Abstract
The objectives of this study were to investigate the relationships between body weight and morpho-structural indices to predict body weight from their orthogonal body shape characters using principal component analysis and to morphologically classify the chicken genotypes using multivariate discriminant analysis. Data used were from 273 randomly selected 12-weeks-old indigenous chickens of normal-feathered (NF), frizzle-feathered (FF), naked-neck (NN) and Anak Titan (AT) genotypes. Phenotypic correlation among body weight and most biometric traits ranged from 0.227–0.876, −0.7–0.901, 0.034–0.968 and −0.207–0.849 for NF, NN and AT chickens, respectively. Factor analysis with varimax rotation of interrelated traits revealed three principal components which accounted for 83.1%, 74.4%, 78.8% and 76.5% of the total variance in NF, FF, NN and AT chickens in the order listed. Breast girth, keel length, thigh length, shank length and wing length were found to be the most discriminating variables to separate the chicken genotypes. The longest distance (72.54) occurred between AT and NF genotypes while the shortest distance (4.27) was recorded for FF and NN genotypes. Classification results showed that 85.2% of AT genotype was correctly classified into their source population. However, 22.7% of NF was misclassified as NN, while 33.3% of NN was misclassified as NF chickens. These results suggest that there is high rate of gene flow between these two indigenous chicken genotypes. Information obtained from this study may be considered useful in breed improvement programmes for selection, characterization, conservation and better management of Nigerian indigenous chickens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Indigenous chickens constitute 80% of the 185 million chickens found in Nigeria (FAOSTAT 2011). They contain a highly conserved genetic reservoir, with high level of heterozygosity, which may provide the biological material for the development of genetic stocks with improved adaptability and productivity. In addition, poultry industry has played an impressive role in providing the teeming populace with a vital source of animal protein and income (Okeno et al. 2011; Osei-Amponsah et al. 2011), thus becoming a veritable tool in reducing hunger and alleviating poverty.
With the increasing attention given to the characterization of the African genetic resource (Yakubu et al. 2011; Osei-Amponsah et al. 2012), the first phase of characterization involves the identification of populations based on morphological descriptors. Morphometric measurements have been used to evaluate the characteristics of various breeds of animals, and could provide useful information on the suitability of animals for selection. Body weight had always been a trait of economic importance to livestock farmers. The increasing need to estimate the weight of chickens in order to study their growth pattern has led to the development of regression equations which were designed to predict the live weight of animals from linear body measurements (Peters et al. 2007). Most of the studies on body weight and morphometric indices have been mainly on univariate analysis of variance. A more reliable assessment of morphometric relationship among livestock breeds has been obtained using multivariate statistical tools such as principal component (PC) analysis (Yakubu et al. 2009), which reduces the dimensionality of a dataset, by finding a new set of variables, smaller than the original set of variables. Discriminant analysis can be defined as a body of procedures that maximizes discrepancies between the groups thereby distinguishing the groups which at first seem intermingled with each other.
Multivariate analyses involving the use of principal components has been reported for extensively managed chickens in the Northern part of Nigeria (Yakubu et al. 2009), but that of Nigerian indigenous and exotic chickens raised under intensive system of management in South West Nigeria has not been conducted. The present study therefore sought not only to estimate body weight from body measurements of chickens using orthogonal conformation traits derived from the PCs but also to establish the morphometric differences among genotypes using multifactorial discriminant analysis. The information obtained will aid the management, conservation and selection of birds towards improved productivity.
Materials and methods
Location of the study
The study was carried out at the Poultry Breeding Unit of the Teaching and Research farm, University of Agriculture, Abeokuta, Nigeria with latitude 7°10′ N and longitude 3°2′ E. Details about data on annual rainfall and vegetation of the location of the study have been described in Adeleke et al. (2011a).
Experimental animals
Two hundred and seventy three birds comprising 147 normal-feathered (NF) (39 males and 108 females), 69 frizzle-feathered (FF) (22 males and 47 females), 30 naked-neck (NN; 10 males and 20 females) Nigerian indigenous chickens and 27 Anak Titan (AT; exotic broiler; comprising of 10 males and 17 females) were generated from 15 sires and 80 dams through artificial insemination. The birds were identified with the aid of wing tag and were raised on a deep litter system in a well-ventilated building. The chicks were fed ad libitum with chicks’ mash that supplied 21.49% crude protein and 11.77 MJ/kg metabolizable energy from 0 to 8 weeks of age, after which they were fed on a grower’s ration that supplied 16.90% crude protein and 11.35 MJ/kg metabolizable energy. Clean water was given to the birds without restriction and the birds were subjected to similar treatment throughout the period of the study.
Traits measured
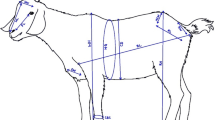
Body weight (BW) (grammes) and seven linear body measurements were measured on each chicken. The body measurements (centimetres) were taken using a measuring tape except for body weight that was taken using a measuring scale in grammes. The parts measured were body length (BL), measured as the distance between the tip of the beak and the longest toe without the nail; wing length (WL), taken as the distance between the tip of the phalanges and the coracoids-humerus joint; wing span (WS), measured as the distance between the left wing tip to the right wing tip across the back of the chicken; shank length (SL), taken as the distance from the hock joint to the tarsometatarsus; thigh length (TL) measured as the distance between the hock joint and the pelvic joint; breast girth (BG), measured as the circumference of the breast around the deepest region of the breast and keel length (KL), taken as the distance between the anterior and posterior ends of the keel.
Statistical analyses
Effects of genotype and sex were analysed using General Linear Model. Pearson’s coefficients of correlation (r) among body weight and various body linear measurements were estimated. Data were generated for principal component factor analysis using the correlation matrix.
Principal component analysis
Everitt et al. (2001) described principal component analysis as a method for transforming the variables in a multivariate data set X 1, X 2, …, X p , into new variables, Y 1, Y 2, …, Y p , which are uncorrelated with each other and account for decreasing proportions of the total variance of the original variables defined as:
with coefficients being chosen so that y 1, y 2, …, y p account for decreasing proportions of the total variance of the original variables x 1, x 2, …, x p . To enhance the interpretability of the principal components, the varimax criterion of the orthogonal rotation method was employed in the rotation of the factor matrix. Prediction of the body weight from linear body measurements using the stepwise multiple regression procedure was carried out using the following model:
A second stepwise multiple regression procedure was also used to obtain models for predicting body weight from principal component factor scores according to the equation below:
Where, BW is the body weight, a is the regression intercept, B i is the ith partial regression coefficient of the ith linear body measurement, X i or the ith principal component. To test the appropriateness of the factor analysis of the data, anti-image correlations and Kaiser–Meyer–Olkin measures of sampling adequacy were computed.
Discriminant analysis
Seven morphometric variables (i.e BL, WL, WS, SL, TL, BG and KL) were stepwisely introduced as variables into the discriminant analysis. The relative importance of the morphometric variables in discriminating the four chicken populations was assessed using the F-to-remove statistic. Collinearity among the variables used in the discriminant model was evaluated using tolerance statistic. The ability of this function to identify the chicken genotypes was indicated as the percentage of individuals correctly classified from the sample that generated the function. Accuracy of the classification was evaluated using split-sample validation (cross-validation). Pairwise group comparison (pairwise distances) was also estimated across the four chicken genotypes. All statistical analyses were done using SPSS (2001).
Results and discussion
Genotype had significant effect (P < 0.05) on all the linear body measurements with AT chickens having significantly highest means compared to Nigerian indigenous chickens (Table 1). This observation not only attested to previous reports that Nigerian indigenous chickens are light breeds (Adeleke et al. 2011a), but suggests the suitability of Anak Titan chickens for crossbreeding programmes which when mated with indigenous stocks will ultimately improve the growth and carcass traits potentials of Nigerian indigenous chickens. The observable differences in body weight among indigenous breeds were similar to that observed by Gunn (2008) who reported better performance of NN birds in comparison to others. Sex-associated (P < 0.05) differences were observed in all the morpho-structural traits examined, with higher values exhibited by male birds. This result is consistent with the findings of earlier workers (Martinho de Almeida and Zuber 2009; Osei-Amponsah et al. 2012). This dimorphism in favour of males might be attributed to the usual between- sex differential hormonal effects on growth.
The correlation coefficients (r) for body weight and zoometric body measurements ranged from 0.227–0.876 for NF, −0.7–0.901 for FF, 0.034–0.968 for NN and −0.207–0.849 for AT chickens (Table 2). Across genotypes, the highest correlation coefficients between BW and morphological traits were observed between BL and BW (r = 0.862), BG and BW (r = 0.845), BG and BW (r = 0.914) and WS and BG (r = 0.798) for NF, FF, NN and AT chickens, respectively. Peters et al. (2007) suggested that breast girth could be used as a reliable predictor of BW of most livestock species. High positive correlations suggest that selection for a trait may lead to a correlated response in the other trait.
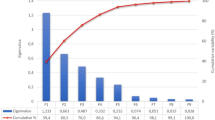
High values of Kaiser–Meyer–Olkin measure of sampling adequacy observed for the four genetic groups as well as those of Communalities gave credence to the appropriateness of the PC analysis (Table 3). There were variations in the pattern of loadings of the body dimensions on each PC. PC1 was characterized by WL, WS, TL, BL and BG in both NF and FF birds. However, PC1 had its loadings for BL and BG in NN birds and WL, WS, BL and BG in AT chickens. SL (PC2) and KL (PC3) were the traits of interest in NF and FF chickens while WL, WS and SL (PC2) and KL (PC3) were loading traits in NN chickens. SL and TL were more associated with PC 2 while KL was the sole loading trait on PC3 of AT birds. In the present study, it was noted that the first factor (PC1) accounted for the largest variance for both indigenous and exotic chickens and this had been the usual trend in studies that used principal component factor analysis as reported by Mendes (2009) in chickens and Shahin and Hassan (2000) in rabbits. The relevance of PC as a multivariate statistical tool was evidenced in the reduction of large number of explanatory variables into components that gave a better description of size and shape. Seven explanatory variables have been reduced to three components. The three principal components (PC 1, PC 2 and PC 3) obtained for each genotype could be useful in evaluating animals for breeding and selection purposes. Since correlation between principal components is zero, the selection of animals for any principal component will not cause a correlated response in terms of other principal components (Pinto et al. 2006). Across the four genetic groups used in this study, PC3 was determined only by KL. From the foregoing, it appears that, selection based on KL would not bring about any correlated response in morpho-structural traits.
The principal component factor score coefficients of NF, FF, NN and AT birds are shown in Table 4. These factor scores could be used instead of the original interdependent linear type traits in predicting the BW of chickens (Mendes 2009).
The interdependent original morphometric indices and their independent principal component factor scores were used for the prediction of body weight (Table 5). This revealed that in NF birds, BL alone accounted for 74% of the variation in BW. This result is comparable with that reported by Yakubu et al. (2009) on Nigerian indigenous chickens managed under extensive system. However, the best prediction equation (R 2 = 0.84) for BW was obtained when BL, SL, TL, BG and WL were included in the model. In FF chickens, BG alone accounted for 71.4% of the variation in BW. The high association between BW and BG might be attributed to large deposits of bones and muscles in breast region of the birds. The proportion of the explained variance increased to 74.9% when thigh length was included in the model. In NN birds, BG contributed 83.6% to the variation in BW. The proportion of the explained variance was further increased to 86.3% when SL was included in the model. WS in AT birds contributed 63.7% to the variation in BW. The proportion of the explained variance was further increased to 77.7% when BG and SL were included in the model.
The use of PC1 as a single predictor in this present study, explained 57.5%, 66%, 53.1% and 68% of the total variability in BW in NF, FF, NN and AT birds in the order listed. However, a combination of PC1, PC2 and PC3 led to a considerable improvement in the amount of variance explained (R 2 = 0.83) in NF birds while PC1 and PC2 led to an improvement in the amount of variance explained (R 2 = 0.744; R 2 = 0.788 and R 2 = 0.765) in FF, NN and AT birds, respectively. The use of principal component scores (orthogonal traits) gave a better and more reliable assessment of body weight since it was able to break multicollinearity, a problem associated with the use of interdependent original body dimensions (Yakubu et al. 2009).
The results from stepwise discriminant procedure highlighting Wilks lambda, F values, tolerance and probability statistics of the variables are presented in Table 6. BG, KL, TL, SL and WL were the most discriminating variables to separate NF, FF, NN and AT chickens based on their significant F values. Some of the discriminating variables of the present study are similar to those reported by Abdelqader et al. (2008). Discriminant analysis was equally applied to chicken performance by Rosario et al. (2008).
The results of all pairwise distances among the chicken genotypes (Table 7) were significant (P < 0.0001). The largest distance (72.54) was observed between NF and AT genotypes while the lowest (4.27) was observed between FF and NN genotypes. The lowest distance between FF and NN genotypes could be adduced to the high rate of intermingling between these two genotypes. Efforts should therefore be geared towards preventing the extinction of the major genes inherent in indigenous chickens knowing well that the NN and the FF chickens had evolved adaptive genetic structures to withstand heat stress which is an important factor that determines the overall success of the local chickens under tropical conditions. The high morphometric differentiation between AT and NF genotypes when compared with their FF and NN counterpart may indeed suggest the possibility of obtaining higher heterotic gains with AT and NF crosses while the cross between AT and other indigenous ecotypes may not result in an appreciable heterosis. In any case, the comparative assessment of the growth and carcass potentials of crossbreds obtained from crosses involving AT and indigenous ecotypes would further clarify these hypotheses. Figure 1 showed that AT was marked distinct from the indigenous counterpart. In any case, this result is expected being an exotic genotype. However, the way the indigenous genotypes are distributed in the canonical discriminant function lends more credence to the existence of genetic admixture among Nigeria’s local chickens which was substantiated by the low level of morphometric differentiation in their pairwise comparison.
Classification results presented in Table 8 showed that most of the AT genotypes were classified into their source population (85.2%) obviously highlighting its morphological distinctness from the indigenous genotypes. However, 22.7% of the NF birds were misclassified as NN while 33.3% of NN were misclassified as NF. This result suggests some kind of introgression between these two genotypes which may constitute a threat to the future of indigenous chickens (Ansari et al. 1997). The high rate of introgression among Nigeria’s indigenous chicken could be adduced to improper management. Genetic introgression among distinct genotypes could possibly lead to the erosion of between-breed diversity thus resulting into more homogenous populations which has negative implication for utilizing genetic variation in future improvement programmes. This result conflicts with the report of Adeleke et al. (2011b). They used blood protein to characterize the local chickens and reported that FF chickens were closely more related to NF than NN birds.
Conclusion
Multivariate analysis using principal components has been shown to be useful in the prediction of body weight using orthogonal conformation traits. BG, KL, TL, SL and WL were found as the most discriminating variables in separating the indigenous and exotic genotypes. Low level of morphometric differentiation among indigenous genotypes revealed by low pairwise distances and proportion of individuals misclassified suggest high level of gene flow among them. The present information provides a simple practical methodological framework suited for management, characterization and conservation of chicken genetic resources in a resource poor developing economy. However, the morphometric assessment using multivariate techniques should be followed up with highly polymorphic DNA-based techniques such as single nucleotide polymorphisms to properly unravel the genetic distinctiveness or similarity among the three local chicken genotypes.
Abbreviations
- AT:
-
Anak Titan
- BW:
-
Body weight
- BG:
-
Breast girth
- BL:
-
Body length
- FF:
-
Frizzle feathered
- KL:
-
Keel length
- NF:
-
Normal feathered
- NN:
-
Naked neck
- PC:
-
Principal component
- SL:
-
Shank length
- TL:
-
Thigh length
- WL:
-
Wing length
- WS:
-
Wing span
References
Abdelqader, A., Wollny, C.B.A. and Gauly, M., 2008. On-farm investigation of local chicken biodiversity and performance potentials in rural areas of Jordan, Animal Genetic Resources Information, 43, 49-58.
Adeleke, M.A., Peters, S.O., Ozoje, M.O., Ikeobi, C.O.N., Bamgbose, A.M. and Adebambo, O. A., 2011a. Growth performance of Nigerian local chickens in crosses involving an exotic broiler breeder, Tropical Animal Health and Production, 43 (3), 643-650.
Adeleke, M.A., Peters, S.O., Ozoje, M.O., Ikeobi, C.O.N., Adebambo, A.O., Olowofeso, O., Bamgbose, A.M. and Adebambo, O.A., 2011b. A preliminary screening of genetic lineage of Nigerian local chickens based on blood protein polymorphism, Animal Genetic Resources, 48, 23-28.
Ansari, S., Edriss, M.A. and Pourreza, J., 1997. Genetic and non-genetic variations of body weight in Iranian native chicken and their crosses with exotic breeds, Iran Agricultural Research, 16, 83-96.
Everitt, B.S., Landau, S. and Leese, M., 2001. Cluster analysis. 4th edn., (Arnold Publisher, London).
FAOSTAT, 2011. FAOSTAT database on Agriculture. www.fao.org/library. Accessed on May 24, 2011.
Gunn, H.H., 2008. Effects of frizzling and naked neck gene on growth, haematology, carcass traits and organ weights of the Nigerian local chicken (unpublished M. Agric. Dissertation, Department of Animal Breeding and Genetics, University of Agriculture, Abeokuta)
Martinho de Almeida, A. and Zuber, U., 2009. The effect of the naked neck genotype (Nana), feeding and outdoor rearing on growth and carcass characteristics of free range broilers in a hot climate, Tropical Animal Health and Production, 42, 99-107.
Mendes, M., 2009. Multiple linear regression models based on principal component scores to predict slaughter weight of broiler, Archiv für Geflügelkunde 73, 139–144.
Okeno, T.O., Kahi, A.K. and Peters, K.J., 2011. Characterization of indigenous chicken production systems in Kenya, Tropical Animal Health and Production, DOI 10.1007/s11250-011-9942-x.
Osei-Amponsah, R., Kayang, B.B., and Naazie, A., 2012. Age, genotype and sex effects on growth performance of local chickens kept under improved management in Ghana, Tropical Animal Health and Production, 44:29–34.
Osei-Amponsah, R., Kayang, B.B., Naazie, A., Arthur, P.E. and Barchia, I.M., 2011. Characterisation of local Ghanaian chickens: growth performance evaluation based on Richards growth model and genetic size scaling. Tropical Animal Health and Production, 43, 1195–1201.
Pinto, L.F.B., Dacker, I.U., De melo, C.M.R., Ledur, M.C. and Coutinho, L.L., 2006. Principal components analysis applied to performance and carcass traits in the chicken, Animal Research, 55, 419-425.
Peters, S.O., Adeleke, M.A., Ozoje, M.O., Adebambo, O.A. and Ikeobi, C.O.N., 2007. Bio-prediction of liveweight from linear body measurement traits among pure and crossbred chicken, Nigerian Poultry Science Journal, 4, 1-6.
Rosario, M.F., Silvia, M.A.N., Coelho, A.A.D., Savino, V.J.M. and Dias, C.T.S., 2008. Canonical discriminate analysis applied to broiler chicken performance. Animal, 2, 419-424.
Shahin, K.A. and Hassan, N.S., 2000. Sources of shared variability among body shape characters at marketing age in New Zealand White and Egyptian rabbit breeds. Annales de Zootechnie, 49, 435-445.
SPSS, 2001. Statistical Package for Social Sciences. SPSS Inc., 444 Michigan Avenue, Chicago, IL60611.
Yakubu, A., Kaankuka, F.G. and Ugbo, S.B., 2011. Morphometric traits of Muscovy ducks from two agro-ecological zones of Nigeria, Tropicultura, 29, 121-124.
Yakubu, A., Kuje, D. and Okpeku, M., 2009. Principal components as measures of size and shape in Nigerian indigenous chickens, Thai Journal of Agricultural Science, 42 (3), 167-176.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ajayi, O.O., Adeleke, M.A., Sanni, M.T. et al. Application of principal component and discriminant analyses to morpho-structural indices of indigenous and exotic chickens raised under intensive management system. Trop Anim Health Prod 44, 1247–1254 (2012). https://doi.org/10.1007/s11250-011-0065-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-011-0065-1