Abstract
The objective of this study was to investigate the relationship between seroprevalence to Theileria parva infection in cattle and potential environmental and farm-level effects in 80 farms under traditional crop–livestock system in Mbeere District, Kenya. A standardized questionnaire was used to collect the effects characteristics as related to T. parva infection epidemiology. Serum samples were collected from 440 cattle of all ages for detection of T. parva antibodies by the enzyme-linked immunosorbent assay technique. The association between the variables was assessed using a generalized estimation equation logistic regression model. The overall T. parva seroprevalence, accounting for correlation of responses, was 19.3% (95% confidence interval (CI) 14%, 25%). Two variables, “administrative division” and “presence of the vector tick on the farm”, were significantly associated with the T. parva seroresponse. Respectively, cattle from farms in Gachoka, Evurore, and Mwea divisions were (and their 95% CI) 1.3 (0.36, 4.8), 4.4 (1.2, 15.9), and 15.2 (4.9, 47.1) times more likely to be seropositive relative to those from Siakago Division (P = 0.000). Cattle from farms in which the vector tick was present were 2.9 (1.2, 6.7) times more likely to be seropositive (P = 0.011). Results of this study suggested that both environmental and farm factors may be associated with T. parva infection epidemiology in Mbeere District. Under such circumstances, characterization of environmental suitability for the vector tick and corresponding environment-specific farm management practices in the district is required both for improved understanding of the disease and in planning disease control programs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Theileria parva is a tick-borne protozoan parasite that is transmitted by the three host tick, Rhipicephalus appendiculatus, to cause a fatal disease in cattle known as East Coast fever (ECF) in eastern, central, and southern Africa (Norval et al. 1992). Whereas about 28 million cattle are at risk in the region, the costs of tick control using acaricides was estimated to range between US $6 and US $36 per adult animal in east Africa (Minjauw and McLeod 2003). In Kenya, T. parva infection is considered a major constraint to improved livestock production (Gitau et al. 1999, 2010; Maloo et al. 2001; Muraguri et al. 2005; Okuthe and Buyu 2006; Chenyambuga et al. 2010).
Previous studies in Kenya have identified environmental factors, particularly agro-ecological conditions, livestock production systems, and farm management practices as the main factors associated with the epidemiology of ECF with reported disease morbidity, mortality, and case fatality rates varying significantly by these factors (Deem et al. 1993; Gitau et al. 1999; Maloo et al. 2001; Okuthe and Buyu 2006). Moreover, these factors were often reported to be strongly associated with each other. Thus, suitable AEZs for the vector tick (mainly the lower elevation zones) are characterized by high T. parva infection prevalence and incidence, low incidence of clinical ECF, and ECF-resistant indigenous zebu cattle breeds reared under open grazing systems that allow exposure to infected ticks. This phenomenon has been termed endemically stable (Norval et al. 1992). In the upper elevation zones, there exist suitable to marginal zones for the tick vector that are characterized by low to moderate T. parva infection prevalence and incidence, moderate to high clinical ECF and ECF-susceptible exotic cattle breeds kept under stall feeding system that restricts exposure to infected ticks (Gitau et al. 1999). In between these two zones are areas characterized by graded tick suitability areas, mixed grazing systems, and cattle crosses between the exotic and indigenous breeds among other characteristics. Previous studies selected few sites in this middle zone as part of larger studies involving diverse AEZs (Gitau et al. 1999).
The present study, therefore, focused on the whole of Mbeere District, an area predominantly characterized by traditional crop–livestock system, indigenous cattle breeds, and moderate to insignificant differences in agro-climatic features (Jaetzold and Schmidt 1983; Onduru et al. 2002). The aim of the study was to investigate the factors associated with T. parva infection seroprevalence in cattle in the district. The information generated will aid in development of disease control practices, particularly in similar areas characterized by homogenous ecosystems and farming systems.
Materials and methods
Study area
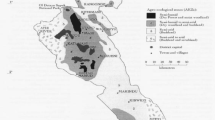
Mbeere District is located in Eastern Province, Kenya, and lies between latitudes 0°20′ and 0°50′ S and longitude 370°16′ and 370°56′ E. The district is covered by three main AEZs: lower midlands 3 (LM3), lower midlands 4 (LM4), and lowlands 5 (L5) (Jaetzold and Schmidt 1983). Mbeere District has no major diverse environments despite large variations in elevation and annual rainfall (Jaetzold and Schmidt 1983).
Study design
Sample size determination
The sample size was determined according to the method described by Martin et al. (1987) as follows: n = [1.962 × p(1 − p)]/L 2, where 1.96 was the z value for the desired confidence level (95%), p was an estimate of the probable prevalence, and L was the tolerable error. As the antibody prevalence to T. parva infection was not known a priori, 50% prevalence and a 5% tolerable error were assumed. A reconnaissance visit in the district in November 2006 indicated that the average number of cattle in farms was around 5. Therefore, we required at least 80 farms to achieve the target sample size (400). Thus, sub-locations (the smallest administrative units in Kenya) and farms were selected by stratified random sampling method by first classifying all sub-locations according to all four divisions (Siakago, Gachoka, Evurore, and Mwea). All sub-locations were eligible for selection. Two sub-locations from each of the four divisions were then selected using random numbers to give a total of eight sub-locations. In the second stage, through collaboration with the assistant chief (the sub-locational administrator) and village elders, a list of all farms owning cattle was composed and ten of them per sub-location were randomly sampled using random number tables to give a total of 80 farms.
Sampling of individual cattle used proportional allocation approach. A constant 50% proportion of animals in each farm were selected using systematic random sampling. In farms that had six or less cattle, all the cattle in that farm were sampled. All cattle ages were eligible for selection except calves less than 4 months of age to minimize the possibility of detecting passively derived colostral antibodies (Gitau et al. 1999). A total of 440 animals were sampled in this study. The study was conducted in March 2007.
Administration of questionnaire
For each farm visited, a standardized questionnaire was used to summarize the farm’s management practices that were likely to be related to T. parva transmission. The grid location of each study farm was geo-referenced and Arcview version 3.3 (ESRI, Buckinghamshire, UK) used to create a detailed map of the study area (Fig. 1).
Collection of ticks, sera, and serology
During the farm visit, all cattle in a farm were first examined for presence of ticks which were categorized into species according to Kaiser et al. (1988). For this study, our main target was R. appendiculatus. After blood collection, serum samples were separated and stored at −20°C until they were analyzed at the International Livestock Research Institute, Nairobi. T. parva indirect enzyme-linked immunosorbent assay (sensitivity, 99%; specificity, 94–98%) was used to detect antibodies to the parasite (Katende et al. 1998).
Statistical analysis
Estimating T. parva seroprevalence
Animal-level T. parva seroprevalence was estimated accounting for correlation of responses (Dohoo et al. 2003). In estimating farm-level T. parva seroprevalence, a farm was considered positive if at least one animal in the farm tested positive for antibodies against T. parva and negative if none was positive.
Associations between explanatory variables and T. parva seroprevalence
Association between explanatory variables (environmental and farm variables; see “Results” section) were calculated using chi-square statistics. The association between the explanatory variables and the response variable (T. parva serostatus) was assessed using generalized estimation equation (GEE) approach of Zeger and Liang (1986) to account for correlation of responses from multiple animals within farms. Univariable analysis was first performed using all explanatory variables. Variables with P ≤ 0.1 were selected for inclusion in the multivariable model which was built by manual backward variable selection process based on the Wald’s chi-square statistic (P < 0.05). Throughout the modeling process, “administrative division” was maintained in the model to account for the study design of random farm selection from within each administrative division. The model regression coefficients were exponentiated into odds ratios (OR). The OR is a relative measure of risk that describes how much more likely it is that an animal which is exposed to the factor under study will develop the outcome as compared to an animal which is not exposed. Confounding and interaction were assessed as described by Dohoo et al. (2003). All statistical analyses were performed using STATA Version 10 (StataCorp 2007).
Results
General results
Serum samples were collected from 440 animals representing 677 cattle from 80 farms. The mean (± SD) number of animals in the 80 farms was 8.5 (± 6.4), ranging from 2 to 30. Table 1 shows the distribution of the major characteristics of the sampled cattle. Although the district is covered by three main AEZs, that is, LM3, LM4, and L5, the selected farms fell into only LM4 and L5 zones after overlaying the selected farms on district’s AEZ map as the sampling had been done by division.
Farm-level characteristics across divisions and AEZs
R. appendiculatus vector was more likely to be found in farms in Mwea and Gachoka divisions (P = 0.000) and in farms in LM4 zone (P = 0.002) relative to other divisions and L5 zone, respectively. Similarly, majority of farmers in Mwea and Gachoka divisions (P = 0.000) and in LM4 zone (P = 0.000) were more likely to initiate tick control on calves at an earlier calf age than those in other divisions and L5 zone, respectively. Calf tick control was more frequent in farms from Mwea and Gachoka divisions (P = 0.000) and indeed was also more likely to have applied tick control measures in the farm within 2 weeks prior to the farm visit (P = 0.000) relative to other divisions. However, there was no significant difference among farms in the two AEZs in terms of application of tick control measures within 2 weeks prior to the farm visit (P = 0.377) or calf tick control frequency (P = 0.671). Farms in Mwea Division (P = 0.000) and in LM4 zone (P = 0.000) reported higher use of acaricides compared to other drugs (multivitamins and antihelmintics) relative to farms in other divisions and L5 zone, respectively. Farmers from Mwea Division (P = 0.000) and in LM4 zone (P = 0.000) were more likely to report previous occurrence of ECF syndrome in cattle (generalized lymphadenopathy, fever, dyspnea, and a characteristic cough) relative to other divisions and L5 zone, respectively. R. appendiculatus was found in 15%, 40%, 10%, and 50% of the sampled farms in Siakago, Gachoka, Evurore, and Mwea divisions, respectively, and the difference across divisions was significant (P = 0.012) whereas the vector tick was found in 34% and 20% (P > 0.05) of the sampled farms in LM4 and L5 zones, respectively. All selected farms in Siakago Division (P = 0.000) and in L5 zone practiced free grazing in comparison with other divisions and LM4 zone (P = 0.000), respectively, which practiced various forms of mixed free and stall systems.
Estimating T. parva infection exposure prevalence
Divisional-specific animal and farm seroprevalence differed significantly across all divisions (P = 0.000). Both AEZ-specific animal and farm seroprevalence also differed significantly (P = 0.001 and 0.028, respectively) across the two zones (Table 2).
Univariate analysis
Univariate analysis returned five significant farm factors and both environmental factors (P < 0.1). The significant factors included presence of vector tick, age at which farmers initiated calf control on calves, the drug most commonly used on the farm, administrative division, and AEZ.
Multivariate analysis
When the seven significant variables in the univariate analysis were offered to the multivariable analysis, two of them were significantly (P < 0.05) associated with T. parva seroprevalence (Table 3). Cattle from farms in which the vector was found during the farm visit were 2.9 times (95% CI 1.2, 6.7) more likely to be seropositive (P = 0.01) than those from farms in which the vector was not found. Respectively, cattle from farms in Gachoka, Evurore, and Mwea divisions were 1.3 times (95% CI 0.36, 4.8), 4.4 times (95% CI 1.2, 15.9), and 15.2 times (95% CI 4.9, 47.1) more likely to be seropositive relative to those in Siakago Division (P = 0.000). We suspected some confounding effect of “administrative division” because removing it from the final model changed the estimate of the variable “presence of vector tick on the farm” by 27% (Dohoo et al. 2003).
As divisional boundaries are administrative in nature, factors that could have been masked by effects of “administrative division” were investigated for their association with T. parva seroprevalence in the district by repeating univariate analysis but in absence of the variable “administrative division”. In addition to the presence of the vector on the farm, two additional variables were significant (P < 0.05) and these included AEZ and calf tick control frequency. However, we chose the model in Table 3 as the most parsimonious for this study because (a) we wanted to retain “administrative division” in the model to account for the study design, (b) the suspicion of the confounding effect of “administrative division”, and (c) the model’s Wald test for composite linear hypotheses was highly significant (P = 0.0000) compared to the one without “administrative division” (P = 0.0002).
Discussion
This study offered baseline population-structured cross-sectional findings on T. parva seroprevalence in cattle in Mbeere District. Although cross-sectional studies are not powerful at showing cause–effect relationships (Dohoo et al. 2003), this study showed evidence of importance of both environmental and farm factors in ECF epidemiology in the district.
The relatively low seroprevalence of 19.3% indicated that T. parva infection most likely existed in the district under endemic instability status. Endemic instability implies an ecological imbalance between the host, tick, and environment where only a small proportion (mostly <70%) of cattle in a population become infected and immune by 6 months of age (Norval et al. 1992). The low prevalence could have arisen out of low infection challenge, probably due to low levels of vector abundance and distribution and/or low tick infection rates among other reasons (Gilioli et al. 2009; Odongo et al. 2009). This is supported by the observation that the low seroprevalence existed under open grazing system that normally permits exposure to infected ticks. However, reliable spatial and/or seasonal data on tick populations in the district are unavailable. Other factors that have been reported to influence distribution of ticks and T. parva transmission and prevalence include rainfall, altitude, host population size and density, habitat modification, vector control programs, and the social environment (Olwoch et al. 2008; Nshimiyimana and Mutandwa 2010).
Farms in divisions and AEZs where the vector was reportedly present or absent practiced higher or lower levels of tick control, respectively, probably as a result of differences in the distribution of (infected) vector ticks and corresponding clinical infection. Farmers tend to apply tick control measures depending on occurrence, perceived incidence, and severity of the clinical disease experiences and economic impacts of the disease at the farm level (Swai et al. 2009; Mugabi et al. 2010; Phiri et al. 2010). Additionally, presence of the tick vector on the farm was a strong predictor of T. parva seroprevalence in this study. This was expected as the distribution of ECF is largely defined by the distribution of the vector. A distinct pattern between R. appendiculatus distribution and T. parva seroprevalence has previously been reported both in cross-sectional studies (Deem et al. 1993) and in longitudinal studies (Rubaire-Akiiki et al. 2006).
Lack of association between tick control practices and T. parva seroprevalence and incidence has been reported previously (Gitau et al. 1999; Rubaire-Akiiki et al. 2006; Swai et al. 2009). However, the finding that frequent application of acaricides to calves was associated with higher exposure to T. parva would normally be unusual; however, it is likely that farmers tended to react to presence of perceived disease and/or vector burdens that may have constituted a potential risk factor for within-farm disease transmission. Indeed, seroconversion to T. parva parasite has been associated with clinical signs of ECF (Magona et al. 2008). We could not, however, establish the effectiveness of this management practice due to limitation of determining temporal effects in cross-sectional studies.
The large and important effects of environmental variables on T. parva seroprevalence in this study have been previously reported with the seroprevalence and incidence varying across administrative districts and AEZs in East Africa (Deem et al. 1993; Gitau et al. 1999; Maloo et al. 2001; Rubaire-Akiiki et al. 2006; Bazarusanga et al. 2007; Chenyambuga et al. 2010). This clearly reflects different levels of exposure to T. parva infection. Differences in both T. parva seroprevalence and distribution of the vector on farms suggested differential vector environmental suitability across the district. This was supported by the fact that modeling without the variable “administrative division” revealed the importance of its environmental counterpart, AEZ. In Mbeere District, the environmental difference in T. parva seroprevalence could, in part, be explained by ecological differences between the two AEZs (Jaetzold and Schmidt 1983). In total, these findings suggested that even in areas with no major ecological diversity, differential micro-environmental effects may indirectly explain variability of T. parva seroprevalence at the farm level in endemically unstable areas and subsequent differential farm disease management practices. It is under these circumstances that we suspected “administrative division” to be a potential confounder implying that environmental variables alternately explained the observed relationship between the presence of the vector and T. parva response.
In conclusion, although T. parva seroprevalence in cattle in Mbeere District was low, suggesting endemic instability, areas where the tick vector was likely to be found corresponded with higher levels of both farm tick control management and levels of exposure to T. parva infection. As cross-sectional studies are not powerful at evaluating such relationships, further information on both the tick vector environmental suitability and environment-specific farm management factors in Mbeere District and other areas with similar ecology and farming systems is required for planning targeted ECF control programs.
References
Bazarusanga, T., Vercruysse, J., Marcotty, T. and Geysen, D., 2007. Epidemiological studies on theileriosis and the dynamics of Theileria parva infections in Rwanda. Veterinary Parasitology, 143, 214–221.
Chenyambuga, S. W., Waiswa, C., Saimo, M., Ngumi, P. and Gwakisa, P.S., 2010. Knowledge and perceptions of traditional livestock keepers on tick-borne diseases and sero-prevalence of Theileria parva around Lake Victoria Basin. Livestock Research for Rural Development., 22, Art. #135. http://www.lrrd.org/lrrd22/7/chen22135.htm. Accessed July 2010.
Deem, S.L., Perry, B.D., Katende, J.M., McDermott, J.J., Mahan, S.M., Maloo, S.H., Morzaria, S.P., Musoke, A.J. and Rowland, G.J., 1993. Variations in prevalence of tick borne diseases in zebu cattle by agroecological zone: implications for East Coast fever immunisation, Preventive Veterinary Medicine, 16, 171–187.
Dohoo, I., Martin, W. and Stryhn, H., 2003. Veterinary epidemiologic research. In: S.M. Mcpike (Ed.), A comprehensive text for the discipline. AVC, Charlottetown, p. 706.
Gilioli, G., Groppi, M., Vesperoni, M.P., Baumgartner, J. and Gutierrez, A.P., 2009. An epidemiological model of East Coast fever in African livestock. Ecological Modelling, 220, 1652–1662.
Gitau, G.K., Perry, B.D. and McDermott, J.J., 1999. The incidence, calf morbidity and mortality due to Theileria parva infections in smallholder dairy farms in Murang’a District, Kenya. Preventive Veterinary Medicine, 39, 65–79.
Gitau, G.K., Aleri, J.W., Mbuthia, P.G. and Mulei, C.M., 2010. Causes of calf mortality in peri-urban area of Nairobi, Kenya. Tropical Animal Health and Production, doi:10.1007/s11250-010-9614-2.
Jaetzold, R. and Schmidt, H., 1983. Farm management handbook of Kenya, Volume II. Natural conditions and farm management information, Ministry of Agriculture, Kenya.
Kaiser, M.N., Sutherst, R.W., Bourne, S.A., Gorrison, L. and Floyd, R.B., 1988. Population dynamics of ticks on Ankole cattle in five ecological zones in Burundi and strategies for their control. Preventive Veterinary Medicine, 6, 199–222.
Katende, J., Morzaria, S., Toye, P., Skilton, R., Nene, V., Nkonge, C. and Musoke, A., 1998. An enzyme-linked immunosorbent assay for detection of Theileria parva antibodies in cattle using a recombinant polymorphic immunodominant molecule. Parasitology Research, 84, 408–416.
Magona, J.W., Walubengo, J., Olaho-Mukani, W., Jonsson, N.N., Welburn, S.C. and Eisler, M.C., 2008. Clinical features associated with seroconversion to Anaplasma marginale, Babesia bigemina and Theileria parva infections in African cattle under natural tick challenge. Veterinary Parasitology, 155, 273–280.
Maloo, S.H., Rowlands, G.J., Thorpe, W., Gettinby, G. and Perry, B.D., 2001. A longitudinal study of disease incidence and case-fatality on smallholder dairy farms in coastal Kenya. Preventive Veterinary Medicine, 52, 17–29.
Martin S.W., Meek, A.H. and Willeberg, P., 1987. Veterinary epidemiology: principles and methods. Iowa State University Press, Ames, p. 343.
Minjauw, B. and McLeod, A., 2003. Tick-borne diseases and poverty. The impact of ticks and tick-borne diseases on the livelihood of small-scale and marginal livestock owners in India and eastern and southern Africa. Research report, DFID Animal Health Programme, Centre for Tropical Veterinary Medicine, University of Edinburgh, UK, p. 116.
Mugabi, K.N., Mugisha, A. and Ocaido, M., 2010. Socio-economic factors influencing the use of acaricides on livestock: a case study of the pastoralist communities of Nakasongola District, Central Uganda. Tropical Animal Health and Production, 42, 131–136.
Muraguri, G.R., McLeod, A., McDermott, J.J. and Taylor, N., 2005. The incidence of calf morbidity and mortality due to vector-borne infections in smallholder dairy farms in Kwale District, Kenya. Veterinary Parasitology 130, 305–315.
Norval, R.A.I., Perry, B.D. and Young, A.S., 1992. The epidemiology of Theileriosis in Africa. Academic, London, p. 234.
Nshimiyimana, J. and Mutandwa, E., 2010. Seasonal dynamics and distribution of ticks in Rwanda: implications for tick control strategy in Rwanda. International Journal of Animal and Veterinary Advances, 2, 21–25.
Odongo, D.O., Ueti, M.W., Mwaura, S.N., Knowles, D.P., Bishop, R.P. and Scoles, G.A., 2009. Quantification of Theileria parva in Rhipicephalus appendiculatus (Acari: Ixodidae) confirms differences in infection between selected tick strains. Journal of Medical Entomology, 46, 888–894.
Okuthe, O.S. and Buyu, G.E., 2006. Prevalence and incidence of tick-borne diseases in smallholder farming systems in the western-Kenya highlands. Veterinary Parasitology, 141, 307–312.
Olwoch, J.M., Reyers, B., Engelbrecht, F.A. and Erasmus, B.F.N., 2008. Climate change and the tick-borne disease, theileriosis (East Coast fever), in sub-Saharan Africa. Journal of Arid Environments, 72, 108–120.
Onduru, D.D., Gachumbi, L., Maina, F., Muchena, F.N. and de Jager, A., 2002. Sustaining agricultural production in the semi-arid areas of eastern Kenya: a case study of Mbeere District. INMASP Report No. Ke-03: ETC-EA, KARI (NARL) and LEI-DLO.
Phiri, B.J., Benschop, J. and French, N.P., 2010. Systematic review of causes and factors associated with morbidity and mortality on smallholder dairy farms in Eastern and Southern Africa. Preventive Veterinary Medicine, 94, 1–8.
Rubaire-Akiiki, C.M., Okello-Onen, J., Musunga, D., Kabagambe, E.K., Vaarst, M., Okello, D., Opolot, C., Bisagaya, A., Okori, C., Bisagati, C., Ongyera, S. and Mwayi, M.T., 2006. Effect of agro-ecological zone and grazing system on incidence of East Coast fever in calves in Mbale and Sironko Districts of Eastern Uganda. Preventive Veterinary Medicine, 75, 251–266.
StataCorp., 2007. Stata Statistical Software: release 10. College Station: StataCorp LP
Swai, E.S., Karimuribo, E.D., Kambarage, D.M. and Moshy, W.E., 2009. A longitudinal study on morbidity and mortality in youngstock smallholder dairy cattle with special reference to tick borne infections in Tanga region, Tanzania. Veterinary Parasitology, 160, 34–42.
Zeger, S. L. and Liang, K.-Y., 1986. Longitudinal data analysis for discrete and continuous outcomes. Biometrics, 42, 121–130.
Acknowledgments
We are grateful to Mbeere cattle farmers who participated in this study. Logistical support from Mbeere District Veterinary Office is acknowledged. The authors are also grateful to BecA—ILRI Hub and in particular Alice Njeri for laboratory technical assistance. We thank Pamela Ochungo, ILRI, for generating the map of the study area. This work was funded by the Association for Strengthening Agricultural Research in Eastern and Central Africa (ASARECA) and Kenya Agricultural Productivity Project (KAPP).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gachohi, J.M., Kitala, P.M., Ngumi, P.N. et al. Environment and farm factors associated with exposure to Theileria parva infection in cattle under traditional mixed farming system in Mbeere District, Kenya. Trop Anim Health Prod 43, 271–277 (2011). https://doi.org/10.1007/s11250-010-9688-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-010-9688-x