Abstract
Cyclic citrullinated peptide (CCP) antibody has been shown recently to be a promising marker for early detection and diagnosis of rheumatoid arthritis (RA). In order to exploit newly developed therapies for RA, early intervention is crucial in preventing irreversible joint damage. Here, we describe use of a plant expression system to produce a CCP antibody that could be used in the early diagnosis of RA. Heavy and light chain gene sequences of a CCP monoclonal antibody (CCP mAb) were cloned from the hybridoma cell (12G1) and introduced into two separate plant expression vectors under the control of the rice α-amylase 3D (RAmy3D) promoter system. The vectors were introduced into rice calli (Oryza sativa L. cv. Dongjin) using Agrobacterium tumefaciens mediated transformation. Integration of the CCP mAb genes into rice chromosomes was confirmed by a genomic DNA polymerase chain reaction and expression was verified by northern blot analysis of mRNA. The in vivo assembly and secretion of CCP mAb occurred in transgenic rice cell suspension culture under the RAmy3D expression system; accumulated CCP mAbs in the medium were purified by protein G affinity chromatography. Immunoblot assays and ELISA showed these plant-produced CCP mAbs successfully bound to a synthetic CCP antigen. Taken together, our results suggest that CCP mAb produced in a transgenic rice suspension culture were easily purified and biologically active against their antigen in the RA, and thus may be used a specific serological marker, which is present very early in the RA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a systemic inflammatory disease characterized by chronic and erosive polyarthritis as a result of abnormal growth of synovial tissue (pannus), which causes irreversible joint disability (Serdaroglu et al. 2008). Disease outcomes vary from mild symptoms to severe systemic disease when joint destruction is accompanied by extra articular manifestations (e.g., rheumatoid nodules, vasculitis), thus leading to a great decrease in the quality of life for disease sufferers. In recent times, new and more effective therapies have been developed; however, as early intervention is crucial in preventing irreversible joint damage (Bukhari et al. 2003; Landewe 2003; Lard et al. 2001), it is increasingly important to diagnose RA at the earliest possible stage. Diagnosis during the early stages of the disease, when not all clinical symptoms are manifested, requires the use of a good serological marker.
Citrullination is the conversion of the amino acid arginine in a protein into citrulline by deamination as a result of the activity of peptidylarginine deiminase during post-translational modification. At a neutral pH, arginine is positively charged whereas citrulline is uncharged, so that conversion from arginine to citrulline may have crucial influence on the structure and function of the protein. Citrullinated molecules have been used as the targets of antibodies in proteins such as filaggrin, keratin, fibrin, and vimentin (Vossenaar and van Venrooij 2004). The antibodies are detected by ELISA, where a synthetic cyclic citrullinated peptide (CCP) is used as the substrate (Alexiou et al. 2007; Itty et al. 2008). Protein citrullination has attracted a lot of interest because of its involvement in physiological and pathological processes. The former include terminal differentiation of epithelial cells, regulation of gene expression, and apoptosis; while pathological processes involving citrullinated proteins have been linked to disease progression in RA, multiple sclerosis, and Alzheimer’s disease (Martinez et al. 2011).
Currently, the diagnosis of RA primarily depends on clinical symptoms; in most cases diagnosis is only carried out after significant progression in joint destruction. Although, rheumatoid factor (RF) has been adopted as a serological parameter for RA diagnosis in the International Classification Criteria established by the American College of Rheumatology. It has poor sensitivity as up to 20% of RA patients are RF negative throughout the progression of RA (Serdaroglu et al. 2008). Further, RF has low specificity and is detected in patients suffering from other rheumatoid disorders, chronic inflammation, or malignant tumors, and has also been detected in some healthy people. A good marker for RA should ideally be able to predict the erosive or non-erosive progression of the disease. The anti-cyclic citrullinated protein antibody, an autoantibody against citrullinated proteins, meets these criteria and is therefore a valuable marker for early RA and is now used as a diagnostic marker (Landewe 2003; Vossenaar and van Venrooij 2004).
Monoclonal antibodies with high affinity for antigens are used in clinical diagnosis, commercial analysis, and passive immunotherapy (Buyel et al. 2017; Casadevall 2002; Weiner 2015). Transgenic plants provide one means to produce full-size monoclonal antibodies of the IgG (Zeitli et al. 1998; Giritch et al. 2006; Huang et al. 2010), secretory IgA (Larrick et al. 2001; Wycoff 2005), and single-chain variable fragment (Brar and Bhattacharyya 2012) types. This approach has the potential for large-scale and low-cost biomass production, with low risk of mammalian pathogen contamination (Buyel et al. 2017; Kolotilin et al. 2014). Plant cell suspension cultures are widely used for production of industrial and pharmaceutical recombinant proteins and can be used to accumulate recombinant proteins secreted into the culture medium (Santos et al. 2016; Xu and Zhang 2014). In cultured cells, the rice α-amylase gene family is regulated by metabolic repression (Huang et al. 1993; Simmons et al. 1991); the rice α-amylase gene promoter, RAmy3D, has been used for recombinant protein production in rice suspension cells. The activity of this promoter is induced by sugar starvation; its signal peptide allows the secretion of recombinant proteins from the cells. Previous studies demonstrated that that the RAmy3D promoter, signal sequence, and terminator can act together to provide a powerful system for recombinant protein production. Moreover, plant cell culture has major advantages, including simplicity of use, safety of the media, and ease of purification (Jung et al. 2016; Kim et al. 2011; McDonald et al. 2005).
In the present study, CCP-specific monoclonal antibodies were produced using an RAmy3D system in a transgenic rice cell suspension culture; the proteins accumulated in the culture medium where they could be easily recovered and purified. The purified monoclonal antibodies showed specific binding to a synthetic CCP peptide antigen.
Materials and methods
Construction of plant expression vectors
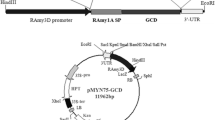
We prepared cDNA from the hybridoma cell line 12G1, which secretes a mAb with high affinity for CCP (kindly provided by Dr. JH Ju, Catholic University of Korea). The cDNA for the CCP heavy chain (1464 bp) and light chain (751 bp) fragments using specific its primer sets (Fig. 1a) were cloned into pGEM-T easy vector (Promega, Madison, WI), and the sequences were confirmed (DNA sequence analysis was kindly carried out by Dr. YS Jang, Chonbuk National University). These DNA fragments harbor a XbaI restriction enzyme site at the 5′ end and a KpnI site at the 3′ end. The fragments were digested with XbaI and KpnI and then introduced to the same restriction sites in the plant expression vector, pCAMBIA1300 (Hajdukiewicz et al. 1994), under the control of the RAmy3D promoter, and with the 3′ untranslated region (UTR) of the RAmy3D gene as the terminator. The expression vectors were designated pMYD319 for the light chain and pMYD320 for the heavy chain; both vectors included the hygromycin phosphotransferase (hpt) gene as a selection marker for plant transformation (Fig. 1). Each of the vectors was transformed into Agrobacterium tumefaciens LBA4404 using the helper plasmid pRK2013 in the tri-parental mating method (Hiei et al. 1994; Van Haute et al. 1983).
Primer sequences to clone CCP mAb from cDNA of hybridoma cell (a) and schematic diagram of plant expression vectors pMYD319 and pMYD320 (b, c). The light or heavy chain of the CCP mAb gene, fused with the Ramy3D signal peptide (3Dsp), is located between the rice amylase 3D promoter (Ramy3D-p) and the 3′-untranslated region (3′-UTR). Transferred DNA (T-DNA) of the final plasmid is shown. RB, T-DNA right border; 3′-UTR, 3′-untranslated region of the rice α-amylase 3D gene; 35S-p, cauliflower mosaic viral (CaMV) 35S promoter; HPT, hygromycin phosphotransferase; 35S polyA, terminator of 35S gene; LB, T-DNA left border
Rice transformation
Scutellum-derived calli from mature rice seeds (Oryza sativa L. cv. Dongin) were prepared and transformed by Agrobacterium-mediated transformation (Hiei et al. 1994). Briefly, The Agrobacterium cells harboring light chain (pMYD319) or heavy chain (pMYD320) vectors were mixed together and re-suspended in N6 liquid medium with 200 µM of acetosyringone. Agrobacterium-infected rice calli were transferred onto N6 co-culture medium containing N6 salts and vitamins supplemented with sucrose (30 g/L), casamino acids (1 g/L), 2,4-D (2 mg/L), glucose (10 g/L), gelrite (2 g/L), acetosyringone (100 µM), pH 5.2, and incubated in the dark at 28 °C for 3–5 days. Putative induced calli were induced after 3–4 weeks on N6 medium containing hygromycin B (50 mg/L) and cefotaxime (250 mg/L).
Confirmation of transgene integration by polymerase chain reaction (PCR) analysis
To confirm integration of both heavy and light chains of the CCP mAb gene into the rice genome, genomic DNA PCR was performed. The putative induced calli were collected for genomic DNA extraction (Aljanabi and Martinez 1997). PCR was carried out in 20 μL of PCR mixture containing genomic DNA (200 ng) and 10 μL of GoTaq® Green Master Mix (Promega). The following primers were used: forward CCP HC1-F1: 5′-TTT TGG TCT CAA GGT TCT AGA ATG GAA TGG AGT GGG GTC-3′, and reverse CCP HC2-R2: 5′-GAG CTC GGT CTC AAA GCG GTA CCA TAC ATT TAC CAG GAG AGT G-3′ for the heavy chain; and forward CCP LC-F: 5′-TTT TGG TCT CAA GGT TCT AGA ATG GTA TCC ACA GCT CAT-3′, and reverse CCP LC-R: 5′-GAG CTC GGT CTC AAA GCG GTA CCT ATC AAC ACT CAT TCC TGT TG-3′ for the light chain. Amplification consisted of 30 cycles of 1 min at 94 °C, 1 min 30 s or 40 s at 58 °C, and 1 min at 72 °C. The PCR products were then separated by electrophoresis on a 1% agarose gel, visualized by staining with ethidium bromide, and analyzed under ultraviolet light.
Establishment and induction of rice cell suspension culture
Transformed rice calli were propagated and cultured in the dark at 28 °C using a rotary shaker with a rotation speed of 100 rpm. To maintain the cell line, a cell suspension was cultured in a 300-mL Erlenmeyer flask using 50 mL N6 medium containing 2 mg/L 2, 4-dichlorophenoxyacetic acid (2,4-D), 0.02 mg/L kinetin, and 3% sucrose (Chen et al. 1994). A 10 mL inoculum was transferred every 7 days for sub-culturing. To induce CCP mAb expression under the control of the Ramy3D promoter, the N6 medium was removed from the cell suspension by aspiration, and the cells were transferred to fresh N6 (-S) medium (without sucrose) at 10% (5 g weight of wet cells/50 mL of medium) concentration. The supernatant from the culture medium of induced rice cells was collected by pouring the induced cell suspension through 2–3 layers of Miracloth (Calbiochem, La Jolla, CA, USA). Total secreted proteins were collected from the medium by centrifugation at 15,000×g at 4 °C for 10 min, to remove the debris.
Northern blot analysis
Total RNA was isolated from a cell suspension grown for 7 days in N6 (S+) and N6 (S−) (with and without sucrose, respectively) liquid media using TRIzol® Reagent (Life Technologies, Van Allen Way, CA, USA), according to the manufacturer’s instructions. RNA was electrophoretically separated on a formaldehyde-containing agarose gel (Sambrook and Russell 2001), and then transferred to a Hybond N+ membrane (Amersham Pharmacia Biotech, Piscataway, NJ, USA). This membrane was hybridized with α 32P-labeled CCP monoclonal antibody HC probe using the Prime-a Gene labeling system (Promega) at 65 °C in a hybridization incubator (FINEPCR Combi-H, Seoul, Korea). The membrane was washed twice in 2 × SSC (3 M NaCl and 0.3 M Na-Citrate) and 0.1% SDS, then twice more with 2 × SSC and 1% SDS for 15 min at 65 °C. Finally, hybridization bands were detected via autoradiography on X-ray film (Fuji Photo Film Co. HR-G30, Tokyo, Japan).
SDS–PAGE and western blot analysis
The expression of CCP mAb was confirmed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and western blot analyses (Laemmli 1970). The culture supernatant was collected on day 7 after sugar starvation and centrifuged at 15,000×g for 5 min to remove the rice suspension cells. For western blot analysis, 5 µg of total secreted protein (20 μL of 250 mg/L of total secreted protein) from the culture medium was separated via 8% (w/v) SDS-PAGE and electroblotted onto a nitrocellulose membrane. The membrane was then incubated in blocking solution [5% (w/v) non-fat dried milk in TBST buffer (20 mM Tris–Cl, pH 7.5, 500 mM NaCl, and 0.05% Tween 20)], followed by goat anti-mouse IgG (whole molecule) conjugated with alkaline phosphate (Sigma, Louis, MO, USA), goat anti-mouse IgG (gamma-chain specific) conjugated with alkaline phosphate (Sigma), and rat monoclonal anti-mouse kappa light chain (Sigma). The rat monoclonal anti-mouse kappa light chain antibody was detected using goat anti-rat IgG (whole molecule) conjugated with alkaline phosphate (Sigma). The gel was stained with 0.25% Coomassie brilliant blue R-250 with 45% methanol and 10% glacial acetic acid. Protein concentration was determined using a protein assay reagent (Sigma) based on the Bradford method, with bovine serum albumin as a standard.
Quantification of rice-produced CCP mAb
To induce CCP mAb expression under the control of the Ramy3D promoter, 50 mL of N6 (+S) medium was removed from the cell suspension by aspiration, and 5 g of rice cells was inoculated into 50 mL of fresh N6 (-S) medium (without sucrose) in a 300-mL Erlenmeyer flask. Time-dependent changes in the amounts of recombinant CCP mAb in the transgenic rice cell suspension culture were measured by quantitative ELISA after sugar starvation. Briefly, wells of microtiter plates (Nalgene NUNC International Corp., Rochester, NY, USA) were coated with goat anti-mouse IgG1 (Sigma) to bind CCP mAb and incubated at 4 °C for overnight. The wells were washed three times with TBST and blocked with 1% bovine serum albumin in PBS buffer at room temperature for 2 h. Culture medium containing secreted CCP mAb collected at 2 day intervals for 15 days after sugar starvation were added to each well and incubated at room temperature for 2 h. The mouse IgG1 isotype was used as a standard protein. After washing the wells with TBST, anti-mouse IgG (H + L specific) conjugated with horseradish peroxidase (HRP) (Promega) was added. The microplate was incubated at room temperature for 2 h and then washed with PBST; 100 µL of 1:1000 diluted avidin-horseradish peroxidase (Avidin-HRP, BD PharMingen Inc., San Diego, CA, USA) was added to each well. Samples were incubated at room temperature for 30 min, washed with PBST, and then each well was loaded with 100 µL of TMB substrate (BD PharMingen Inc.). The absorbance of the samples at 430 nm was recorded with a microplate reader.
Purification of monoclonal antibody
Recombinant CCP mAb secreted in rice cell suspension culture was purified using a HiTrap Protein G HP column (GE Healthcare, Huston, TX) following the manufacturer’s instructions. A 50 mL of 9 days culture medium after sugar starvation from a 300-mL Erlenmeyer flask was centrifuged at 15,000×g at 4 °C for 30 min, filtered through Miracloth (Calbiochem), then dialyzed against binding buffer (20 mM sodium phosphate buffer, pH 7.0). The dialyzed culture medium was passed through a 0.2 µm filter and loaded onto the column previously equilibrated with binding buffer. The column was washed with five volumes of binding buffer (20 mM sodium phosphate, pH 7.0). Bound antibodies were eluted using elution buffer (0.1 M glycine–HCl, pH 2.7) and the pH was neutralized by adding 1 M Tris–HCl (pH 9.0). Fractions were collected and purity was assessed by SDS-PAGE and western blot analysis. The purified plant-produced CCP mAbs were quantified using the Bradford protein assay (Bio-Rad, Hercules, CA, USA) and identified by separate SDS-PAGE and western blot analysis.
Affinity determination of rice derived CCP mAb
The binding activity of the rice-derived CCP mAb with synthetic CCP antigen was investigated via western blot analysis (Fig. 6c, d) and ELISA (Fig. 6e, f). The CCP derived filaggrin (Schellekens et al., 2000) as an antigen, and C-reactive protein (CRP) as a negative control were synthesized (Fig. 6a). To clarify the optimal concentration of CCP antigen against rice derived CCP mAb, various concentrations of CCP antigens {2.0, 1.0, and 0.2, 0 (PBS buffer)} were tested by Dot bot analysis (Fig. 6b). In order to determine the reactivity of rice-derived CCP mAb with synthetic CCP or CRP (negative control) peptide (Fig. 6a), 2 µg of CCP or CRP peptide were loaded onto 15% acrylamide gels. For ELISA, CCP or CRP antigen was diluted to 50 µg/mL in coating buffer and 50 µL were coated onto each well of an ELISA plate. The wells were washed three times with TBST and blocked with 1% bovine serum albumin in PBS buffer at room temperature for 2 h. Each culture medium containing secreted rice derived CCP mAb (rice), or hybridoma (12G1), or transfected Chinese Hamster Ovary (CHO) cell was added to each well and incubated at room temperature for 2 h. PBS buffer was added as a negative control (NC) (from next procedures see description above).
Results
Plant expression vector construction and genomic DNA PCR analysis
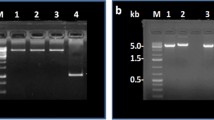
cDNAs encoding the heavy and light chains gene of CCP mAb were introduced into a plant expression vector under the control of the Ramy3D promoter system (Fig. 1b, c). Expression vectors for pMYD319 (light chain) and pMYD320 (heavy chain) were inserted into A. tumefaciens LBA4404 using the helper plasmid pRK2013 and then transformed into embryogenic rice calli. Genomic DNA PCR was performed to confirm successful insertion of heavy and light chain genes in the induced transgenic rice calli (Fig. 2a, b). Seven of eight lines of calli generated a PCR product for the light chain gene sequence, and eight generated PCR products for the heavy chain gene sequence. In Line 7, only insertion of HC was detected because transfer DNA of Agrobacterium may subsequently randomly integrate into rice genome (Gelvin, 2017). The positive PCR signals had the expected sizes for the light chain gene (0.7 kb) and heavy chain gene (1.4 kb) (Fig. 2a, b). No PCR products were obtained from non-transgenic (wild type) rice calli. This analysis indicated that the CCP mAb genes had successfully integrated into the chromosomes of the transgenic rice calli. Seven rice callus lines were selected for further analysis and established as cell suspensions.
Genomic DNA PCR to confirm the integration of CCP mAb genes into rice chromosomes. a The heavy chain (a) and light chain (b) genes of CCP mAb were amplified by genomic DNA PCR with specific primer sets. Lane M shows a 1 Kb + 100 bp DNA size marker; lane PC shows pMYD320 and pMYD319 plasmids used as a positive PCR control for heavy and light chain genes; lane NC shows non-transgenic callus genomic DNA used as a negative control; lanes 1–7 show PCR products from transgenic callus genomic DNA. c northern blot analysis showing expression of CCP mAb mRNA in transgenic rice cells. Lane NC shows total RNA extracted from non-transformed rice cells (negative control); lanes 1–7 contain RNA extracts of transgenic rice cells. d Loading standards are indicated by ethidium bromide stained ribosomal RNA (rRNA)
Presence of the monoclonal antibody in transgenic rice calli
To confirm expression of the CCP mAb mRNAs, seven transgenic rice calli induced of CCP mAb for 7 days using sugar starvation conditions. At the end of this period, total RNAs were extracted from wild-type and transgenic calli for northern blot analysis (Fig. 2c, d). Variations in transcript signals were detected in all transgenic cell lines; no signal was detected in wild-type cell line (Fig. 2c).
Production of CCP mAb in transgenic rice cell suspension culture
To determine whether CCP mAb was synthesized and secreted into the culture medium in a sucrose-dependent manner, transgenic cell suspensions were assessed using SDS-PAGE stained with Coomassie blue R250 (Fig. 3a, b) and western blot analysis (Fig. 3c–e) at 7 days after induction. The heavy chain and light chain assembled antibodies were detected in the transgenic rice cell suspension culture by western blot analysis using heavy chain-specific or light chain-specific antibodies under reducing and non-reducing conditions (Fig. 3c–e). A 44–46-kDa band of rice α-amylase that was induced by sugar depletion (Chen et al., 1994) was strongly expressed and observed in all lanes except the whole IgG lane (Fig. 3a). The CCP mAb bands detected by the heavy chain-specific antibody were located at the same position as those detected by the light chain-specific antibody, and also those detected by the whole molecule antibody. These results indicated that the heavy and light chains were assembled into the whole antibody under non-reducing conditions (Fig. 3c). Additionally, under reducing conditions, monomers of the heavy and light chains were detected 55-kDa (Fig. 3d) and 25-kDa bands (Fig. 3e), respectively. The highest expression was present in the cell line 4 in the western blot analysis; this cell line was selection for analysis of time-dependent expression.
SDS-PAGE (a, b) stained with Coomassie blue R250 and western blot analysis (c–e) of transgenic rice cell suspension medium. Whole assembled CCP mAb was detected under non-reducing conditions (a, c). Heavy and light chains of CCP mAbs were detected by heavy chain (d) and light chain-specific (e) antibodies under reducing conditions. Lane M shows a pre-stained protein marker, lane PC shows whole IgG protein, lane NC shows wild-type rice cell culture medium; lanes 1, 2, 3, 4, 5, 6, and 8 show culture medium of transgenic rice suspension cells. * indicate the 44-46 kDa of rice amylase under sugar starvation
Quantitative assays of the cell line showing high CCP mAb expression
To determine the period when maximum production of the monoclonal antibody occurred in the high expression line four, a time series experiment was conducted. Culture medium was collected at 2-day intervals for 15 days after sugar depletion; the samples were subjected to SDS-PAGE, western blot analysis (Fig. 4a, b), and ELISA (Fig. 4c). For a specific ELISA, the isotype of the CCP mAb was determined by western blot analysis with mouse monoclonal antibody isotyping reagents (IgG1, IgG2a, IgG2b, IgG3) (Fig. 4d). The western blot results showed that the CCP mAb bound with anti-mouse IgG1 antibody, indicating that the CCP mAb belongs to the IgG1 isotype (Fig. 4d). The amounts of CCP mAb in the transgenic rice callus suspension were measured by ELISA using anti-mouse IgG1 antibody. The highest level was 22 mg/L on day 9 after sugar starvation (Fig. 4c).
Time course study of recombinant CCP mAb production in transgenic rice cell suspension culture medium. The accumulated CCP mAbs in the suspension culture medium were detected over a period of 15 days after sugar starvation via western blot analysis (a, b) and ELISA (c). For specific ELISA, IgG isotypes (IgG1, IgG2a, IgG2b, IgG3) bound with CCP mAb were determined by western blot analysis (d). Error bars indicate standard errors from triplicate cultures. * indicate the 44–46 kDa of rice amylase under sugar starvation
Purification and biological activity of monoclonal antibody
The accumulated CCP mAbs on day 9 of sugar starvation in the transgenic rice cell suspension cultures were purified using a protein G affinity column for further experiments. The purified CCP mAbs were examined by SDS-PAGE stained with Coomassie blue R250 (Fig. 5a) and western blot analysis (Fig. 5b) under non-reducing conditions. Whole assembled antibodies were detected over 170-kDa because of N-glycosylation of Fc region in heavy chain (Higel et al. 2016). Additional bands were also detected and may indicate complex and truncated forms of antibodies produced by proteolytic degradation in the culture medium (Fig. 5b) (Magy et al. 2014).
Purification of rice-produced CCP mAb using affinity chromatography on a protein G column. Purified CCP mAb was separated by SDS-PAGE stained with Coomassie blue R250 (a) and visually assessed by western blot analysis with anti-mouse IgG1 antibody (b). Lane M contains pre-stained molecular weight markers; lane PC shows an IgG standard protein; lane NC shows the non-transgenic rice cell suspension culture medium sampled on day 9 after induction of sucrose starvation, used as a negative control; lane C, crude; lane FT, culture medium column flow-through; lane W, column flow-through washing buffer; lanes E1and E2, eluted fractions; arrow indicates 170-kDa purified recombinant CCP mAb
The binding activity of the CCP mAb from the culture medium was tested using synthetic CCP and CRP antigens (Fig. 6a); synthetic CCP antigens, at a concentration of 0.5, 1, and 2 µg, were dot-blotted onto a Hybond™ C nitrocellulose membrane. A negative control (PBS) was also dot-blotted onto the membrane. As shown in Fig. 6b, the most intense signal was found at the 2 µg concentration of the synthetic CCP antigen; the signals showed a concentration-related reduction in intensity and no signal was detected for the negative control (PBS). Synthetic CCP (or CRP) antigen was also used for SDS-PAGE stained with Coomassie blue R250 (Fig. 6c), western blotting (Fig. 6d), and ELISA (Fig. 6e, f). The analyses showed that the synthetic CCP antigen bound to rice-derived CCP mAb on the western blot and produced a band at approximately 2.3-kDa; a positive result was also found by ELISA.
Antigen-specific binding activity of rice-produced CCP mAb. a indicates the synthetic CCP and CRP peptide sequences. b Shows the result of a dot-blot analysis against synthetic CCP peptide using optimization of the loading amount. The synthetic CCP peptide, at concentrations of 0.5, 1, or 2 µg, were dot-blotted onto Hybond™ C nitrocellulose membrane. A negative control (PBS buffer) was also dot-blotted onto the membrane. c and d show rice-produced CCP mAb bound with synthetic CCP peptides of approximately 2.3 kDa via SDS-PAGE stained with Coomassie blue R250 and western blot analysis. Panels E and F show results of ELISA. CCP or CRP (negative control) peptides were coated onto ELISA plates. Anti-CCP mAbs derived from supernatant of hybridoma cells (Hybridoma 12G1), supernatant of transfected CHO cells (CHO), purified rice-produced CCP mAb (Rice), and PBS buffer (NC) were loaded into the wells. Error bars indicate standard errors from triplicate cultures
Discussion
The present study provides the first report of production of a CCP mAb specific for the CCP autoantigen peptide using the RAmy3D system in a transgenic rice cell suspension culture. Full-size CCP mAb was successfully produced at a level of 22 mg/L at 9 days after sugar starvation. In our previous study, it was demonstrated that the Ramy3D promoter has a strong promoter activity under sugar starvation conditions in transgenic rice cell suspension cultures: a 1000-fold increase in human GM-CSF protein expression levels were obtained using this promoter system compared to the 35S CaMV promoter expression system (Shin et al. 2003). Various recombinant proteins, including antibodies, can be produced in cells using the RAmy3D system in suspension culture and are secreted in high quantities into the culture medium, for example, granulocyte macrophage-colony stimulating factor at 288 mg/L (Kim et al. 2013), acid alpha-glucosidase at 37 mg/L (Jung et al. 2016), tumor-associated glycoprotein 72 antibody at 30 mg/L (Hong et al. 2008), and FimA monoclonal antibody at 17.3 mg/L (Kim et al. 2014).
The rice-produced CCP mAbs accumulated in the culture medium where they could be easily purified. The western blot analysis (under non-reducing conditions) showed that CCP mAbs were secreted into the suspension culture medium and also that heavy chain, and light chain specific antibodies located at the position corresponding to the size of the whole antibody, indicating that the two chains assembled to form a complete CCP mAb after secretion (Fig. 3b). For the production of antibody and therapeutic proteins, transgenic plants and plant cell cultures are gaining interest as attractive hosts, as they offer a way to replace animal-derived proteins with a safe and economical alternative (Lallemand et al. 2015; Schillberg et al. 2013). In addition, the entire manufacturing process is free from any animal-derived components, complementing the safety advantages associated with plant culture processing.
The antigen binding specificity of purified rice-derived CCP mAb was confirmed by both western blot analysis and ELISA (Fig. 6d, e). The CCP mAb showed binding activity to a synthetic CCP peptide but not with a synthetic non-citrullinated peptide (CRP). Both synthetic antigens contain 21 amino acids and differ only at the 9th amino acid from the N-terminal: non-citrullinated CRP has an R (arginine) residue, whereas citrullinated peptide CCP has an X. Analyses of sequences derived from filaggrin showed that peptides containing citrulline flanked by neutral and relatively flexible residues, such as glycine and serine, showed greater sensitivity for identification of RA than those containing strongly charged residues or those that gave a rigid structural conformation, such as proline (Schellekens et al. 1998, 2000). Therefore, we expect that the CCP mAb produced by our rice suspension culture system will be of use for detection of CCP antigens in RA patient sera and should open a new approach for diagnosis of the disease.
Extensive research has been directed towards the development of diagnostic markers for RA, and this resulted in the identification the citrullination of proteins and the presence of an anti-citrullinated protein antibody (ACPA), an autoantibody against citrullinated proteins, in rheumatoid joints. Hence, ACPA is now used as a diagnostic marker for RA. The currently available commercial anti-CCP assay kits for diagnosis of RA operate in the following manner: a blood sample from a patient is allowed to bind to CCP peptides coated on commercially supplied microtiter plates; color development is then performed to qualitatively or quantitatively determine an autoantibody in the blood sample. However, there are many irrelevant autoantigens and autoantibodies in the blood of RA patients, and not all autoantigen candidates are citrullinated. Further, the secondary antibodies used in the anti-CCP assay kits are usually antibodies specific against mouse antigens, but may also recognize non-specific antigens present in the specimen sample, giving rise to false positive errors. To overcome this problem, Ju and Kim developed an ACPA from hybridoma cells for use in a kit for RA diagnosis (Ju and Kim 2015; Kim et al. 2015). These ACPA assay kits are designed to directly detect an antigen of specimen of RA onto micro titer plate coated with ACPA against RA. It is more accurate and provides a more rapid diagnosis based on the detection of citrullinated autoantigens in a test sample.
In this study, we demonstrated that CCP mAb was produced efficiently in transgenic rice cell suspension culture and was shown to be biologically active by binding to a specific-citrullinated antigen. Thus, we suggest that transgenic rice cell suspension culture can be employed for high-yield production of monoclonal antibodies with biological activity, and the monoclonal antibodies to RA produced in the suspension culture may be used in the early diagnosis of RA.
References
Alexiou I, Germenis A, Zioqas A, Thedoridou K, Sakkas LI (2007) Diagnostic value of anti-cyclic citrullinated peptide antibodies in Greek patients with rheumatoid arthritis. BMC Musculoskelet Disord 8:37
Aljanabi SM, Martinez I (1997) Universal and rapid salt-extraction of high quality genomic DNA for PCR-based techniques. Nucl Acids Res 25:4692–4693
Brar HK, Bhattacharyya MK (2012) Expression of a single-chain variable-fragment antibody against a Fusarium virguliforme toxin peptide enhances tolerance to sudden death syndrome in transgenic soybean plants. Mol Plant Microbe Interact 25:817–824
Bukhari MA, Wiles NJ, Lunt M, Harrison BJ, Scott DG, Symmons DP, Silman AJ (2003) Influence of disease modifying therapy on radiographic outcome in inflammatory polyarthritis at five years: results from a large observational inception study. Arthritis Rheum 48:46–53
Buyel JF, Twyman RM, Fischer R (2017) Very-large-scale production of antibodies in plants: the biologization of manufacturing. Biotechnol Adv 35:458–465
Casadevall A (2002) Passive antibody administration (immediate immunity) as a specific defense against biological weapons. Emerg Infect Dis 8:833–841
Chen MH, Liu LF, Chen YR, Wu HK, Yu SM (1994) Expression of α-amylases, carbohydrate metabolism, and autophagy in cultured rice cells is coordinately regulated by sugar nutrient. Plant J 6:625–636
Gelvin SB (2017) Integration of Agrobacterium T-DNA into the plant genome. Annu Rev Genet 51:195–217
Giritch A, Marillonnet S, Engler C, van Eldik G, Botterman J, Klimyuk V, Gleba Y (2006) Rapid high-yield expression of full-size IgG antibodies in plants coinfected with noncompeting viral vectors. Proc Natl Acad Sci USA 103:14701–14706
Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6:271–282
Higel F, Seidl A, Sorgel F, Friess W (2016) N-glycosylation heterogeneity and the influence on structure, function and pharmacokinetics of monoclonal antibodies and Fc fusion proteins. Eur J Pharm Biopharm 100:94–100
Hong SY, Lee TS, Kim J, Jung JH, Cho CW, Kim TG, Kwon TH, Jang YS, Yang MS (2008) Tumor targeting of humanized fragment antibody secreted from transgenic rice cell suspension culture. Plant Mol Biol 68:413–422
Huang N, Chandler J, Thomas BR, Koizumi N, Rodriguez R (1993) Metabolic regulation of α-amylase gene expression in transgenic cell cultures of rice (Oryza sativa L.). Plant Mol Biol 23:737–747
Huang Z, Phoolcharoen W, Lai H, Piensook K, Cardineau G, Zeitlin L, Whaley KJ, Arntzen CJ, Mason HS, Chen Q (2010) High-level rapid production of full-size monoclonal antibodies in plants by a single-vector DNA replicon system. Biotechnol Bioeng 106:9–17
Itty S, Pulido JS, Bakri S, Baratz KH, Matteson EL, Hodge DO (2008) Anti-cyclic citrullinated peptide, rheumatoid factor, and ocular symptoms typical of rheumatoid arthritis. Trans Am Ophthalmol Soc 106:75–81
Ju JH, Kim YK (2015) Rheumatoid arthritis diagnosis kit. United States Patent Application Publication. US2015/0080245 A1
Jung JW, Kim NS, Jang SH, Shin YJ, Yang MS (2016) Production and characterization of recombinant human acid α-glucosidase in transgenic rice cell suspension culture. J Biotechnol 226:44–53
Kim NS, Yu HY, Chung ND, Shin YJ, Kwon TH, Yang MS (2011) Production of functional recombinant bovine trypsin in transgenic rice cell suspension cultures. Protein Expr Purif 76:121–126
Kim NS, Jang SH, Yu HY, Chung ND, Kwon TH, Yang MS, Kim TG (2013) Amylase and cysteine proteinase gene knockdown in rice cells using RNA interference for enhancing production of recombinant proteins. Plant Cell Tiss Organ Cult 114:97–107
Kim BG, Kim SH, Kim NS, Huy NX, Choi YS, Lee JY, Jang YS, Yang MS, Kim TG (2014) Production of monoclonal antibody against FimA protein from Porphyromonas gingivalis in rice cell suspension culture. Plant Cell Tiss Organ Cult 118:293–304
Kim YK, Lee J, Jung H, Hyoju Yi, Rim YA, Jung SM, Ju JH (2015) Development of synthetic anti-cyclic citrullinated peptide antibody and its arthritogenic role. Clin Transl Immunol 4:e51
Kolotilin I, Topp E, Cox E, Devriendt B, Conarad U, Joensuu J, Stöger E, Warzecha H, McAllister T, Potter A, McLean MD, Hall JC, Menassa R (2014) Plant-based solutions for veterinary immunotherapeutics and prophylactics. Vet Res 45:117
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head bacteriophage T4. Nature 227:680–685
Lallemand J, Bouche F, Desiron C, Stautemas J, De Lemos Esteves F, Perilleux C, Tocquin P (2015) Extracellular peptidase hunting for improvement of protein production in plant cells and roots. Front Plant Sci 6:1–10
Landewe RB (2003) The benefits of early treatment in rheumatoid arthritis: confounding by indication, and the issue of timing. Arthritis Rheum 48:1–5
Lard LR, Visser H, Speyer I, vander Horst-Bruinsma IE, Zwinderman AH, Breedveld FC, Hazes JM (2001) Early versus delayed treatment in patients with recent-onset rheumatoid arthritis: comparison of two cohorts who received different treatment strategies. Am J Med 111:446–451
Larrick JW, Yu L, Naftzger C, Wycoff K (2001) Production of secretory IgA antibodies in plants. Biomol Eng 18:87–94
Magy B, Tollet J, Laterre R, Boutry M, Navarre C (2014) Accumulation of secreted antibodies in plant cell cultures varies according to the isotype, host species and culture conditions. Plant Biotechnol J 12:457–467
Martinez EO, Ramirez DFH, Nunez-Alvarez CA, Cabiedes J (2011) Citrullinated proteins in rheumatoid arthritis. Reumatol Clin 7:68–71
McDonald KA, Hong LM, Trombly DM, Xie Q, Jackman AP (2005) Production of alpha-1-antitrypsin from transgenic rice cell culture in a membrane bioreactor. Biotechnol Prog 21:728–734
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, vol 1, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Santos RB, Abranches R, Fischer R, Sack M, Holland T (2016) Putting the spotlight back on plant suspension cultures. Front Plant Sci 7:297
Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, van Venrooij WJ (1998) Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest 101:273–281
Schellekens GA, Visser H, de Jong BA, van den Hoogen FH, Hazes JM, Breedveld FC, van Venrooij WJ (2000) The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum 43:155–163
Schillberg S, Raven N, Fischer R, Twyman RM, Schiermeyer A (2013) Molecular farming of pharmaceutical proteins using plant suspension cell and tissue cultures. Curr Pharm Des 19:5531–5542
Serdaroglu M, Cakirbay H, Deger O, Cengiz S, Kul S (2008) The association of anti-CCP antibodies with disease activity in rheumatoid arthritis. Rheumatol Int 28:965–970
Shin YJ, Hong SY, Kwon TH, Jang YS, Yang MS (2003) High level of expression of recombinant human granulocyte-macrophage colony stimulating factor in transgenic rice cell suspension culture. Biotechnol Bioeng 82:778–783
Simmons CR, Huang N, Cao Y, Rodriguez RI (1991) Synthesis and secretion of α-amylase by rice callus: evidence for differential gene expression. Biotechnol Bioeng 38:545–551
Van Haute E, Joos H, Maes M, Warren G, Van Montagu M, Schell J (1983) Intergeneric transfer and exchange recombination of restriction fragments cloned in pBR322: a novel strategy for the reversed genetics of the Ti plasmids of Agrobacterium tumefaciens. EMBO J 2:411–417
Vossenaar ER, Van Venrooij WJ (2004) Anti-CCP antibodies, a highly specific marker for (early rheumatoid arthritis. Clin Applied Immunol Rev 4:239–262
Weiner GJ (2015) Building better monoclonal antibody-based therapeutics. Nat Rev Cancer 15:361–370
Wycoff KL (2005) Secretory IgA antibodies from plants. Curr Pharm Des 11:2429–2437
Xu J, Zhang N (2014) On the way to commercializing plant cell culture platform for biopharmaceuticals: present status and prospect. Pharm Bioprocess 2:499–518
Zeitli L, Olmsted SS, Moench TR, Co MS, Martinel BJ, Paradkar VM, Russell DR, Queen C, Cone RA, Whaley KJ (1998) A humanized monoclonal antibody produced in transgenic plants for immunoprotection of the vagina against genital herpes. Nat Biotechnol 16:1361–1364
Acknowledgements
This research was supported by the Advanced Production Technology Development Program, Ministry for Food Agriculture, Forestry and Fisheries (312037-05) and Do Van Giap was supported by the BK21 plus program in the Department of Bioactive Material Sciences, Chonbuk National University, Republic of Korea. The authors are appreciative of Dr. JH Ju of Catholic University for providing hybridoma cell line and also grateful to Dr. YS Jang of Chonbuk National University for performing the cloning of heavy and light chain of CCP from hybridoma cell.
Author information
Authors and Affiliations
Contributions
DV and JW carried out almost all experiments in this study and prepared the manuscripts. NS provided suggestions for experiments and wrote the manuscript. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Van Giap, D., Jung, JW. & Kim, NS. Production of functional recombinant cyclic citrullinated peptide monoclonal antibody in transgenic rice cell suspension culture. Transgenic Res 28, 177–188 (2019). https://doi.org/10.1007/s11248-019-00113-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-019-00113-w