Abstract
The study was carried out to detect Theileria annulata, the causative agent of theileriosis, and Babesia bovis, the causative agent for babesiosis, in Friesian cattle by PCR and conventional blood smear examination. One hundred blood samples obtained from diseased Friesian cattle kept on private livestock farms at Pattoki, District Kasur, Pakistan were collected in addition to 20 blood samples obtained from non-diseased animals. The disease manifestations observed clinically included high fever, swelling of sub mandibular and sub scapular lymph nodes, weakness, increased respiration and pulse, anorexia, loss of condition and rough hair coat. Neurologic sign of in coordination was also seen in weak animals. Signs of lacrimation, pale conjunctiva, diarrhoea, dyspnea and frothy nasal discharge were observed in only one animal. Clinically nine animals showed signs of haemoglobinuria. Diagnosis of bovine theileria and babesia species was based on finding many intraerythrocytic piroplasms of both blood protozoa with clinical signs associated with anaemia, lymph node hyperplasia and haemoglobinuria. One hundred samples of ticks were also collected for identification of vector. Results showed that the prevalence of Hyalomma tick was highest (15%) followed by Boophilus (12%), Haemaphysalis (5%) and Rhipicephalus (3%). The blood smear examination showed 21% (21/100) samples positive for blood parasites out of which 66.6% (14/ 21) samples were positive for theileriosis while 42.8% (9/21) were positive for babesiosis. It was also recorded that 66.66% (6/9) samples were positive for B.bigemina while 33.33% (3/9) were positive for B.bovis. The results showed that 60% (60/100) samples were positive for blood parasites by PCR test. Out of these 60% (36/60) were positive for T.annulata while 33.33% (20/60) were positive for babesia. The specificity and sensitivity of PCR test was higher than blood smear examination. The blood parameters in haemoparasites infection were also analyzed and the results showed significant decrease in total erythrocyte count and haemoglobin while MCV, MCH values increased and MCHC was slightly less than normal indicating macrocytic hypochromic anaemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Theileria annulata and Babesia bovis, the protozoan parasite of cattle and domestic buffalo (Bubalus bubalis), are transmitted by ticks of the genus Hyalomma, Ixodes and Dermacenter respectively. Tropical Theileriosis, a disease which is present in northern Africa and southern Europe, extending through the Middle East, India, and Southern Russia into China (Neitz 1957) while Babesiosis is prevalent all over the world (Fahrimal et al. 1992). The hemoparasitic diseases threatens an estimated 250 million cattle and acts as a major constraint on livestock production and improvement in many developing countries. Due to Tropical Theileriosis mortality varies from 90% (in introduced exotic breeds) to 5% or less (in indigenous breeds) (Neitz 1957) while incase of babesiosis 90% mortality % in severe cases has been reported in untreated cases (Radostitis et al., 2000). Theileria parasites enter the bovine host during tick feeding as porozoites and rapidly invade lymphocytes. Here, they mature into macroschizonts and induce proliferation of the host cell. Macroschizonts develop further into microschizonts and ultimately into merozoites, which are released from the lymphocytes. The merozoites invade erythrocytes and develop into piroplasms (Uilenberg 1981). The life cycle of Babesia bovis has two phases. In the vertebrate host they multiply by merogony in erythrocytes and in ticks by sporogony (Susan and Asa 1999). Theileriosis is characterized by a marked anaemia and fever while in Babesiosis the clinical symptoms of anaemia, haemoglobinuria, ictrus and fever occur. Diagnosis of clinical T. annulata and B. bovis infections in cattle is usually based on Giemsa’s-stained blood smears but the detection of agent is not reliable and is almost impossible in carrier stage. After recovery, a long-lasting carrier state occurs, in which low numbers of erythrocytes remain infected with theileria and babesia piroplasms (Neitz 1957). Babesia bovis carrier cattle as well as ticks are reservoir of pathogenic babesia species. Carriers animals are important contributors to the infection within ticks (Oliveira and Jongejan 2005). Hence, detection of developmental stages of protozoa in carrier animals is an important epidemiological parameter. The advent of PCR has allowed the development of sensitive diagnostic assays (Saiki et al. 1988). Primers were derived from the gene encoding the 30-kDa major merozoite surface antigen for T. annulata (Allsopp et al., 1993) while for B. bovis SSU rRNA gene sequences were used (Guido et al. 2002). In the present study Polymerase chain reaction test (PCR) was compared with conventional microscopic blood smear examination (MBE detecting hemoparasites in carrier animals. The sensitivity and specifity of PCR and MBE was also determined.
Materials and methods
Categorization of animals and collection of blood samples
One hundred blood samples were collected from Frisian cattle imported from Australia and maintained at twenty private farms in District Kasur, Pakistan. The reference cattle population at livestock farms had history of relapses of fever, decreased milk production, tick infestation and sudden death. The disease manifestations observed clinically in five animals included high fever, swelling of sub mandibular and sub scapular lymph nodes, weakness, increased respiration and pulse. Anorexia, loss of condition and rough hair coat were also observed. Neurologic sign of in coordination was also seen in three weak animals. Signs of lacrimation, pale conjunctiva, diarrhoea, dyspnea and frothy nasal discharge were observed in only one animal. Clinically nine animals showed signs of haemoglobinuria. The 100 animals in this group were designated as clinically “diseased” animals i.e exposed to ticks. For polymerase chain reaction test, 3 ml each, blood sample were collected in EDTA coated vacutainers. The blood smears were also prepared and stained with Giemsa’s stain according to method described by Benjamin, (1986). Twenty blood samples and blood smears were collected from Friesian animals (non-diseased), monitored for a period of one month by taking daily rectal temperature and blood smears examination and without any ectoparasites infestation.
Polymerase chain reaction tes
Total DNA was extracted from samples with the help of DNA Extraction Kit (PUREGENE® USA, GENTRA) according to prescribed method. Analysis of extracted DNA was made by spectrophotometer and agarose gel electrophoresis based analysis.
-
1.
Spectrophotometric analysis: For this purpose 10 ul of extracted DNA was mixed with 99 ul of autoclaved distilled water. Ratio between 260 nm and 280 nm wavelength was calculated to analyze the quality of extracted DNA.
-
2.
Agarose gel electrophoresis based analysis: 0.7% agarose gel was used for this purpose.
The Primers used in the present study are describe in Table 1. PCR analysis of the samples was done with two concentration of MgCl2, 5 ul & 6 ul for T.annulata while for B. bovis and B. bigemina concentration of MgCl2, was 3 ul. Amplification was done with the help of thermal cycler set for 30 cycles at following cycling conditions. Samples were denatured at 94°C for 5 min., 94°C for 30 sec. Temperature was lowered for several minutes to allow both forward and backward (right or left) primers to anneal with the complementary sequences. At this stage three conditions 50°C, 55°C and 60°C for 30 seconds were checked for each primer set. For extension temperature was raised to 72°C for 45 seconds and completed with 72°C for 7 min. Analysis of amplified product by electrophoresis was done with a 1% agarose gel. The results were photographed.
Estimation of blood parameters of all animals was done by hematology analyzer.
Survey of vectors
Collection of ticks
A systematic survey of various species of ticks occurring at selected livestock farms was conducted by collecting 100 specimen of ticks randomly from Friesian cattle, 5 ticks from each farm. Specimen were brought to the laboratory of Department of Clinical Medicine and Surgery, University of veterinary and Animal Sciences, Lahore and identified according to keys described by Soulsby 1982.
Results
Vector
Out of total 100 tick specimen the prevalence of Hyalomma tick was highest (15%) followed by Boophilus (12%), Haemaphysalis (5%) and Rhipicephalus (3%).
Blood smear
Erythrocytes on the blood films were examined for intracellular piroplasms of the theileria and Babesia species. The blood films with theileria piroplasms, included cocci, and signet-ring forms with diameter of 0.5–1.5 micrometer. The babesia species were identified as B. bigemina with paired structure at an acute angle to each other with the dimensions of 3-3.5 × 1–1.5 um while incase of B. bovis the paired structure forms were at an obtuse angle to each other with dimension of 1-1.5 X 0.5–1.0 um. Abnormalities in erythrocyte structure were also observed in both theileria and babesia positive smears which included anisocytosis, poiklocytosis, basophilic stippling and presence of reticulocytes (Figure 1).
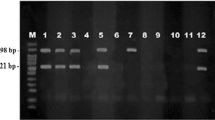
The blood smear examination showed 21 %( 21/100) samples positive for blood parasites. Out of these 66.6 %( 14/ 21) samples were positive for theileria while 42.8% (9/21) were positive for babesiosis. It was also recorded that 66.66% (6/9) samples were positive for B. bigemina 33.33% (3/9) were positive for B. bovis. The results showed that blood smear examination gave 25% false positive and 79 % false negative results. The sensitivity of blood smear examination was 21% while specificity was 75%. The positive predictive value recorded for blood smear examination was 80.77% while negative predictive value was 15.96 % (Table 2). Amplified DNA of both theileria and T. annulata is shown in Fig. 2.
Blood parameters
The results of complete blood cell count (CBC) for a samples collected from infected animals showed regenerative anaemia, lymphocytosis, monocytosis. In samples collected after 20 days, CBC results revealed increased MCV, MCH values and slightly decreased MCHC value indicating persistence of macrocytic hypochromic anaemia and increased values of WBC’s indicating enhanced inflammatory response. The result of blood parameters of total erythrocyte count, total leukocyte count, packed cell volume and hemoglobin showed significant decrease in affected cattle.
PCR
Data recorded on ten DNA extracted samples by spectrophotometeric analysis showed the ratio between 260 nm and 280 nm in the range of 1.6–2.2. It was found out that 6 ul of MgCl2 gave successful results than 5 ul concentration for T. annulata while for Babesia 3 ul gave successful results. It was found out that primers set A (T.annulata specific) anneal at Tm 55°C while Primer set B (Theileria specific) anneal at Tm 60°C. Primer set C (B. bovis specific) and D (B. bigemina specific) anneal at Tm.50°C. The amplified product was photographed as shown in 2. Primer set A amplified the expected 721-bp fragment while primer set B amplified at 1098 bp. The primers set C and D amplified the expected 541 bp and 1124 bp band. The specificity of the amplified DNA fragment was confirmed by positive control. The results showed that 60% (60/100) samples were positive for blood parasites by PCR test. Out of these 60 %(36/60) were positive for T. annulata. 33.33 %(20/60) were positive for babesia species out of which 13/20 (65%) samples were positive with B. bigemina while 7/20 (35%) samples were positive for B. bovis.The results showed that PCR test gave 15% false positive with 40% false negative result. The sensitivity of PCR test was 60% while specificity was 85%.The positive predictive value recorded for PCR test was 95.2% while negative predictive value was 29.8% (Table 3). It was found also out that blood samples with 0.00040% parasitemia, corresponding to 20 parasites per ml were detected.
Statistical analysis
The differences in sensitivity and specificity of tests were found to be statistically significant (P < 0.01) by applying Randomized Complete Block Design (RCBD) (Sher 1992).
Discussion
Theileriosis and Babesiosis are the pasture infections and linked to vector tick. The prevalence of theileriosis depends upon the dynamics of tick population. During the present study the prevalence of Hyalomma tick was highest (15%) followed by Boophilus (12%).Similar observations were made by Khan et al., (1996) who recorded tick prevalence of 28.2% (1269/4500) in cattle and 14.7% (662/4500) in buffaloes in Faisalabad district, Pakistan. They reported seven species of ticks i.e Rhipicephalus sanguineus, Boophilus microplus, B.annulatus, Hyalomma a. anatolicum, H.marginatum marginatum, Hyalomma aegyptium and Dermacentor. The incidence of ticks in Pakistan is also reported by Ali, (1986) who reported Hyalomma aegyptium, Hyalomma a a.anatolicum, Boophilus microplus and Hypoderma lineatum in cattle in Northern areas of Pakistan. Loria et al. (1996) also reported that Hyalomma marginatum and Boophilus annulatus were the most common ticks collected in April and November 1998 respectively.
CBC results revealed the persistence of macrocytic hypochromic anaemia and an enhanced inflammatory response. Abnormalities in erythrocyte structure were present including anisocytosis, poikolicytosis, basophilic stippling and presence of reticulocytes. The findings are in accordance with findings of Conrad et al., (2000) who reported macrocytic normochromic anaemia with acanthocytosis and spherocytosis. Similar results were reported by Dhar and Gautam (1979), Obi and Anosa (1980), Rajan and Nagarajan (1980), Mishera et al. (1983). Goddeeris (2004) also recorded significant decrease in total leukocyte count, red blood cell count, hemoglobin concentration and packed cell volume. Friedhoff (1999) reported general anaemia and gradual fall in total erythrocyte count, packed cell volume and hemoglobin concentration and significant increase in total leukocyte count in cross bred calves infected with blood parasites.
The clinical manifestations reported in the present study are in accordance with the findings of Srivastava et al., (2003) who also reported cerebral involvement. The first symptom of fever manifested by the animal in theileriosis was in accordance with the findings of Manickan and Dhar (1984). Gill et al., (1977), Levine (1985) but Friedhoff (1999) reported swelling of lymph nodes as the first sign in all calves along with lacrimal and nasal secretions. Salama and Gaabarya (2007) reported eye lesions which were not observed in the present study.
The results regarding efficacy of diagnostic test applied in present study is supported by the findings of Shiels et al. (1994) who reported that diagnosis of clinical T. annulata infection in cattle is usually based on the detection of macroschizonts in Giemsa’s-stained lymph node biopsy smears. After recovery, a long-lasting carrier state occurs, in which low numbers of erythrocytes remain infected with Theileria piroplasms. In the present study the efficacy of blood smear examination was less than PCR test. Similar findings were made by Irvin 1987 who reported that it was difficult to differentiate theileria and babesia species solely on the basis of the morphology, developmental stages and confusion may arise if mixed infections occur. Under field conditions, it is important to be able to distinguish infections caused by T. annulata from those caused by B.bovis which occur worldwide. Bishop et al. (1993) was also of the view that geographic distribution and vector specificity may help in the identification of a theileria & babesia species, and in most cases, it is necessary to use a combination of methods. With the availability of sequenced parasite genes and PCR, it is possible to detect parasites within samples of blood. The lowest detection limit of the PCR was two to three parasites per ml of infected blood, which corresponds with a parasitemia of 0.000048% (Christine et al. 1995) while our results demonstrate that PCR assay detects parasitemia of 0.000040% in carrier cattle.
References
Ali, W. F. (1986). Incidence and chemotherapy of ectoparasites of cattle, sheep, goat and poultry in Northern Areas. Vet. Parasitol, 67(4):125–131.
Allsopp, B. A., H. A. Baylis, M. T. E. P. Smith, R. P. Bishop (1993). Discrimination between six species of Theileria using oligonucleotide probes which detect small subunit ribosomal RNA sequences. Parasitology 107(2):157–165
Benjamin, M. M. (1986). Outline of veterinary clinical Pathology, 3rd Edition, The Iwoa State Uni.press, Ames, Iwoa, USA:7–8, 29–30.
Bishop, D. M. Carrington, B. Sohanpal, and P. Spooner. 1993. Discrimination between six species of Theileria using oligonucleotide probes which detect small subunit ribosomal RNA sequences. Parasitology 107:157–165.
Christine D.O., I. M.V.D. Weide, I. Miguel, A. Habela, P. Jacquiet, and F. Jongejan. (1995). Detection of Theileria annulata in blood samples of carrier cattle by PCR. J. Clinical Microbiol. 33(10). 2665–2669
Conrad, P. A., K. Iams, W. C. Brown, B. Sohanpal and O. K. ole-MoiYoi (2000). DNA probes detect genomic diversity in Theileria parva stocks. Mol. Biochem. Parasitol. 25:213–226.
Dhar, S. and O.P. Gautam (1979) Serum protein in experimental Theileria annulata infection of cattle. Ind. J. Animal. Sci., 49(7);511–516.
Fahrimal, Y., W. L. Goff, and D. P. Jasmer. 1992. Detection of Babesia bovis carrier cattle by using polymerase chain reaction amplification of parasite DNA. J. Clin. Microbiol. 30:1374–1379.
Friedhoff, K.T. (1999) Clinical and haematological infestations of T.annulata infection in cattle. Tropenmed Parasit., 32: 227–233.
Gill B. S,Y. Bhattacharyulu and D. Kaur (1977). Symptoms and pathology of experimental bovine tropical theileriosis (Theileria annulata infection). Ann. Parasitol. Hum. Com., 52(6):592–608.
Goddeeries, B.M. (2004). Theileria annulata in cattle, Characterization of infected lymphocytes and the immune response they provoke. Vet. Immun. Immuno. Path. 20:213–217.
Guido, F.C.L, P.S. Angela, H.L. Liyod and R.M. Claudio (2002) Assessment of primers designed from small ribosomal subunits RNA for specific discrimination between Babesia bigemina and Babesia bovis by PCR. Ciencia Animal Brasileria V. 3(2):27–32.
Irvin, A. D. 1987. Characterization of species and strains of Theileria. Adv. Parasitol. 26:145–197.
Khan, N.M., C.S. Hayat, Z. Iqbal, B. Hayat and A. Naseem (1996). Prevalence of ticks on Livestock in Faisalabad, Pak. Vet. J., 13(4):182–184.
Levine, N.D., 1985. Veterinary Protozoology. Iowa State University Press, Ames.
Loria GR, S. Riili, F.Vitale, A. Greco, O. Sparagano (1996). Clinical and laboratory studies on Theileriosis outbreak in Sicily, Italy. Vet Parasitol. 1996 Oct 25;65(3–4):199–211
Manickan, R. and S.S. Dhar (1984). Blood histamine level in acute tHeileria annulata infection of calves. Ind. Vet. J. 61(3):192–194.
Mishera, K.C., K.C. Achar and M.D. Lama (1983) Efficacy of some chemotherapeutic agents against clinical Theileria annulata infection in exotic cattle. Ind. Vet. J., 60(4):313–318.
Neitz, W. O. 1957. Theileriosis, gonderioses and cytauxzoonoses: a review. Onderstepoort J. Vet. Res. 27:275–430.
Obi, T.U. and V.O. Anosa (1980) Haematological studies of domestic animals in Nigeria. Trypanosomiasis, Theileriosis,Anaplasmosis, Erythrozoonosis and Helminthiasis Zentralblatt fur Veterinary Medizin, 27 (9):789–797.
Oliveira, C., and F. Jongejan. (2005). PCR based detection of Babesia bovis and Babesia bigemina in their natural host Boophilus microplus and cattle. Int. J. Parasitol., 35(1):105–111.
Radostitis, O.M.C. Blood, C.C.Gay and K.W. Hinchcliff (2000).Veterin ary Medicine, 9th Edition. W.B. Saunders, London, Pp:1289–1296.
Rajan, T.S.S. and V.V. Nagarajan (1980) Blood transfusion in cases of tropical theileriosis in cows. J. Vet. Sci. 9(1):60–62.
Saiki, R. K., D. H. Gelfand, S. Stoffel, S. J. Scharf, R. Higuchi, G. T. Horn,K. B. Mullis, and H. A. Erlich. 1988. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 239:487–491.
Salama A. O. and M. H. A. Gaabarya (2007). Clinical, haematological and therapeutic studies on tropical theileriosis in water buffaloes (Bubalus bubalis) in Egypt. Vet. Parasitol. 146(3–4):337–340.
Sher M.C., (1992) Introduction to statistical theory Part 2.5th Edition.Markazi Kutub Khana, Urdu Bazar, Lahore.
Shiels, B. R., A. Smyth, J. Dickson, S. McKellar, L. Tetley, K. Fujisaki, B.Hutchinson, and J. H. Kinnaird. 1994. A stoichiometric model of stage differentiation in the protozoan parasite Theileria annulata. Mol. Cell. Differ. 2:101–125.
Soulsby, E.J.L. (1982). Helminths, Arthropods and Protozoa of domesticated animals. 7th Edition. Baillier Tindall and Cassel Ltd. London.
Srivastava, C., N. M. Khan, J. W. Tyler (2003). Study of the host range and incidence of Hyalomma dromedarii Report by Division of Parasitology, Indian Veterinary Research Institue, Izatnagar. 24(3): 1–22
Susan, E.A. and M. Asa (1999). The Merk Veterinary Manual, 8th Edition. Merk & Co. Inc. Pp:23–25.
Uilenberg, G. 1981. Theilerial species of domestic livestock, p. 4–37. In A. D.Irvin, M. P. Cunningham, and A. S. Young (ed.), Advances in the control of theileriosis. Martinus Nijhoff, The Hague, The Netherlands.
Acknowledgments
This work was funded and supported by the Higher Education Commission, Government of Pakistan in connection with the research project titled “Epidemiology of Theileriosis in Bovine”. The authors are also thankful to Nestlé, Pakistan limited for extending support in the collection of blood samples from Nestlé’s Dairy farms.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Durrani, A.Z., Kamal, N. Identification of ticks and detection of blood protozoa in friesian cattle by polmerase chain reacton test and estimation of blood parameters in district Kasur, Pakistan. Trop Anim Health Prod 40, 441–447 (2008). https://doi.org/10.1007/s11250-007-9117-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11250-007-9117-y