Abstract
Poly (vinyl alcohol) (PVA) hydrogel is one of the anticipated materials for artificial cartilage. In our previous studies, wear of PVA hydrogel depended on content of proteins in lubricants. The secondary structures of bovine serum albumin (BSA) and human gamma globulin (HGG) were investigated in circular dichroism spectroscopy to clarify the influence of the proteins on frictional properties. BSA and HGG were mainly composed of the α-helix and the β-sheet, respectively. BSA containing the α-helix structure showed low friction compared to HGG composed of the β-sheet structure in mixed or boundary lubrication mode. The α-helix structure forms low shear layer because the α-helix structure is easily released from surfaces and low cohesive strength. HGG forms uniform adsorption layer, but showed higher friction than BSA in the rubbing with single protein. In the repeated rubbing with changing of lubricants from HGG to BSA, however, the final friction was reduced, because an optimum layered structure of proteins was formed. Hence, layered structure of proteins appears to play an important role to protect rubbing surfaces and to reduce friction. In heat treatment tests, heat-induced BSA showed very low friction because of reduction of the α-helix structure. Heat-induced HGG did not show large differences from native HGG, but could not bring low friction with heat-induced BSA. Thus it was shown that the protein conformation has effective influences on friction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For many years joint prostheses have been applied to clinical use and have resulted in recovery of daily activities and releasing from pains for patients with osteoarthritis, rheumatoid arthritis, or related damage. Joint prosthesis is mainly composed of a sliding pair of ultra-high molecular weight polyethylene (UHMWPE) and corrosion-resistant metal or ceramics. In most patients, wear of UHMWPE determines a limit of durability for joint prosthesis, which is about 10–20 years. The wear problem of UHMWPE is not fully overcome but still a major issue for biotribologists and orthopedists [1–4]. Recently as a useful solution of this matter, a pair of hard-on-hard materials, i.e., ceramic material or corrosion-resistant metal against itself was used as rubbing surfaces of artificial joints. Many researchers study on the effect of gamma ray irradiation to UHMWPE to enhance the wear resistance by increasing cross-linking of molecules [5]. These prostheses could reduce wear of rubbing materials. In the prostheses composed of these materials, however, the full fluid film lubrication is not expected in various daily activities, and thus certain wear is inevitable. In contrast, the application of compliant artificial cartilage is expected to enhance the effective fluid film formation in various activities as pointed out by Dowson [6]. Although elastic deformation of soft artificial cartilage is effective in elastohydrodynamic lubrication film formation, the considerable wear occurs due to a lack of mechanical strength under severe conditions with local direct contact. Therefore, the study for effective and well-directed protection of compliant artificial cartilage is required.
It is pointed out that the existence of protein and lipid in lubricant is important for joint prosthesis lubrication [7–9]. It is known in certain cases that lubricants containing proteins and/or lipids show low friction and low wear in a sliding pair of materials for joint prostheses. Proteins and lipids adsorb on material surfaces and construct a film of proteins or lipids as an effective boundary layer. Therefore lubricants containing appropriate proteins or lipids show low friction and low wear. In other cases, however, the existence of proteins and/or lipids increases friction and wear depending on frictional test conditions; the severity is controlled by concentration of proteins or lipids in lubricants, applied load, and sliding speed. Proteins and lipids can interfere or react with rubbing surfaces, under some conditions. It is noticed that protein has two kinds of different roles that enhance lubricating ability in some cases but reinforce adhesion between rubbing surfaces in other cases [7–9]. Therefore, it is important to establish rubbing conditions to suppress adverse effects of proteins and lipids. In this paper, we focus the influence of adsorbed protein film on friction and wear of artificial cartilage.
In our previous study [10] on poly (vinyl alcohol) hydrogel (PVA hydrogel) with an optimum material properties [11] as one of candidate materials for artificial cartilage, we showed that albumin and gamma-globulin both of which are included in natural synovial fluid, adsorbed on rubbing surfaces and had remarkable effects on wear of PVA hydrogel as shown in figure 1 [10]. The wear grade for a sliding pair of PVA hydrogel against itself is defined as the summed values of wear severity for the upper and lower specimens, where the wear severity was classified as 5 grades based on the observation of rubbing surface by optical microscope. The wear of PVA hydrogel depended on content and A/G ratio (ratio of albumin to gamma-globulin) of proteins in hyaluronate (HA) solution. For lubricants containing single protein, an increase in concentration of protein increased the wear grade as indicated by arrows in figure 1. For lubricants containing both albumin and gamma-globulin, excessive protein concentration in lubricants at 2.8 wt% showed severe wear because of random formation of adsorbed protein film. However, wear of PVA hydrogel was remarkably reduced at both of total content of 2.1 wt% and A/G ratio of 1/2 or 2/1. Then, we observed adsorption of proteins on a glass plate in fluorescent microscope by labeling proteins with fluorescent dye [12]. In these low wear cases the adsorbed protein film formed a regular structure of layered proteins of albumin and gamma-globulin as shown in figure 2(a). In contrast, in high wear case for higher protein concentration, the adsorbed film was formed as a separated structure as shown in figure 2(b). Therefore it is concluded that construction of layered protein film of albumin and gamma-globulin is essential for reducing wear of PVA hydrogel [10].
Influence of protein constituents on wear for sliding pair of PVA hydrogel in the previous study [10].
Fluorescent images of adsorbed protein film. Green and red area means γ-globulin and albumin, respectively. (a) Low wear condition (0.7 wt% albumin and 1.4 wt% γ-globulin) (b) High wear condition (1.4 wt% albumin and 1.4 wt% γ-globulin) [12].
At the present stage, the detailed mechanisms of formation of protein-adsorbed layer on the surfaces of artificial cartilage are not well known. It is important to clarify the formation mechanisms of protein boundary film with good lubricating ability. It is considered that adsorptive abilities of proteins are affected by various properties such as molecular weight of proteins, electrical charge, hydrophilic, or hydrophobic properties of proteins and artificial materials, pH of solutions and other characteristics of proteins. But it is difficult to change the only one of the properties, for example, a change of pH modifies electric charge property. Therefore, we have to investigate comprehensive mechanisms of protein adsorption. Although the complete primary, secondary, tertiary, and quaternary structures of intact albumin and gamma-globulin are known [13–15], the protein conformation is affected by the above properties, and is closely related with other properties. Hence, we considered that the protein conformation is the key factor of protein adsorption and focused on the secondary conformation of proteins. For this purpose, CD data can indicate the changes in conformation of globular proteins as the secondary structure.
The aim of this study is to clarify the mechanisms of protein adsorption as boundary film in order to design the boundary film with lubricating ability of reducing friction and wear for PVA hydrogel.
Experimental method
Protein solution
Bovine serum albumin (BSA) (Wako Pure Chemical Industries Ltd., Japan) and human serum gamma-globulin (HGG) (Wako Pure Chemical Industries Ltd., Japan) were used as additives. Proteins were mixed into saline and stored at 4 °C in a refrigerator for one night to diffuse proteins for circular dichroism (CD) measurement and for friction test as lubricants. For the measurement and evaluation of conformational change of protein, bottles filled with protein solution were kept in an oven at 60, 70, and 80 °C for 2 h.
CD measurement
Conformational changes of protein were measured by the CD method. The CD spectra were recorded from 250 to 190 nm on a CD spectrophotometer (J-720, JASCO Co. Ltd., Japan), using a synthesis quartz cuvette with 1 mm optical path length at room temperature. Samples were prepared at the content of 0.7 mg/mL solution. Each sample was scanned for five times, and averaged CD signal was used in analysis on the secondary structure of proteins.
The change in the α-helix content of BSA was considered to be an indicator of the denatured degree of native structure of BSA, and the β-sheet content of HGG was considered to be as the denatured degree of native structure of HGG.
The content of secondary structure and denature degree were determined from the ellipticity at 208 nm for BSA and at 217 nm for HGG. The ellipticity of poly-l-lysine was used as the standard of α-helix [14]. The loss of α-helix was calculated using the following equation [16,17]:
Here, θ is the ellipticity in deg cm2/dmol, L is the optical path length in cm, C is the molar concentration in M, C o is the concentration (μg/mL), and M o is the mean residue weight. The content of the β-sheet was calculated using the results of Vermeer et al. [13].
Frictional tests
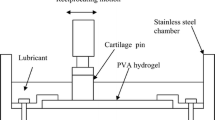
The reciprocating friction apparatus (shown in figure 3) [11] was used to investigate the influence of the lubricants containing proteins on the frictional properties. The sliding surfaces are composed of PVA hydrogel, and a glass plate. PVA hydrogel which has water content of 79 wt% and elastic modulus of 1.2 MPa, is the stationary upper specimen of elliptical geometry with diameters of 25 mm and 40 mm, and the glass plate is the reciprocating lower one. Frictional force was measured by strain gauges attached with supporting plate for the upper specimen. The frictional test conditions were chosen according to the previous study [11] and the frictional conditions were realized by the mixed or boundary lubrication mode to evaluate the lubricating properties of adsorbed protein films on rubbing surfaces under severe condition as follows: load: 2.94 N, average contact pressure: 0.24 MPa, sliding speed: 20 mm/s, sliding distance: 800 m.
Saline solution containing BSA or HGG at concentration of 0.7 wt% was used as lubricant. The pH of BSA solution and HGG solution was 7.1 and 7.2, respectively. The pH of saline solution containing heat-induced protein was not changed from native protein solution. The lubricant was changed either from BSA solution to HGG solution or from HGG solution to BSA solution after each 200 m of sliding distance.
Results
CD measurement
CD spectra of native BSA had peaks at 208 and 222 nm as shown in figure 4. These were typical α-helix spectra and showed that native BSA is mainly constructed from the α-helix. The α-helix content of BSA is from 60 to 67% [14,15]. Heat-induced BSA showed smaller peaks compared to native BSA. This means that heat-induced BSA changes secondary conformation from α-helix to random coil [16].
CD spectra of native HGG had peaks at 217 nm as shown in figure 5. It is known that the β-sheet has peak at 217 nm [13]. Therefore we showed that much of HGG is constructed from the β-sheet. Heated HGG was changed with secondary structure as shown in figure 5. The spectra of heated HGG at 60 and 70 °C showed an increase of a peak at 217 nm. This means that heat-induced HGG changes secondary conformation from β-sheet to random coil.
Table 1 summarizes the loss of the α-helix and the β-sheet as a function of temperature. Both of proteins changed the secondary structure, and the content of initial secondary structure was reduced with an increase of temperature. It was confirmed that the secondary structure is changed due to heating.
Frictional test
The changes in friction coefficient of lubricant containing native protein are shown in figure 6. The friction test was started with the first lubricant containing either native BSA or native HGG. BSA lubricant showed lower friction coefficient than HGG lubricant at the first sliding distance of 200 m. With Changing of protein solution after unloading at 200 m, 400 m and 600 m, friction was initially decreased but gradually increased with rubbing. However, the test, which initially started from HGG, showed lower friction at 800 m than the final friction at 600 m sliding with HGG. The lowering of friction suggests the formation of a layered structure, as discussed in the following section. In contrast, the test starting from BSA solution showed a gradual increase in friction even after repeated sliding of 800 m.
Figure 7 shows the changes in coefficient of friction of lubricants containing heated proteins. Coefficient of friction started from BSA solution showed excellent low friction even after 800 m sliding with changing of lubricants three times. BSA was denatured by heating and the secondary structure was changed from the α-helix to random coil. Denatured BSA adsorbed probably uniformly and strongly compared to native BSA. In contrast, denatured HGG showed almost similar coefficient of friction to native HGG during the first sliding distance of 200 m. After 800 m sliding, however, the final friction attained higher level than native HGG. This fact suggests that an appropriate layered structure was not formed.
Discussion
PVA hydrogel is one of the anticipated materials for artificial cartilage [18–25]. PVA hydrogel in this study has high water content and a low elastic modulus of 1.2 MPa similar to natural cartilage, which is effective on the formation of elastohydrodynamic lubrication film, but its major disadvantage is its lower strength. Although PVA hydrogel showed low coefficient of friction under walking condition in knee joint simulator [7], PVA hydrogel experienced rapid wear under severe conditions such as mixed or boundary lubrication. Therefore, the existence of a protective surface film with low friction and wear becomes important to prevent surface failure. In our previous study [10], we showed that adsorbed protein film which has an effect of reducing wear of PVA hydrogel, is composed of layered protein film of BSA and HGG as shown in figure 2(a) [12]. On the contrary, the protein film without ability of reducing wear showed separate adsorption of BSA and HGG as shown in figure 2(b) [12]. Consequently it is important to form a layered structure of BSA and HGG for reducing wear of PVA hydrogel. The possibility of forming the protein boundary film which reduces wear, depended on the content of protein in lubricants. Wear grade of PVA hydrogel was contingent on the A/G ratio (ratio of BSA to HGG) even at the same total content of protein as shown in figure 1 [10]. Therefore the elucidation of mechanisms on protein adsorption becomes important to design for excellent protein boundary film to reduce wear and friction for long durability of PVA hydrogel as artificial cartilage.
Protein adsorption on artificial materials is reported by many researchers [13,14,16,26–29]. Adsorption of protein depends on many factors i.e., pH, salt content, temperature, time, concentration and size of proteins, hydrophilicity and other properties of proteins. It is known that amount of protein adsorption also depends on surface wettability as pointed out by Sigal et al. [30]. They evaluated the protein adsorption to various surface moieties. They reported that the adsorption amount of BSA and HGG is quite different for hydroxyl groups. PVA hydrogel has a hydroxyl groups on surface. The glass plate has a hydroxyl groups in immersed condition in water. Therefore, it is considered that the amount of adsorption of BSA is lower than HGG and the difference of adsorption quantity is dependent on the conformation of protein.
Native BSA and HGG on hydroxyl groups are considered to be easily released from surfaces of materials because of its weak adsorption strength. Sethuraman et al. [14] showed that protein adsorbs to hydroxyl group in small adhesion force comparing to hydrophobic surface. In this study, however, serum proteins, particularly HGG adsorbed strongly on surfaces, and it was hard to exfoliate from surfaces by rinses, because the rubbing process with shearing appears to enhance adsorption and formation of boundary film. In general, an adsorbed protein changes conformation from native structure to denatured one [13,17,31,32]. Tanaka et al. showed that adsorbed BSA onto polymers changes its conformation and decreases the α-helix content depending on surface hydrophilicity of polymers [17]. We referred to these reports, and hence, experimented both native and heat-induced proteins in friction test.
We showed that BSA is mainly composed from the α-helix as shown in figure 4 and it is considered that adsorptive ability of BSA depends on content of the α-helix structure, that is, native or denatured structure has an effect on BSA adsorption. As shown in figure 7, heat-induced BSA decreased friction coefficient and showed better lubrication ability. Water as lubricant showed higher friction coefficient (not shown in figure). Decreasing friction coefficient with heat-induced BSA means the formation of uniform protein boundary film. It is considered that heat-induced BSA adsorbed strongly compared to native BSA due to reduction of the α-helix structure. The α-helix structure is hard to adsorb on material surfaces in this condition. Hence, protein containing the α-helix is hard to exhibit good lubricating ability in boundary lubrication. Heuberger et al. reported that denatured BSA adsorbs strongly on surfaces and increases friction in a sliding pair of UHMWPE and ceramics [32]. In this study for PVA hydrogel against glass plate, however, denatured BSA, which experienced heat treatment showed low friction. The secondary structure of heat-induced denatured protein is different from that of adsorbed protein and after heat treatment of the adsorbed molecules the difference is less pronounced [13]. The detailed mechanism of low friction in the reported tests started with heat-induced albumin should be examined in our future study.
Native HGG is mainly produced from the β-sheet structure as shown in figure 5. Native HGG is likely to adsorb intensely on PVA surface compared with native BSA with plenty of the α-helix. Heat-induced HGG with random coil did not show large difference in friction from native HGG at initial 200 m sliding but deteriorated without friction reduction in repeated rubbing process after changing of protein solution as shown in figure 7. The increase in random coil in heat-induced HGG may interact with heat-induced BSA. These phenomena indicate that the lubricating ability of adsorbed film in coexistence of heated HGG and heated BSA depends on the construction of beneficial hierarchical structure.
We showed that the existence of a uniform protein film as a bottom layer like adsorbed HGG film is important to maintain low friction coefficient after 800 m sliding as shown in figure 6. The uniform firm film of adsorbed HGG exists under the condition of friction test starting with HGG solution, and in the following rubbing process with BSA, BSA adsorbed onto the HGG layer. This condition can form a layered structure of adsorbed HGG and BSA as shown in figure 8(a). This adsorbed film is capable of protecting rubbing surfaces by directly adsorbed HGG layer and maintaining low shearing stress with sliding at the boundary between HGG and BSA layer or within BSA layer with lower cohesive strength. In contrast, in initial rubbing lubricated with BSA solution, the native BSA adsorbs weakly and is exfoliated during rubbing. In the next rubbing after changing lubricant, if HGG accesses near the surfaces, HGG adsorbs onto uncovered surfaces. This condition constructs uneven structure of adsorbed protein shown in figure 8(b). Uneven adsorbed film has a less boundary lubrication ability because it is hard to protect surfaces and slide at appropriate boundary between BSA and HGG.
In comparison of frictional tests with native and heat-treated proteins, a proposal is given in the following. If heat-treated BSA has higher adsorption ability and higher cohesive strength than heat-treated HGG, beneficial adsorbed film can be formed with a condition of initial supply of heat-treated albumin followed by heat-treated HGG similar to a condition of initial native HGG followed by native albumin. To clarify this model for heat-treated proteins, we have to validate the structure of adsorbed film by application of direct observation such as fluorescence microscopy by labeling proteins with fluorescent dye [12] and total internal reflection fluorescence microscopy [33].
We consider that the protein boundary film is essential for long life of artificial cartilage in a clinical use. Albumin and gamma-globulin, which are contained in natural synovial fluid, can easily adsorb onto the surfaces of joint prosthesis. An optimum control of protein constituents in joint fluids is expected to improve both friction and wear properties of artificial cartilage.
Conclusion
The effect of conformation of BSA and HGG on frictional ability was investigated. BSA and HGG were mainly constructed from the α-helix and the β-sheet structure, respectively. The α-helix structure is lack of adsorption ability, and the β-sheet structure is strongly adsorbed on PVA hydrogel. Therefore the roles of BSA and HGG were different in lubrication. It is important to form a layered structure of adsorbed protein for protecting rubbing surfaces and maintaining low friction by combination of different proteins. In heat treatment tests, it was indicated that the denatured BSA has a potential of low friction. In the next study we plan to examine the detailed structure of adsorbed film started with denatured albumin.
References
A. Unsworth, Tribol. Int., 28(7) (1995) 485
G. Lewis, J. Biomed Mater Res Appl Biomater, 38 (1997) 55
T.M. McGloughlin, A.G. Kavanagh, Proc. Instn. Mech. Engrs., 24 (Part H) (2000) 349
H.C. Amstutz, P. Campbell, N. Kossovsky, I.C. Clarke, Clin. Orthop., 276 (1992) 7
L.C. Sutula, J.P. Collier, K.A. Saum, Clin. Orthop., 319 (1995) 28
D. Dowson, Proc. Inst. Mech. Eng., Int. Conf. The Changing Role of Engineering in orthopedics, C384/KN1 (1989) 1
T. Murakami, Y. Sawae, H. Higaki, N. Ohtsuki and S. Moriyama, Elatohydrodynamics ‘96, edited by D. Dowson et al., (Elsevier, 1997) 371
T. Murakami, H. Higaki, Y. Sawae, N. Ohtsuki, S. Moriyama and Y. Nakanishi, Proc. Instn. Mech. Engrs. 212, Part H: J. Engng. in Medicine (1998) 23
T. Murakami, Proc. Biotribology Satelllite Forum of ITC Nagasaki, 2000 and 21st Biotribology Symposium (2000) 1
K. Nakashima, T. Murakami, Y. Sawae, Trans. JSME. Ser. C (in Japanese), 70(697) (2004) 218
K. Nakashima, T. Murakami, Y. Sawae, Proc. The International Tribology Conference Nagasaki, 2000 (2001) 1537
K. Nakashima, Y. Sawae, T. Murakami, JSME International Journal, 48(4) (2005) 555
A. Vermeer, M. Bremer, W. Noede, Biochim. Biophys .Acta., 1425 (1998) 1
A. Sethuraman, M. Han, R. Kane, G. Belfort., Langmuir, 20(18) (2004) 7779
A. Wittemann, M. Ballauff, Anal. Chem., 76 (2004) 2813
N. Greenfield, G. Fasman, Biochem., 8(10) (1969) 4108
M. Tanaka, T. Motomura, M. Kawada, T. Anzai, Y. Kasori, T. Shiroya, K. Shimura, M. Onishi, A. Mochizuki, Biometerials, 21 (2000) 1471
J. Bray, E. Merrill, J. Biomed. Mater. Res., 7 (1973) 431
T. Noguchi, T. Yamamoto, M. Oka, J. Appl. Biomater., 2 (1991) 101
T. Murakami, N. Otsuki and H. Higaki, Thin Films in Tribology, edited by D. Dowson et al., (Elsevier, 1993) 673
Gu. Zhen-Oiu, Xiao Jiu-Mei, Zhang Xiang-Hong, Biomed. Mater. Eng., 8 (1998) 75
M Oka, K Usio, K Ikeuchi, S H Hyon, T Nakamura, H Fujita, Proc. Instn. Mech. Engrs., 214(Part H) (2000) 59
J.P. Gong, M. Higa, Y. Iwasaki, Y. Katsuyama, Y. Osada, J. Phys. Chem. B, 101 (1997) 5487
J.P. Gong, Y. Iwasaki, Y. Osada, K. Kurihara, Y. Hamai, J. Phys. Chem. B, 103 (1999) 6001
J.P. Gong, G. Kagata, Y. Osada, J. Phys. Chem. B, 103 (1999) 6007
K. Ishihara, H. Nomura, T. Mihara, K. Kurita, Y. Iwasaki, N. Nakabayasi, J. Biomed. Mater. Res., 39 (1998) 323
J. Kardos, D. Okuno, T. Kawai, Y. Hagihara, N. Yumoto, T. Kitagawa, P. Zavodszky, H. Naiki, Y. Goto, Biochim. Biophys. Acta., 1753 (2005) 108
A. Vermeer, W. Norde, Biophys. J., 78 (2000) 394
C. Chang, C. Wu, J. Yang, Anal. Biochem., 91 (1978) 13
G. Sigal, J. Am. Chem. Soc., 120 (1998) 3464
M.R. Widmer, M. Heuberger and N.D. Spencer, Boundary and mixed lubrication: Science and applications, edited by D. Dowson et al., (Elsevier 2002) 361
M.P. Heuberger, M.R. Widmer, E. Zobeley, R. Glockshuber and N.D. Spencer, Biomaterials, 26 (2005) 1165
Y. Sawae and T. Murakami, Proc. 5th World Congress of Biomechanics (2006) in CD
Acknowledgments
Authors would like to thank Dr. Masayuki Takeuchi for his technical advices in the CD measurement and the CD measurement was conducted at the Center of Advanced Instrumental Analysis, Kyushu University. This study was partly supported by the Grant-in-Aid for Scientific Research (A) No. 15200037, No. 16760112 from Japan Society for the Promotion of Science, and No.15086212 from Ministry of Education, Culture, Sports, Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakashima, K., Sawae, Y. & Murakami, T. Influence of protein conformation on frictional properties of poly (vinyl alcohol) hydrogel for artificial cartilage. Tribol Lett 26, 145–151 (2007). https://doi.org/10.1007/s11249-006-9185-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11249-006-9185-6