Abstract
Native chicken breeding station of Mazandaran was established in 1988 with two main objectives: genetic improvement through selection programs and dissemination of indigenous Mazandarani birds. (Co)variance components and genetic parameters for economically important traits were estimated using (bi) univariate animal models with ASREML procedure in Mazandarani native chicken. The data were from 18 generations of selection (1988–2009). Heritability estimates for body weight at different ages [at hatch (bw1), 8 (bw8), 12 (bw12) weeks of ages and sex maturation (wsm)] ranged from 0.24 ± 0.00 to 0.47 ± 0.01. Heritability for reproductive traits including age at sex maturation (asm); egg number (en); weight of first egg (ew1); average egg weight at 28 (ew28), 30 (ew30), and 32 (ew32) weeks of age; their averages (av); average egg weight for the first 12 weeks of production (ew12); egg mass (em); and egg intensity (eint) varied from 0.16 ± 0.01 to 0.43 ± 0.01. Generally, the magnitudes of heritability for the investigated traits were moderate. However, egg production traits showed smaller heritability compared with growth traits. Genetic correlations among egg weight at different ages were mostly higher than 0.8. On the one hand, body weight at different ages showed positive and relatively moderate genetic correlations with egg weight traits (ew1, ew28, ew30, ew32, ew12, and av) and varied from 0.30 ± 0.03 to 0.59 ± 0.02. On the other hand, low negative genetic correlations were obtained between body weight traits (bw1, bw8, bw12, and wsm) and egg number (en). Also, there is low negative genetic correlation (−24 ± 0.04 to −29 ± 0.05) between egg number and egg weight. Therefore, during simultaneous selection process for both growth and egg production traits, probable reduction in egg production due to low reduction in egg number may be compensated by increases in egg weight.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
About 50% of the poultry breeds are classified as being at risk. Indigenous chickens could form the basis for genetic improvement and diversification to produce breeds adapted to local conditions (Hoffmann 2005). Indigenous chickens appear to have an inherent scavenging and nesting habit, are more resistant to various diseases, and can survive under harsh nutritional and environmental conditions (Minga et al. 2004).
Iranian indigenous chicken are meat–egg type or dual purpose. Growth rate and egg production under conventional rearing system in villages are very low. During the past several decades importation of exotic breeds have increased risk of extinction (Ghazikhani Shad et al. 2007). The initial endeavor for breeding and extension of Iranian native fowls, in the frame of the national project, has been relatively successful. This project started back in 1984 in several places. All in all, six breeding stations in different regions of Iran (Mazandaran, Fars, Esfahan, West Azarbaijan, Yazd, and Khorasan) have been established. Native chicken breeding station of Mazandaran located in the North of Iran debuted in 1988 with two main objectives, namely extension and genetic improvement. Genetic improvement is done by selecting the best 100 roosters and 800 hens as parents of the next generations. On the other hand, 8-week-old chicks get distributed among rural communities with the aim of increasing the population of native fowls in Northern provinces of Iran (Enayati and Rahimi 2009). Mazandaran province is situated between latitudes 35°46′ and 36°56′, and longitude 50°21′ to 54°8′. The province has a humid subtropical climate with an average temperature of 25°C in summer and about 12°C in winter. Winters are cool and rainy while summers are hot and humid.
In many animal breeding situations, accurate estimates of genetic and phenotypic (co)variance components are required for the formation of selection schemes aimed at maximizing genetic improvement of specific traits (Danbaro et al. 1996). Phenotypic and genetic (co)variance components are extensively used in animal breeding for estimation of heritability, breeding value estimation, planning breeding programs, and interpretation of the genetic mechanism of quantitative traits (Henderson 1986).
Although there is much evidence that local chicken production plays an important role in the lives of rural households, not much work has been done in terms of improving the productivity of local chickens. Improvement in the productivity of indigenous breeds requires attention to nutritional, breeding, health, and management aspects (Norris and Ngambi 2006). From the breeding point of view, genetic improvement through selection within local chickens seems to be an attractive option (Lwelamira et al. 2009).
Despite some sporadic studies (Kianimanesh et al. 2002; A. Nejati Javaremi, personal communication, 2002; Ghazikhani Shad et al. 2007), no comprehensive work relevant to genetic and phenotypic parameters estimation has yet been published for Mazandaran native chicken. On the other hand, genetic parameters are liable to change in a population under continuous selection (Falconer and Mckay 1996). Therefore, as the aim of the current study, we used phenotypic information of 18 consecutive generations of Mazandaran native chicken to estimate heritabilities and correlations of the recorded traits in this indigenous chicken breed.
Material and methods
Experimental population
In 1986, about 5,000 males and females were purchased from rural regions across the Mazandaran province and kept in a quarantine farm for a year. In 1987, about 2,500 birds of both sexes were kept to produce hatching eggs and chicks produced from these eggs were transferred to the station in 1988. Since then birds have been individually tagged and trap-nested for pedigree recording. Genetic evaluation of the birds for body weight at 8 weeks, age of the hens at first egg, average egg weight, and total number of eggs laid during first 12 weeks after flocks maturity (when 5% of the flock are in egg production) have been performed. Economic indexes are calculated for these traits and birds of both sexes are selected based on their aggregate genotypes for these traits (Khadem et al. 2010). Parents of each generation (100 cocks and 800 hens) are selected from among 6,000 pedigreed and performance-recorded birds produced each generation (Enayati and Rahimi 2009).
Traits measured
The data file considered in this study consisted of three registered fixed effect factors (generation, sex, and hatch) and 11 recorded traits including body weight at hatch (bw1), body weight at age of 8 (bw8) and 12 (bw12) weeks, and body weight at sexual maturity (wsm); age at sexual maturity (asm); egg number (en); first egg weight (ew1); average egg weight at age of 28 (ew28), 30 (ew30), and 32 (ew32) weeks; and average egg weight for the first 12 weeks of production (ew12). Bw1, bw8, and bw12 have been measured in both males and females. Traits of bw1, ew28, ew30, and ew32 have been recorded since generation nine in the station. No information for the bw12 and ew1 traits in the first four generations was recorded. Moreover, three combined traits consisting of av (average of ew28, ew30, and ew32), intensity of egg production [eint = (egg number/days recording) × 100], and egg mass (em = en × ew12) were analyzed and (co)variance components were also estimated for them. Description of data set is shown in the Table 1.
Statistical analyses
Pedigree and data file were prepared using Visual FoxPro 9.0 software, the relational database management system. Pedigree information was obtained using PEDIGREE software version 1.01. SAS 9.1 package was used for statistical analysis and model fitting. Fixed effect factors and their interactions were considered in an animal model provided they were having significant effect.
Genetic analyses were performed using ASREML software (Gilmour et al. 2006). Heritability of growth and egg production traits were estimated by univariate (1) procedure and correlations among traits by bivariate (2) animal model. The model used for the analysis is as follows:
Where, for trait i (i = 1, 2); y i = vector of observations; b i = vector of fixed effects of generation, sex, and hatch; a i = vector of random direct genetic effects; e i = vector of random residual effects; and X i and Z i are incidence matrices relating the observations to the respective fixed and direct genetic effects.
Results
Descriptive statistics
Pedigree information is shown in Table 1. Number of recorded animals for growth and egg production traits, their mean, and CV% are presented in Table 2.
Heritability of growth and egg production traits
Heritability of growth and egg production traits are shown in Table 3. Heritability estimates for body weight at different ages varied from 0.24 to 0.47. Lower heritability values were obtained for traits of bw8 and bw12 as compared with bw1 and wsm. Magnitude of heritability estimates for age at first egg (asm) and egg number (en) were 0.36 and 0.17, respectively. Among egg weight traits, ew32 and ew1 had the highest and lowest heritability with the magnitudes of 0.43 and 0.17, respectively. As the birds get older, in general, heritabilities increase. The same heritability values of 0.16 were obtained for both egg mass and egg production intensity traits. Generally, heritability estimates for investigated traits were moderate. However, growth traits seem more heritable than egg production traits.
Correlations among traits
Genetic and environmental correlations among investigated traits are presented in Table 3. Generally, genetic correlations among body weight traits were moderate to high and also were positive, varying from 0.36 to 0.91. Bw8 and bw12 appear to be genetically strongly correlated (0.91). There was also moderate environmental correlation between them (0.47). Although body weight traits had low negative genetic correlations with egg number, but they were correlated with egg weight traits positively moderately and ranged from 0.30 to 0.59. Moderate antagonistic genetic relationship was found between sex maturation age and egg number (−0.41). Egg number and egg weight traits were correlated genetically negatively. However, the coefficient values were rather moderate changing from −0.24 to −0.29. Favorable strong and positive genetic correlations were observed between ew1 and the other egg weight traits. Genetic correlations among traits of ew28, ew30, ew32, and ew12 were close to unity (0.97–0.99). Whereas, from environmental viewpoint, these traits are correlated moderately positively. Additionally, there were genetic relationships close to unity between egg number with em and eint traits, 0.96 and 0.98, respectively. Also, environmental correlations among en, em, and eint were higher than 0.9.
Discussion
Phenotypic means
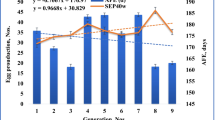
Phenotypic means of the considered traits (Table 2) were compared to some previous reports on indigenous chicken breeds. Birth weight mean of Mazandaran native chicken was higher than that of the local Venda (Norris and Ngambi 2006) and Horro chicken of Ethiopia (Dana et al. 2011). Although, Horro (Dana et al. 2011), two ecotypes of Tanzania chicken (Kuchi and Medium) (Lwelamira et al. 2009), and Fars native chicken (Ghazikhani Shad et al. 2007) seem to have less bw8 and bw12 in comparison with our studied breed, two of Iranian indigenous chicken (Esfahan and Azarbaijan) (Ghazikhani Shad et al. 2007) exhibited a larger bw12. Favorably, age at first egg for Mazandaran native chicken seems to be smaller relative to those that have been presented in the literature (Ghazikhani Shad et al. 2007; Lwelamira et al. 2009; Dana et al. 2011). Phenotypic mean of egg weight in the current study was in the range which has been reported previously (Francesch et al. 1997; Ghazikhani Shad et al. 2007; Lwelamira et al. 2009). Implicit phenotypic differences may be due to breeds’ diversity, long-term selection in Mazandaran native birds (18 full generations), different environmental conditions, etc. An increasing trend in egg weight was observed with increased age in this population. Such results were showed in some previous studies (Abdallah et al. 1995; Sabri et al. 1999).
Heritabilities
Heritability estimates for all investigated traits (Table 3) were in general moderate. Estimated heritability for bw1 was higher than that obtained before by Norris and Ngambi (2006) and Dana et al. (2011). The higher estimate found in our study could be due to not considering maternal and permanent environmental effects in the model. Heritability estimates of other body weight traits are at the lower end of the range reported in previous studies (Danbaro et al. 1996; Ghazikhani Shad et al. 2007; Kamali et al. 2007; Lwelamira et al. 2009; Dana et al. 2011). Age at first egg or sexual maturity in our study seems to be more heritable than in two Iranian chicken ecotypes (Azarbaijan and Esfahan) (Ghazikhani Shad et al. 2007), Korean native chickens (Sang et al. 2006), and Horro chicken of Ethiopia (Dana et al. 2011) but less than other reported estimates (Danbaro et al. 1996; Kamali et al. 2007; Lwelamira et al. 2009). Most of reported heritability estimates for egg number (Besbes et al. 1992; Francesch et al. 1997; Sabri et al. 1999; Sang et al. 2006; Kamali et al. 2007; Lwelamira et al. 2009; Dana et al. 2011) are higher than that obtained in our study. In the current research, heritability estimates of egg weight traits ranged from 0.17 to 0.43, which are less than the heritability values found in the literature (Besbes et al. 1992; Danbaro et al. 1996; Francesch et al. 1997; Sabri et al. 1999; Kamali et al. 2007; Lwelamira et al. 2009) except for those reported by Ghazikhani Shad et al. (2007) in Azarbaijan and Esfahan chicken ecotypes.
As age advances, a general increasing trend could be seen in heritability magnitudes of body weight and egg weight traits (Table 3). Review of literature shows that additive genetic variation increased with age for egg number (Engström et al. 1992; Ledur et al. 2003), egg weight, and albumen height (Ledur et al. 2003). Also, Ledur et al. (2000) stated that genetic variance of egg production increased with age. Changes of heritability over time may result from activation of different genes during the production cycle. Early stages of production are under the influence of sexual maturity whereas after the seventh month of production, genes related to persistency of egg production could be more influential (Wolk and Szwaczkowski 2009). Besides breed and environmental differences, lower heritability estimates in Mazandaran local fowls in comparison to previous studies are probably due to the long-term selection of 18 generations. Heritability estimates for some egg production traits showed a decline with the advancement of the selection (Sharma et al. 1996). The observed heritability estimates promise to support genetic improvement of growth and egg production traits through selection.
Genetic and environmental correlations
Genetic correlations among body weight traits varied from relatively moderate to high (0.36–0.91; Table 3), which are mostly at the low end of the range existing in the previous reports with the exception of genetic correlation between bw8 and bw12 (0.91) (Sang et al. 2006; Lwelamira et al. 2009; Dana et al. 2011). There were genetic relationships close to zero among body weight traits and age at sexual maturation except between wsm and asm, which was 0.41, while low negative genetic correlations have been reported by previous researchers (Ghazikhani Shad et al. 2007; Kamali et al. 2007; Lwelamira et al. 2009). Weak genetic antagonisms among egg number and body weight traits were observed, which agreed with former findings in sign but slightly lower in magnitudes in general (Sang et al. 2006; Kamali et al. 2007; Lwelamira et al. 2009). As observed, all of egg weight traits appeared to be correlated positively and moderately with body-weight-related traits (0.30 to 0.59). This range is consistent with that presented in the literature (Sang et al. 2006; Ghazikhani Shad et al. 2007; Kamali et al. 2007). However, Lwelamira et al. (2009) had estimated slightly lower values for such relationships. Therefore, simultaneous selection for body-weight- and egg-weight-related traits could potentially improve both traits. The genetic correlations indicate an antagonistic relationship between sexual maturity and egg number. In other words, birds that are genetically inclined to attain sexual maturity earlier to some extent tend to lay higher egg number. Such negative correlations though with higher (Sang et al. 2006; Ghazikhani Shad et al. 2007; Kamali et al. 2007) and lower (Lwelamira et al. 2009) values have been reported. Also, traits of egg mass and egg production intensity are moderate in antagonist association with sexual maturity age. In accordance with previous studies (Sang et al. 2006; Ghazikhani Shad et al. 2007; Lwelamira et al. 2009), moderate positive correlations are existing among age at first egg and egg-weight-related traits. Therefore, delayed sexual maturation may reduce egg number but it is expected to increase egg weight. Genetic correlations between egg number and egg weight at all ages are negative and relatively low, varying from −0.24 to −0.29. There is a lower range in the literature for this relationship generally (Besbes et al. 1992; Francesch et al. 1997; Sabri et al. 1999; Ghazikhani Shad et al. 2007; Kamali et al. 2007; Lwelamira et al. 2009). Nevertheless, genetic correlations among egg number with egg mass and egg production intensity is close to unity. It may indicate that genetic improvement of egg number through selection aside from diminishing effect on egg weight could increase egg mass per bird. High positive genetic relationships as Besbes et al. (1992) observed, were obtained among egg weight traits (ew28, ew30, ew32, and ew12), as the majority of correlation values are close to unity. Weight of first egg showed high genetic correlations (≥0.77) with other subsequent measurements of egg weight. Since, selection based on early expressed egg production traits, up to 40 weeks of age, could increase egg production of chickens (Fairful and Gowe 1990; Poggenpoel et al. 1996). Early expressed traits can be used as selection criteria to improve egg production. So, genetic gain per unit of time would be maximized through reduction in generation interval (Ayyagari et al. 1980). Additionally, Hicks et al. (1998) also showed that selection based on partial records of the individual and all available ancestral records resulted in the shortest generation interval and was the most efficient strategy for maximizing egg production in laying hens compared to other strategies using full records (Hicks et al. 1998). Consequently, ew1 may be a good indicator of egg weight and could be used in selection criteria rather than average egg weight at first 12 weeks of production (ew12) or later measurements to reduce generation interval and cost.
Considerable environmental correlations were seen between em with em and eint. Also, egg weights at different ages were correlated with each other environmentally and moderately. Therefore, management and environmental improvement may ameliorate phenotypic mean of the population. Other environmental correlations, regardless of sign, were low in general.
Conclusion
The standard errors of estimates in this study were low indicating that the estimates may have sufficient precision and reliability. Since, chickens under rural production systems are kept for both meat and egg production, selection criteria should include both body weight and egg production traits simultaneously. Moderate heritability for both growth and egg traits on the one hand, and positive genetic correlations between growth and egg weight on the other hand, may let to make such strategy efficient.
References
Abdallah, A. G., Harms, R. H. and Russell, G. B., 1995. Effect of age and resting on hens laying eggs with heavy or light shell weight, Journal of Applied Poultry Research, 4, 131–137
Ayyagari, V. S. C., Mohapatra, A., Venkatramaiah, T., Thiagasundaram, D., Choudhuri, D. C., Johri, D. and Renganathan, P., 1980. Selection for egg production on part records, Theoretical and Applied Genetics, 57, 277–283
Besbes, B., Ducrocq, V., Foulley, J. L., Protais, M., Tavernier, A., Tixier-Boichard, M. and Beaumont, C., 1992. Estimation of genetic parameters of egg production traits of laying hens by restricted maximum likelihood applied to a multiple-trait reduced animal model. Genetic Selection Evolution, 24, 539–552
Dana, N., Vander Waaij, E. and Van Arendonk, J., 2011. Genetic and phenotypic parameter estimates for body weights and egg production in Horro chicken of Ethiopia, Tropical Animal Health and Production, 43, 21–28
Danbaro, G., Oyama, K., Mukai, F., Tsuji, S. and Miyata, T., 1996. Estimation of genetic parameters by restricted maximum likelihood under multi-trait animal models in selected layer lines, Japanese Journal of Poultry Science, 33, 185–192
Enayati, B. and Rahimi,G., 2009. Genomic growth hormone, growth hormone receptor and transforming growth factor β-3 gene polymorphism in breeder hens of Mazandaran native fowls, African Journal of Biotechnology, 8, 3154–3159
Engström, G., Liljedahl, L. E., Wilhelmson, M. and Johansson, K., 1992. The pattern of genetic and environmental variation in relation to ageing in laying hens, Genetic selection evolution, 24, 265–275
Fairful, R. W. and Gowe, R. S., 1990. Poultry Breeding and Genetics, (Elsevier Science, Amsterdam)
Falconer, D. S. and Mckay, T. F. C., 1996. Introduction to Quantitative Genetics, (Longman Group, Essex, UK)
Francesch, A., Estany, J., Alfonso, L. and Iglesias, M., 1997. Genetic parameters for egg number, egg weight, and eggshell color in three Catalan poultry breeds, Journal of Poultry Science, 76, 1627–1631
Ghazikhani Shad, A., Nejati Javaremi, A., Mehrabani Yeganeh, H., 2007. Animal model estimation of genetic parameters for most important economic traits in Iranian native fowls, Pakistan Journal of Biological Sciences, 10, 2787–2789
Gilmour, A. R., Gogel, B. J., Cullis, B. R. and Thompson, R., 2006. ASReml User Guide (NSW Department of Primary Industries, Australia)
Henderson, C. R., 1986. Recent developments in variance and covariance estimations. Journal of Animal Science, 63, 208–216
Hicks, C., Muir, W. M. and Stick, D. A., 1998. Selection index updating for maximizing rate of annual genetic gain in laying hens, Journal of Poultry Science, 77, 1–7
Hoffmann, I., 2005. Research and investment in poultry genetic resources-challenges and option for sustainable use. World’s Poultry Science Journal, 61, 57–70
Kamali, M. A., Ghorbani, S. H., Sharbabak, M. M. and Zamiri, M. J., 2007. Heritabilities and genetic correlations of economic traits in Iranian native fowl and estimated genetic trend and inbreeding coefficients, British Poultry Science, 48, 443–448
Khadem, A., Hafezian, H. and Rahimi-Mianji, G., 2010. Association of single nucleotide polymorphisms in IGFI, IGF-II and IGFBP-II with production traits in breeder hens of Mazandaran native fowls breeding station, African Journal of Biotechnology, 9, 805–810
Kianimanesh, H. R; Nejati Javaremi, A; Rahimi, G., 2002. Estimation of economic indices for important production traits of the Iranian native fowls, First seminar on genetics and breeding applied to livestock, Tehran, Iran
Ledur, M. C., Fairfull, R. W., McMillan, I. and Asseltine, L., 2000. Genetic effects of aging on egg production traits in the first laying cycle of White Leghorn strains and strain crosses, Journal of Poultry Science, 79, 296–304
Ledur, M. C., Liljedahl, L. E., McMillan, I., Asselstine, L. and Fairfull, R. W., 2003. Genetic effects of aging on fitness and nonfitness traits in laying hens housed three per cage, Journal of Poultry Science, 82,1223-1234
Lwelamira, J., Kifaro, G. and Gwakisa, P., 2009. Genetic parameters for body weights, egg traits and antibody response against Newcastle Disease Virus (NDV) vaccine among two Tanzania chicken ecotypes, Tropical Animal Health and Production, 41, 51–59
Minga, U., Msoffe, P. L. and Gwakisa, P. S., 2004. Biodiversity (variation) in disease resistance and in pathogens within rural chicken, World Poultry Congress, Istanbul
Norris, D. and Ngambi, J., 2006. Genetic parameter estimates for body weight in local Venda chickens. Tropical Animal Health and Production, 38, 605–609
Poggenpoel, D. G., Ferreira, G. F., Hayes, J. P. and Preez, J. J. d., 1996. Response to long-term selection for egg production in laying hens, British Poultry Science, 37, 743–756
Sabri, H. M., Wilson, H. R., Harms, R. H. and Wilcox, C. J., 1999. Genetic parameters for egg and related characteristics of white Leghorn hens in a subtropical environment, Genetics and Molecular Biology, 22, 183–186
Sang, B. D., Kong, H. S., Kim, H. K., Cho, C. H., Kim, D., Cho, Y. M., Sang, B. C., Lee, J. H., Jeon, G. J. and Lee, H. K., 2006. Estimation of genetic parameters for economic traits in Korean native chickens, Asian-Australasian Journal of Animal Sciences, 19, 319–323
Sharma, D., Johari, D. C., Kataria, M. C., Singh, B. P., Singh, D. P. and Hazary, R. C., 1996. Effect of long term selection on genetics of economic traits in white leghorn, Asian-Australasian Journal of Animal Sciences, 9, 455–459
Wolk, A. and Szwaczkowski, K., 2009. Estimation of genetic parameters for monthly egg production in laying hens based on random regression models, Journal of Applied Genetics, 50, 41–46
Acknowledgments
We sincerely thank the farm attendants who worked on data collection at the breeding station of Mazandaran native chicken. Thanks are also extended to two anonymous reviewers for their valuable comments on a previous version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Niknafs, S., Nejati-Javaremi, A., Mehrabani-Yeganeh, H. et al. Estimation of genetic parameters for body weight and egg production traits in Mazandaran native chicken. Trop Anim Health Prod 44, 1437–1443 (2012). https://doi.org/10.1007/s11250-012-0084-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-012-0084-6