. CCHFV RNA was detected in both leucocytes and ticks which were collected from two sheep in Gerze province. Four ticks (two R. bursa, two R. turanicus) were collected from CCHFV RNA positive sheep. All of them are unfed adult ticks. Each tick was homogenized separetely. Although CCHFV RNA was detected from both R. bursa tick homogenates, no genomic RNA was detected from R. turanicus tick specimens. One of the positive R. bursa ticks was male. Seven ticks (two D. marginatus, three R. turanicus, one R. bursa, one R. annulatus) were collected from other CCHFV RNA positive sheep. Each species homogenized separetely. Only R. bursa homogenate was found as a positive. The tick species (R. bursa and R. turanicus) collected from four CCHFV RNA positive sheep were found as negative. In additon, no CCHFV RNA was detected in tick samples collected from CCHFV RNA positive animals in Resadiye province. On the other hand, 17 tick homogenates (R. bursa, H. marginatum marginatum, H. detritum) collected from ten CCHFV RNA negative animals were found as CCHFV RNA positive. In additon, six tick homogenates (R. bursa, H. marginatum marginatum, H. detritum) from Yildizeli, three tick homogenates (R. turanicus) from Gerze and one tick homogenate (H. marginatum marginatum) from Vezirkopru collected from CCHFV RNA negative animals were found as CCHFV RNA positive (Tables 1, 2, and 3).
Serological examination of serum samples for anti-CCHFV infection revealed that CCHF IgG antibody was present in 42 of 63 (66.66%) and 36 of 42 (85.71%) goat and sheep, respectively (Tables 1, 2, and 3). The provincial distribution of chosen serum samples in this study was as follows: Yildizeli 82.05%, Gerze 100%, V.kopru 96% and Resadiye 26.92%. No anti-CCHFV antibodies were detected in CCHFV positive animals in Resadiye. In contrast, anti-CCHFV antibodies were found in all of the CCHFV positive animals in Gerze. Although all of the animals collected CCHFV RNA positive ticks were found to be anti-CCHFV antibodies positive in Yildizeli, Gerze and Vezirkopru provinces, some of the animals collected CCHFV RNA positive ticks were found to be anti-CCHFV antibodies negative in Resadiye.
Discussion
Crimean–Congo hemorrhagic fever infection is reported in many countries in Africa, Europe and Asia (Maclachlan and Dubovi 2011). The periodic changes in the population of the tick vectors make for notable differences in the rates of infected animals. The distribution and abundance of potential vectors, which are influenced by climatic conditions, in particular rainfall, in turn determine the distribution of CCHFV (Maclachlan and Dubovi 2011).
The prevalence of antibodies in small ruminants varied among our study sites from intense to sparse, suggesting that CCHFV seroprevalence was either spatially focal or temporally sporadic. Seroprevalence of CCHFV in small ruminants were most intense in the Gerze, Yildizeli and Vezirkopru provinces. The antibodies found in small ruminants of all ages in many regions suggest the endemic transmission throughout four mentioned provinces. The lowest prevalence (26.92%) of antibodies in our sample of small ruminants from Resadiye province where the highest number of human cases was reported during last period, probably because periodic or focalized transmission of virus at a very low level in small ruminants is impossible to detect with this method. The results of the present study indicate that perhaps cattle or other wild animals are the main reservoir and source of transmission of CCHFV to human and more important than small ruminants in this province. In this study, no anti-CCHFV antibodies were detected in CCHFV positive animals in Resadiye. In contrast, anti-CCHFV antibodies were found in all of the CCHFV positive animals in Gerze. This may suggest that some of these animals have exposured to virus for the first time.
The serosurvey studies for CCHFV infection in the region was very limited, but the transmission dynamics and the basic epidemiological measures such as the attack rate should be described (Gunes et al. 2009; Ozkaya et al. 2010; Hekimoglu et al. 2012). The first serosurvey on the presence of CCHFV in Turkey from domestic animals was reported by the Turkish Ministry of Agricultural and Rural Affairs. According to this report, the presence of antibody against CCHFV in 79% of cattle in the some part of the region. In this study, prevalence of anti-CCHFV antibody in small ruminants (74.28%) diagnosed is different from that in previous study. It is commonplace knowledge that the result of the seroprevalence studies are influenced by many factors such as the number of sampled animals, the age of the animals, the time of sampling, the conditions of care and feeding, individual differences and so on. In this respect, when the result of these two studies were evaluated extensively, similar findings were found on the existence/prevalence of infection in this area.
Various ecological factors affect the distribution and abundance of tick vectors, which play a key role in CCHF epidemiology. Although CCHFV has been isolated from at least 30 species of ticks, Hyalomma spp. ticks are considered as the main vector of the virus. Actually, the occurrence of CCHF coincides with the global distribution of Hyalomma spp. ticks (Albayrak et al. 2010a; Ergunay et al. 2010; Gargili et al. 2010; Hekimoglu et al. 2012). However, the high prevalence of H. m. marginatum was surprising, because it is not considered a common tick on domestic ruminants in the middle Black Sea region of Turkey (Albayrak et al. 2010a, b; Hekimoglu et al. 2012). Despite serological evidence of infection, the virus was not recognized in Turkey until 2002 and an increasing number of human cases were reported between 2002 and 2011 as reported by the Turkish Ministry of Health.
The first research on the presence of CCHFV from domestic animals in Turkey was carried out by Tonbak et al. (2006), who found that 47% and 46% of ticks collected from sheep and cattle were R. bursa and H. marginatum marginatum, respectively, and four tick pools (three R. bursa and one H. marginatum marginatum) were CCHFV RNA positive (Tonbak et al. 2006). In the same region, CCHFV RNA was found in seven of 11 tick species by Albayrak et al. (2010a). They also found that positive reactions to CCHFV were present in 29 of 421 tick pools (6.88%).
In this study, prevalence of CCHFV RNA in tick pools diagnosed (11.76%) is different from that in previous studies (Tonbak et al. 2006; Albayrak et al. 2010a). This difference may be influenced by several factors. In this study, the prevalence of study CCHFV RNA has been investigated only in four provinces where CCHF human cases were observed in the past years in the Black Sea region. However, previous studies were carried out on a broad geographical area. The CCHFV RNA was detected in unfed adult ticks species (R. bursa, R. turanicus, H. marginatum marginatum, H. detritum) collected from CCHFV RNA positive and negative animals. Even though Hyalomma spp. is the main vector for CCHFV, R. turanicus, R. bursa, and H. detritum may play a role in CCHFV maintenance and transmission.
Further studies of the CCHFV prevalence in small ruminants as well as cattle and detection of tick vectors in other provinces and climatic areas can show the role of small ruminants, cattle, and tick vectors in transmission of CCHFV to human in Turkey. The results could be helpful to public health authorities in assessing the risk for the disease in humans.
Abbreviations
- CCHFV:
-
Crimean–Congo hemorrhagic fever virus
- ELISA:
-
Enzyme-linked immunosorbent assay
- Nested RT-PCR:
-
Nested reverse transcriptase polymerase chain reaction
References
Albayrak, H., Ozan, E. and Kurt, M., 2010a. Molecular detection of Crimean–Congo hemorrhagic fever virus (CCHFV) but not West Nile virus (WNV) in hard ticks from provinces in northern Turkey. Zoonoses and Public Health, 57, e156–e160
Albayrak, H., Ozan, E. and Kurt, M., 2010b. An antigenic investigation of Crimean–Congo haemorrhagic fever virus (CCHFV) in hard ticks from provinces in northern Turkey. Tropical Animal Health and Production, 42, 1323–1325
Ergunay, K., Whitehouse, C.A. Ozkul, A., 2010. Current status of human arboviral diseases in Turkey. Vector-Borne and Zoonotic Diseases, 11, 731–741
Gargili, A., Midilli, K., Ergonul, O., Ergin, S., Alp, H.G., Vatansever, Z., Iyisan, S., Cerit, C., Yilmaz, G., Atlas, K. and Estrada-Pena, A., 2010. Crimean–Congo hemorrhagic fever in European part of Turkey: genetic analysis of the virus strains from ticks and a seroepidemiological study in humans. Vector-Borne Zoonotic Diseases, 11, 747–752
Gunes, T., Engin, A., Poyraz, O., Elaldi, N., Kaya, S., Dokmetas, I., Bakir, M. and Cinar, C., 2009. Crimean–Congo hemorrhagic fever virus in high-risk population, Turkey. Emerging Infectious Diseases, 15, 461–464
Hekimoglu, O., Ozer, N., Ergunay, K. and Ozkul, A., 2012. Species distribution and detection of Crimean Congo Hemorrhagic Fever Virus (CCHFV) in field-collected ticks in Ankara Province, Central Anatolia, Turkey. Experimental and Applied Acarology, 56, 75–84
Kubar, A., Haciomeroglu, M., Ozkul, A., Bagriacik, U., Akinci, E., Sener, K. Bodur, H., 2011. Prompt administration of Crimean–Congo hemorrhagic fever (CCHF) virus hyperimmunoglobulin in patients diagnosed with cchf and viral load monitorization by reverse transcriptase-PCR. Japanese Journal of Infectious Diseases, 64, 439–443
Maclachlan, N.J. and Dubovi E.J., 2011. Fenner’s Veterinary Virology. Academic Pres, London
MOH, 2012. Ministry of Health of Turkey. The reports of the Communicable Diseases Department, Annual Report, http://www.kirim-kongo.saglik.gov.tr. accessed 16/01/2012
Ozkaya, E., Dincer, E., Carhan, A., Uyar, Y., Ertek, M., Whitehouse, C.A. and Ozkul, A., 2010. Molecular epidemiology of Crimean–Congo hemorrhagic fever virus in Turkey: occurrence of local topotype. Virus Research, 149, 64–70
Randolph, S.E. and Rogers, D.J., 2007. Ecology of tick-borne disease and the role of climate. In: O. Ergonul and C. A. Whitehouse (eds), Crimean Congo Hemorrhagic Fever: A Global Perspective. Springer, Dordrecht, The Netherlands, pp. 167–186
Tonbak, S., Aktas, M., Altay, K., Azkur, A.K., Kalkan, A., Bolat, Y., Dumanli, N. and Ozdarendeli, A., 2006. Crimean–Congo hemorrhagic fever virus: genetic analysis and tick survey in Turkey. Journal of Clinical Microbiology, 44, 4120–4124
Acknowledgements
We are grateful to Fiona J. May (University of Texas Medical Branch, Galveston, TX, USA) for her helpful comments (linguistic correction). Funding for this research was provided by the Samsun Veterinary Control and Research Institute (SVCRI) general budget.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Albayrak, H., Ozan, E. & Kurt, M. Serosurvey and molecular detection of Crimean–Congo hemorrhagic fever virus (CCHFV) in northern Turkey. Trop Anim Health Prod 44, 1667–1671 (2012). https://doi.org/10.1007/s11250-012-0122-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-012-0122-4