Abstract
The effects of n-pentanol vapor on friction and wear of hydrogenated diamond-like carbon (DLC) films during sliding against a 440C stainless steel (SS) ball were investigated with a reciprocating pin-on-disc tribometer. Under dry sliding conditions, the friction coefficient is initially high (>0.2) for a so-called run-in period and then gradually subsequently decreases to an ultra-low value (<0.025). During the run-in period, a carbon transfer film is formed on the SS ball side, which seems to be the key for the ultra-low friction behavior. In n-pentanol vapor environments, the friction coefficient remained nearly constant at ~0.15 throughout the entire test cycles without any noticeable run-in period. Although the friction coefficient is high, there is no visible wear on rubbing surfaces when examined by optical microscopy, and the transfer film forming tendency on the SS ball side was much reduced. In humid environments, the wear prevention effect is not observed and transfer films do form on the ball side. These results imply that the n-pentanol layer adsorbed on DLC film from the vapor phase provides a molecularly thin lubrication layer which can prevent the substrate from wear.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Diamond-like carbon (DLC) is one of the most widely tested hard coatings for wear prevention and lubrication [1]. DLC films with certain structures and hydrogen contents are known to provide a desirable level of high hardness and low friction [2]. Thus, it can be used as a solid lubricant film for a large variety of applications under conditions where utilization of liquid lubricants is neither suitable nor appropriate. However, there are several challenges that have to be resolved in order for these films to be practical in a variety of applications. One of them is the environmental sensitivity of the friction and wear behavior of all DLC films. For example, the friction coefficients lower than 10−2 are observed only in ultra-high vacuum or dry nitrogen environments for highly hydrogenated DLC films [3–7].
In the literature, the friction coefficients of hydrogenated DLC films with self-mated interfaces (DLC against DLC) are reported to vary over wide ranges depending on the test environments [8–12]. They typically span a range of 0.007–0.02 in vacuum or dry nitrogen environments and 0.1–0.4 in ambient air [3–12]. The large spread in the friction coefficient values are mainly due to variations in the structure and composition of the films. As one may expect, the consequence of high friction in ambient air is accompanied by wear of the DLC surface. In contrast, the ultra-low friction coefficient in vacuum or dry nitrogen environments results in an extremely low-wear rate of the DLC film. However, the ultra-low friction coefficient is observed after an initial induction period (or called a run-in period) during which the friction coefficient is high and gradually decreases to a low value as the friction test cycle repeats [13]. It is believed that the surface contaminants or the layers with sub-optimum compositions are removed during the induction or run-in period [13].
When the friction is measured with a counterface made of different material (such as oxide, metals, nitride) sliding against the DLC surface, the induction or run-in period is inevitably observed before the sliding interface enters the steady-state low-friction and low-wear regime [14]. When the friction coefficient is measured in inert environments (vacuum or dry nitrogen), it is initially high (typically higher than 0.2) and then gradually decreased to an ultra-low value (less than 0.05). When the ball surface is analyzed after friction test, there is almost always a carbon transfer film in and around the contact area of the counter-surface [15]. Once the carbon transfer film is formed on the counter-surface, then the friction coefficient and the wear rate are very low. So, it is generally accepted that the transfer film formation is inevitable and necessary to attain low friction and low wear for foreign materials rubbing against DLC. But, it should be noted that the transfer film formation on the counter-surface of foreign materials is the consequence of the initial small wear of the DLC surface.
This article reports an environmental effect that defies this commonly observed friction and wear behaviors of the DLC film in atmospheric conditions. The alcohol vapor-phase lubrication (VPL) effect was studied for a stainless steel ball (SS440C grade) sliding against two types of DLC films—one is highly hydrogenated (atomic hydrogen content = ~40%; previously noted as NFC-6) and the other mildly hydrogenated (atomic hydrogen content = ~25%; noted as NFC-10) [16]. In an Ar environment containing n-pentanol vapor, it was observed that the wear and transfer film formation of the DLC surface is remarkably suppressed although the friction coefficient is not ultra-low (<0.05) but in the vicinity of ~0.15. In the n-pentanol vapor environment, the initial change in friction coefficient is insignificant. These results are contrast to the friction and wear behaviors of the SS440C/DLC interface in the dry and humid argon environments.
2 Experimental Details
Two types of DLC films were used in this study: mildly hydrogenated sample (atomic H percent =~25%; NFC-10) and highly hydrogenated sample (atomic H percent = ~40%; NFC-6). The DLC films were prepared by plasma-enhanced chemical vapor deposition at using a self-bias voltage of −500 V and a chamber pressure of ~10 mTorr. The source gases used for NFC-10 and NFC-6 were C2H2 and a mixture of 25% CH4 and 75% H2, respectively. The deposition procedure has been described previously in Refs. [1, 7] and will not be elaborated further here. The deposited films consisted of an approximately 1-μm thick DLC layer deposited onto a ~100-nm thick Si bond layer which was previously deposited onto clean Si wafers. The roughness of the DLC film surfaces was measured to be ~3.1 nm for NFC-6 and ~4.8 nm for NFC-10 nm with optical profilometry. The sliding counterface was a 440C grade stainless steel (SS440C) ball purchased from TRD Specialties Inc. The ball diameter was 3 mm and the surface roughness was measured to be ~30 nm with optical profilometry. The DLC films and the balls were cleaned with ethanol prior to tribological testing.
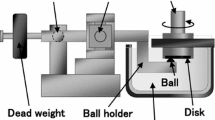
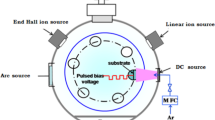
An environment-controlled linear-motion pin-on-disc tribometer was used to measure friction coefficient for sliding the SS ball on the DLC surface at an applied normal load of 1 N and a sliding speed of 2 mm/s. The maximum Hertzian contact pressure and diameter are calculated to be 0.63 GPa and ~45 μm, respectively. The lateral force exerted to the pin during the slide was measured with a strain gauge sensor. The strain gauge sensor was calibrated by placing known weights on top and measuring the voltage signal corresponding to the given load. The friction coefficient was calculated using the Amonton’s law (friction force = friction coefficient × normal load). The friction coefficient was measured for a bi-directional linear motion and averaged over 200 data points per cycle. The wear of the substrate and transfer films on the ball were imaged with an optical microscope. The sample was kept inside a continuous flow vapor chamber. The ball was in contact with the substrate and slid through an opening in the upper cap. When the ball was engaged on the substrate surface, the opening for the vapor vent was ~0.8 cm2. An ultra-high purity Ar gas (O2 < 8 ppm, H2O < 10 ppm) was used as a carrier gas. The gas flow rate was 3 L/min. The Ar gas streams with n-pentanol vapor and 40% relative humidity were produced by the method described in previous literatures [17, 18].
The water and n-pentanol adsorption isotherm thickness was measured with ellipsometry (Ellipsotechnology, wavelength = 632.8 nm, incidence angle = 70°). Initially the refractive index of the DLC substrate was modeled to account for its initial Δ and Ψ angles. The refractive index of DLC was determined to be n = 2.70 ± 0.12 and k = 0.26 ± 0.04. The adsorption isotherm thicknesses were determined by first measuring the changes in the Δ and Ψ angles as a function of relative partial pressures of both water and n-pentanol. Then, these changes in Δ and Ψ were modeled mathematically to calculate the effective adsorption isotherm thicknesses.
3 Results and Discussion
The effects of alcohol vapor in the Ar environment can be seen most drastically in comparison of the friction and wear behaviors of the mildly hydrogenated DLC film (NFC-10). Figure 1a displays the friction coefficient changes over sliding cycles in dry Ar gas and the Ar gas containing n-pentanol vapor with varying partial pressures relative to the saturation vapor pressure (p/p sat = 0.1, 0.4 and 0.8; p sat at 25°C = 2.2 Torr). In the dry Ar condition, the friction coefficient measured with a SS440C ball is initially at 0.15–0.2 and then gradually decreases to <0.025 over ~30 cycles. After that the friction coefficient remains low with some small fluctuations. After 400 cycles of sliding, some wear debris can be found at the end of the sliding track on the substrate (Fig. 1b) and a significant amount of the carbon transfer film can be seen on the SS440C ball surface (Fig. 1c), which is consistent with the previous report [14, 15].
The diameter of the transfer film area is ~60 μm, which is much larger than the Hertzian contact diameter 45 μm (marked with a dotted circle). This implies that during the friction test, the DLC surface is worn to yield a contact area larger than the initial Hertzian contact area between the pristine DLC film and the ball surface. Although small and faint, the wear on the DLC substrate could be seen visually with an optical microscopy (Fig. 1b). The wear of the DLC surface occurs mostly at the beginning of the friction test. The transfer film diameter measured after 10 cycles of sliding is the same, within the experimental error range, as that measured after 400 cycles. Once the carbon transfer film is fully formed on the SS440C ball, then the shear plane is now between low-friction carbon surfaces, not between the nascent steel and DLC surfaces. No growth of the transfer film diameter after first few cycles implies that the wear of DLC is extremely small after the completion of the transfer film formation on the ball surface.
The friction behavior of the same mildly hydrogenated DLC film (NFC-10) in the alcohol-vapor-containing Ar environment is quite different from the behavior observed in the dry case (Fig. 1a). In the n-pentanol vapor environment, the changes during the induction (run-in) period are negligible. The friction coefficient starts at ~0.13 and decreased to ~ 0.1 after a few cycles, but it gradually recovers to and maintains at ~0.13 after about 30 cycles. The friction does not seem dependent strongly on the n-pentanol vapor pressure. More surprisingly, the carbon transfer film formed on the SS440C ball surface (Fig. 1c) as well as the substrate wear (Fig. 1b) are significantly reduced although the average friction coefficient of the entire sliding cycles is much higher than the case tested in the dry Ar environment. The diameter of the area in which small patches of carbon transfer films can be found is not larger than the Hertzian contact diameter. The transfer film does not cover the entire contact area completely; the ball surface can be seen inside the transfer film area even after 400 cycles of sliding. The wear mark on the DLC film is barely found and negligible compared to the dry Ar environment case.
Figure 2 compares the friction coefficient as a function of cycles for the SS440C ball sliding against the highly hydrogenated DLC film (NFC-6) in dry and n-pentanol-contacting Ar environments. Once again, in the dry condition, the friction coefficient is initially high (0.2 ~ 0.25) and then decreases to <0.025 after ~40 cycles of sliding. Unlike NFC-10, the wear mark on the substrate is barely visible. Although small, the carbon transfer film on the ball surface can be clearly seen in the optical microscopy picture. The size of the transfer film is exactly the same as the Hertzian contact diameter. As in the case of the mildly hydrogenated DLC film (NFC-10), the transfer film is formed during the initial run-in period. The size of the transfer film on the ball surface does not grow with repetition cycles after the initial run-in period. When the same friction experiment is carried out in the n-pentanol vapor, the measured averaged friction coefficient is much higher; but the transfer film formation on the ball is negligible. Only a faint rim of the contact area can be observable in the optical microscope images. Inside this rim, the ball surface looks very clean and not much different from the outside region. It is very hard to find the wear track on the DLC film under optical microscopy. The wear of the DLC surface appears to be completely suppressed for the highly hydrogenated film (NFC-6) in the n-pentanol vapor environment.
The friction coefficient of the highly hydrogenated DLC film (NFC-6) is somewhat higher (~ 0.185) at p/p sat = 0.1 and then decreases to ~0.16 at p/p sat = 0.4 and ~0.13 at p/p sat = 0.8. The vapor partial pressure dependence of friction coefficient is not fully understood yet, but it seems to vary depending on the surface roughness. The mildly hydrogenated DLC film surface is somewhat rougher than the highly hydrogenated DLC film surface. This could also be related to incomplete suppression of the transfer film for the mildly hydrogenated DLC film. The difference in surface chemistry or film structure (for example, carbon sp2/sp3 ratio) between these two DLC films could influence the partial pressure dependence.
The steady-state friction coefficient measured in the alcohol vapor does not seem to be affected by the initial surface conditions (Fig. 3). When the 40% p/p sat n-pentanol vapor is introduced after the run-in period of the dry friction test (during which the carbon transfer film is formed on the SS440C ball surface), the friction coefficient increases quickly and then gradually approaches ~0.16, which is the same as the value measured for the clean SS440C ball test in the same pressure of n-pentanol vapor (Fig. 2a). The initial quick rise must be due to the adsorption of alcohol molecules at the sliding interface, and the subsequent gradual increase is believed to be due to some tribochemical reactions. Upon removal of the n-pentanol vapor and switching to the dry environment, the friction coefficient increases slightly and then decreases gradually to an asymptotic value which is higher than the initial steady-state value observed in the dry condition. The reason that the asymptotic value of the friction coefficient is higher than the initial dry value must be due to the presence of some residual tribochemical reaction products, formed during the sliding in the alcohol vapor environment, in the sliding track. After four cycles of vapor changes (total 2000 sliding runs), the ball surface is covered with some deposits that do not look like black carbon (Fig. 3b). Similar deposits are also observed at both ends of the wear track on the DLC substrate (Fig. 3b). The chemical nature of these deposits is not fully analyzed yet and will be the subject of future study.
It is important to note that the wear prevention effect of the alcohol vapor is different from the effect of humidity. Figure 4 exhibits the friction coefficients in the humid Ar environment (RH = 40%) and the images of the transfer film on the ball taken after 400 cycles of sliding. Similar to the dry case, the humid environment shows the run-in period behavior. The friction coefficient is initially high and then decreases slowly to a steady-state value. However, the decrease in the friction coefficient is not as drastic as the dry case and the induction period is much longer. For the SS440C ball sliding against the mildly hydrogenated DLC film (NFC-10), the friction coefficient is initially ~0.22 and then decreases to ~0.15 over ~100 cycles. The value is still decreasing slowly even after 200 cycles. In the case of the highly hydrogenated DLC film (NFC-6), the friction coefficient is initially ~0.27 and then drops to ~0.08 over ~60 cycles. These results indicate that the sliding interface conditions are gradually changing as the test cycles are repeated.
The morphology of the transfer films (wear products) formed on the ball in the humid environment is quite different from that observed in the dry case. The ball rubbed against the mildly hydrogenated DLC film (NFC-10) has a fluid-like deposit, rather than solid carbons. On the ball rubbed against the highly hydrogenated DLC film (NFC-6), there are some black marks along the periphery of the contact area. They are deposited mostly at the front and tailing edges of the sliding direction, instead of being accumulated inside the contact region. These results clearly show that the water vapor adsorption cannot prevent the wear of the DLC substrate while the alcohol vapor adsorption can do.
The vapor is simply a means to deliver lubricant molecules to the surface and keep a certain thickness of the lubricant molecules at the surface. The thickness of the adsorbed layer measured with in situ ellipsometry for n-pentanol and water is shown in Fig. 5. Both n-pentanol and water show the type-II adsorption isotherm behavior in which the adsorbate layer thickness increases fast as the vapor p/p sat rises from zero, increases slowly in the intermediate p/p sat range, and then finally increases fast again as p/p sat approaches unity. In the case of n-pentanol, the adsorbate layer thickness is about one monolayer for p/p sat = 0.1 and 0.4 and a slightly thicker than one monolayer at p/p sat = 0.8 [19]. Since the size of individual water molecule is ~0.3 nm, the data shown in Fig. 5b indicates that at RH = 40% (p/p sat = 0.4), there is at least 2–3 layers of water molecules on the DLC surface [18, 20]. So, the poor wear prevention performance of water is not due to the lack of the adsorbed molecules. The difference between the n-pentanol and water vapor lubrications must be due to the difference in their chemical natures.
Some plausible explanations for the environmental effect can be found in the literature. The dangling bonds created at the carbon surface due to friction and wear readily react with other atoms at the counter-face or molecules impinging from the gas phase [21, 22]. Unless these dangling bonds are properly passivated, the carbon materials will show high friction and be highly susceptible to wear. For example, diamond and graphite show high friction coefficients in vacuum due to carbon–carbon bond formation between two sliding surfaces [23, 24]. Their friction coefficients decrease to ~0.1 in ambient conditions due to adsorption and reaction of water from the gas phase [23, 24]. The highly hydrogenated DLC film shows an ultra-low friction coefficient after the run-in period in vacuum, but its friction coefficient increases to ~0.1 in ambient condition again due to adsorption and reaction of water from the gas phase [12]. The ultra-low friction after the run-in period must be because the hydrogen-terminated carbon surface structure is assumed on both the DLC substrate and the transfer film on the ball [21]. During the run-in period, the surface contaminants or the oxidized layers formed due to air exposure are removed.
The good alcohol VPL efficiency for wear prevention and negligible run-in period implies that the alcohol molecules readily adsorb and modify the DLC surface. An X-ray photoelectron spectroscopy analysis finds that the DLC surface is oxidized before the friction test begins. Once the oxidized carbon surface is fully covered with the adsorbed alcohol layer (or chemisorbed alkoxide molecules at some surface sites), then the shear stress of the interface mediated with alcohol molecules seem to be low enough to prevent the rupture of the underlying carbon surface. As long as the vapor pressure of alcohol is kept above a certain level, the substrate surface is always fully covered with the adsorbate molecules (Fig. 5). It should be noted that the friction coefficient of most self-assembled monolayers (SAMs) of organic molecules on solid surfaces exhibits a friction coefficient of 0.1–0.2 [25–28], which is comparable to the friction coefficient observed for the n-pentanol vapor lubricated SS440C/DLC interface. The alcohol VPL of the SiO2 surface also shows a friction coefficient of ~0.15 [29–32]. These comparisons of the friction coefficient values imply that the SS440C/DLC interface is well lubricated with the adsorbed alcohol molecules so that the DLC wear is suppressed although the friction coefficient is not ultra-low.
4 Conclusion
The environmental effects on the friction and wear behaviors of hydrogenated DLC films rubbed with a stainless steel ball were studied in dry Ar, n-pentanol vapor, and humid conditions. In dry Ar conditions, the DLC samples exposed and cleaned in air always show a run-in period during which the friction coefficient decreases from an initial high value (>0.2) to an ultra-low value (<0.025), and the carbon transfer film is formed on the ball surface. This run-in period is suppressed in the presence of the n-pentanol vapor in the ambient. In the n-pentanol vapor environment, the friction coefficient is not ultra-low (~0.15), but the wear of the DLC film is significantly suppressed. The water adsorption cannot provide the wear prevention effect observed for the n-pentanol vapor, although the total adsorbate thicknesses were similar and the friction coefficient in the humid environment is lower than that in the n-pentanol vapor environment. There seems to be different tribochemical effects for water and n-pentanol molecules on DLC.
References
Erdemir, A., Eryilmaz, O.L., Nilufer, I.B., Fenske, G.R.: Synthesis of superlow-friction carbon films from highly hydrogenated methane plasmas. Surf. Coat Technol. 133, 448–454 (2000)
Robertson, J.: Diamond-like amorphous carbon. Mater. Sci. Eng. R 37, 129–281 (2002)
Grill, A.: Review of the tribology of diamond-like carbon. Wear 168, 143–153 (1993)
Grill, A.: Tribology of diamondlike carbon and related materials: an updated review. Surf. Coat Technol. 94–95, 507–513 (1997)
Grill, A.: Diamond-like carbon: state of the art. Diamond Relat. Mater. 8, 428–434 (1999)
Erdemir, A., Donnet, C.: Tribology of diamond-like carbon films: recent progress and future prospects. J. Phys. D Appl. Phys. 39, R311–R327 (2006)
Eryilmaz, O.L., Erdemir, A.: Surface analytical investigation of nearly-frictionless carbon films after tests in dry and humid nitrogen. Surf. Coat Technol. 201, 7401–7407 (2007)
Donnet, C., Grill, A.: Friction control of diamond-like carbon coatings. Surf. Coat Technol. 94–5, 456–462 (1997)
Ronkainen, H., Koskinen, J., Likonen, J., Varjus, S., Vihersalo, J.: Characterization of wear surfaces in dry sliding of steel and alumina on hydrogenated and hydrogen-free carbon films. Diamond Relat. Mater. 3, 1329–1336 (1994)
Voevodin, A.A., Donley, M.S., Zabinski, J.S.: Pulsed laser deposition of diamond-like carbon wear protective coatings: a review. Surf. Coat Technol. 92, 42–49 (1997)
Harris, S.J., Weiner, A.M., Meng, W.J.: Tribology of metal-containing diamond-like carbon coatings. Wear 211, 208–217 (1997)
Kim, H.I., Lince, J.R., Eryilmaz, O.L., Erdemir, A.: Environmental effects on the friction of hydrogenated DLC films. Tribol. Lett. 21, 53–58 (2006)
Eryilmaz, O.L., Erdemir, A.: Investigation of initial and steady-state sliding behavior of a nearly frictionless carbon film by imaging 2-and 3-D TOF-SIMS. Tribol. Lett. 28, 241–249 (2007)
Erdemir, A., Bindal, C., Pagan, J., Wilbur, P.: Characterization of transfer layers on steel surfaces sliding against diamond-like hydrocarbon films in dry nitrogen. Surf. Coat Technol. 77, 559–563 (1995)
Erdemir, A., Bindal, C., Fenske, G.R., Zuiker, C., Wilbur, P.: Characterization of transfer layers forming on surfaces sliding against diamond-like carbon. Surf. Coat Technol. 86–7, 692–697 (1996)
Liu, A. C. Y., Arenal, R., Miller, D. J., Chen, X. D., Johnson, J. A., Eryilmaz, O. L., Erdemir, A., Woodford, J. B.: Structural order in near-frictionless hydrogenated diamondlike carbon films probed at three length scales via transmission electron microscopy. Phys. Rev. B: Condens. Matter 75:205402 (2007)
Strawhecker, K., Asay, D.B., McKinney, J., Kim, S.H.: Reduction of adhesion and friction of silicon oxide surface in the presence of n-propanol vapor in the gas phase. Tribol. Lett. 19, 17–21 (2005)
Asay, D.B., Kim, S.H.: Evolution of the adsorbed water layer structure on silicon oxide at room temperature. J. Phys. Chem. B 109, 16760–16763 (2005)
Barnette, A.L., Asay, D.B., Janik, M.J., Kim, S.H.: Adsorption isotherm and orientation of alcohols on hydrophilic SiO2 under ambient conditions. J. Phys. Chem. B 113, 10632–10641 (2009)
Asay, D.B., Barnette, A.L., Kim, S.H.: Effects of surface chemistry on structure and thermodynamics of water layers at solid-vapor interfaces. J. Phys. Chem. B 113, 2128–2133 (2009)
Erdemir, A.: The role of hydrogen in tribological properties of diamond-like carbon films. Surf. Coat. Technol. 146, 292–297 (2001)
Eryilmaz, O.L., Erdemir, A.: On the hydrogen lubrication mechanism(s) of DLC films: an imaging TOF-SIMS study. Surf. Coat Technol. 203, 750–755 (2008)
Yen, B.K.: Influence of water vapor and oxygen on the tribology of carbon materials with sp(2) valence configuration. Wear 192, 208–215 (1996)
Chandrasekar, S., Bhushan, B.: The role of environment in the friction of diamond for magnetic recording head applications. Wear 153, 79–89 (1992)
Hsiao, E., Kim, D., Kim, S.H.: Effects of ionic side groups attached to polydimethylsiloxanes on lubrication of silicon oxide surfaces. Langmuir 25, 9814–9823 (2009)
Sambasivan, S., Hsieh, S., Fischer, D.A., Hsu, S.M.: Effect of self-assembled monolayer film order on nanofriction. J. Vac. Sci. Technol. A 24, 1484–1488 (2006)
Khatri, O.P., Biswas, S.K.: Friction of octadecyltrichlorosilane monolayer self-assembled on silicon wafer in 0% relative humidity. J. Phys. Chem. B 111, 2696–2701 (2007)
Choi, J., Kawaguchi, M., Kato, T.: Possibility of organic monolayer films as lubricants for disk drives: comparative study of PFPE and organosilane. J. Tribol. Trans. ASME 125, 850–853 (2003)
Asay, D.B., Dugger, M.T., Ohlhausen, J.A., Kim, S.H.: Macro- to nanoscale wear prevention via molecular adsorption. Langmuir 24, 155–159 (2008)
Asay, D.B., Dugger, M.T., Kim, S.H.: In situ vapor-phase lubrication of MEMS. Tribol. Lett. 29, 67–74 (2008)
Barnette, A.L., Asay, D.B., Kim, D., Guyer, B.D., Lim, H., Janik, M.J., Kim, S.H.: Experimental and density functional theory study of the tribochemical wear behavior of SiO2 in humid and alcohol vapor environments. Langmuir 25, 13052–13061 (2009)
Barnette, A.L., Asay, D.B., Ohlhausen, J.A., Dugger, M.T., Kim, S.H.: Tribochemical polymerization of adsorbed n-pentanol on SiO2 during rubbing: when does it occur and is it responsible for effective vapor phase lubrication? Langmuir 26, 16299–16304 (2010)
Acknowledgment
This work was supported by the Air Force Office of Scientific Research (Grant No. FA9550-08-1-0010).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marino, M.J., Hsiao, E., Bradley, L.C. et al. Is Ultra-Low Friction Needed to Prevent Wear of Diamond-Like Carbon (DLC)? An Alcohol Vapor Lubrication Study for Stainless Steel/DLC Interface. Tribol Lett 42, 285–291 (2011). https://doi.org/10.1007/s11249-011-9771-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11249-011-9771-0