Abstract
One of the factors that may influence the rate of cross-fertilization is the relative size of the pollen donor and receptor fields. We designed a spatial distribution with four varieties of genetically-modified (GM) yellow maize to generate different sized fields while maintaining a constant distance to neighbouring fields of conventional white kernel maize. Samples of cross-fertilized, yellow kernels in white cobs were collected from all of the adjacent fields at different distances. A special series of samples was collected at distances of 0, 2, 5, 10, 20, 40, 80 and 120 m following a transect traced in the dominant down-wind direction in order to identify the origin of the pollen through SSR analysis. The size of the receptor fields should be taken into account, especially when they extend in the same direction than the GM pollen flow is coming. From collected data, we then validated a function that takes into account the gene flow found in the field border and that is very useful for estimating the % of GM that can be found in any point of the field. It also serves to predict the total GM content of the field due to cross fertilization. Using SSR analysis to identify the origin of pollen showed that while changes in the size of the donor field clearly influence the percentage of GMO detected, this effect is moderate. This study demonstrates that doubling the donor field size resulted in an approximate increase of GM content in the receptor field of 7%. This indicates that variations in the size of the donor field have a smaller influence on GM content than variations in the size of the receptor field.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The rapid adoption of GM (genetically modified) technology could give rise to landscapes with a large quantity of GM plots, which may have repercussions for coexistence with conventional plots in terms of gene flow. According to EU legal requirements, Member States need to implement coexistence legislation in order to ensure that genetically modified (GM) and non-GM crops can develop side-by-side without excluding either agricultural option. Tolerance thresholds for the unintentional or technically unavoidable presence of approved GM material in non-GM products have already been put in place for food, animal feed and organic products (European-Commission 2003a; European-Commission 2003b).
According to Devos et al. (2009), the main factors that determine the adventitious presence of GM material in a conventional field are: accidental seed impurity, sowing equipment and practices, cross-fertilization between GM and non-GM crops, the presence of volunteers or product mixtures during harvesting, and transport and storage processes. Of these, cross-fertilization is the major potential biological source of on-farm mixing in the case of maize.
The maize plant is monoecious, with separate male and female flowers on the same plant. Maize is also protandrous, with pollen being shed before the silks are receptive; but as there is some overlap, self-pollination may occur in up to five per cent of cases (Purseglove 1972). Numerous trials have investigated maize pollen dispersal (for a review, see Brookes and Barfoot 2004; Devos et al. 2005; Emberlin et al. 1999; Sanvido et al. 2008; Treu and Emberlin 2000; Van De Wiel and Lotz 2006). These studies clearly show that even though maize pollen is relatively large and heavy compared to other species, it can travel long distances with the airflow when suitable meteorological conditions occur. These studies also show that most cross-fertilization events occur within 50 m of the pollen source, while vertical wind movements or gusts during pollen shedding only lead to very low levels of cross-fertilization over longer distances.
The rate of cross-fertilization between fields also depends on pollen viability, flowering synchronicity, climate conditions and the relative concentration of GM/non-GM pollen in receptor plots, which—in turn—may depend on the distance between the fields and on the relative size of the pollen donor and receptor fields. In recent years, several studies have sought to estimate the utility of using separation distances to control or minimize the adventitious presence of GM maize due to cross-fertilization (Bannert et al. 2008; Henry et al. 2003; Ma et al. 2004; Melé et al. 2004; Pla et al. 2006; Weber et al. 2007). From these studies, it can be concluded that a separation distance of 20–25 m is generally enough to maintain the GM content below the permitted 0.9% threshold in the yield of neighbouring fields of non-GM maize. Occasionally, however, and particularly in the case of small fields of less than 0.5 ha and/or of long, narrow fields that are located downwind from a larger GM maize field, the isolation distance may need to be extended to 50 m or more (Devos et al. 2005; Hüsken et al. 2007; Messéan et al. 2006). Based on a statistical analysis of different datasets on cross-fertilisation rates, Riesgo et al. (2010) concluded that a separation distance of 40 m is sufficient to reduce admixture in maize cultivation to below the legal threshold of 0.9%. However, these separation distances can be reduced if the GM crop in question is surrounded by a buffer of non-GM maize plants, as the effect of cross-fertilization is reduced much more effectively by a pollen barrier than by an isolation distance of bare ground of the same width (Della Porta et al. 2006; Pla et al. 2006). Bearing in mind the fact that gene flow accumulates at the border of the field, it may be more effective to surround the recipient field with a few rows of maize to reduce the adventitious GM content due to cross-fertilization (Della Porta et al. 2008).
Synchronization of pollen dispersal and silking is crucial to achieving high levels of out-crossing in maize, as demonstrated by several authors (Bassetti and Westgate 1993; Bassetti and Westgate 1994; Uribelarrea et al. 2002; Westgate et al. 2003). For maize grown in a temperate area, a difference in sowing date resulted in a difference in flowering time that limited cross-fertilization. This strategy could be effective in improving coexistence (Messeguer and Melé 2006; Palaudelmàs et al. 2008; Messeguer et al. 2006).
Another factor that may influence the rate of cross-fertilization is the size of the transgenic pollen donor field in relation to the receptor, but few studies have, as yet, been published to demonstrate to what extent the size of the donor field may influence the GMO content of a conventional maize field. Melé et al. (2004) showed that with a Bt pollen donor field of 0.25 ha, the level of cross-fertilization decreased from 1.77 to 0.83% when the size of the recipient field was increased from 0.25 to 1 ha. Weber et al. (2007) undertook a coexistence study in Germany in which 27 sites were sown with transgenic Bt Mon810 and surrounded by near isogenic conventional maize. The planting scheme included maize fields of very different sizes (from 0.3 to 23 ha). No relationship was found between Bt maize field size and the percentage of GM DNA. The xenia effect can be defined as the influence of the genes from the male parent on the development of the fruit or seeds. Based on this Xenia effect, Bannert et al. (2008) found that in small field environments, widely varying ratios of donor to receptor field size (around 4:1–1:8) did not influence the cross-pollination rate at distances of 0–20 m from the pollen donor. Using the MAPOD model, several case scenarios were simulated (Messéan et al. 2006) considering a 15 ha GM field and different sized non-GM field areas: 3, 5, 7.5, 12.5 and 15 ha. It seems clear that the larger the recipient field, the more its own pollen mass acts as competitor for any incoming pollen, but more studies are needed to identify to what extent the size of a donor transgenic field may influence the GMO content of a conventional one.
Here, we present a field trial aimed at estimating the effect of receptor field size by measuring the Xenia effect at different distances from the Bt maize field. The trial also sought to estimate the effect of the size of the donor transgenic field by identifying the origin of transgenic pollen received from fields of increasing sizes through simple sequence repeat (SSR) analysis.
Molecular markers allow genotype analysis at the polymorphism level. Simple sequence repeats (SSR) are highly polymorphic, co-dominant markers, which contain a short nucleotide sequence (1–6 bp) repeated in tandem (Hamada et al. 1982). SSR markers have proven useful in parentage analysis and in the genetic characterization of cultivars and have been used as genetic markers for varietal identification and to construct genetic maps in plants such as Arabidopsis (Depeiges et al. 1995), wild strawberry (Sargent et al. 2006), wheat (Plaschke et al. 1995) and maize (Senior and Heun 1993; Smith et al. 1997). Many different electrophoresis techniques can be used to separate SSR alleles. The method most currently accepted is based on PCR with dye-labelled primers, with the fragments being analyzed by capillary electrophoresis in automatic sequencers.
Here, polymorphic microsatellites (SSR) were used to identify the origin of the maize pollen from a neighbouring field. Four varieties of yellow GM maize were spatially distributed to give different size fields. The origin of the yellow kernels in conventional cultivars as a result of cross-pollination was analyzed and attributed to demonstrate the influence of field size.
Materials and methods
Plant material
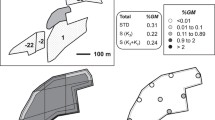
Maize samples were obtained from a field trial conducted at Pla de Foixà, Girona (Spain). Four different Bt yellow kernel commercial hybrids derived from event Mon810 (Monsanto Co) were sown in the centre of the field, along an axis following the dominant wind direction and forming rectangles of increasing area (Fig. 1). These areas were comprised of the following plots: Aristis Bt (Nickerson-Senasa) about 0.25 hectares (ha); DKC6575 (Monsanto Co) about 0.75 ha; PR33P67 and PR32P76 (Pioneer Hi Bred) about 1.25 and 1.75 ha, respectively. Non-transgenic white kernel hybrid PR32Y52 maize (Pioneer Hi Bred) was sown in the surrounding area to fill a total area of approximately 27 ha (Fig. 1). The field trial was situated a few kilometres from the sea, on the plain at the mouth of the River Ter. During the flowering period, a midday sea wind is fairly usual due to differential heat accumulation between the sea and land.
All the GM varieties were sown on 23rd April, while the surrounding conventional varieties were sown from 24th to 26th April. The fields were worked following normal agricultural practices in the zone. During the initial growing stages, occasional volunteers in the whole field were pulled out by hand.
An automated weather station (Weather Wizard III, Davis Instruments, Hayward, CA) in the experimental field recorded temperature, wind strength and wind direction during the flowering period. The software WeatherLink for Windows V 5.4 (Davis Instruments, Hayward, CA) was used to transfer the data to the computer. Daily rainfall data were acquired from the National Environmental Services Station close to the experimental site. The wind register during the flowering period (from 5th to 25th July 2004), measured by the weather station in the centre of the transgenic field, was expressed in wind run values (km), which is measurement of the “amount” of wind passing the station during a given period, expressed in “kilometres of wind”. This measure takes into account the speed and frequency of the wind in each direction.
During the flowering stage, flowering dynamics were visually evaluated for 150 plants of each transgenic variety and for fields A1, A2 and B which contained conventional white maize. The flowering date for each variety corresponded to the moment at which 50% of the plants evaluated entered the flowering stage.
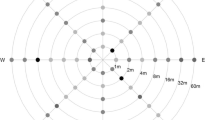
Three sampling methods were applied in the receptor field (see Fig. 1); (1) a stratified method which drew a 10 × 10 m grid in the main direction of the wind, that is, in the north-western part of the conventional field (field A1). Samples (three cobs each one) were collected every 10 m in both orientations, abscises and ordinates and, in addition, at 0, 2, 5 and 10 m from the nearest border to the GM field in abscises direction on the conventional field. (2) a stratified method, similar to that previously described, but using a 20 × 20 m grid (fields A2, B, C and D, i.e. the remainder of the conventional crop that was not in the main direction of the wind). The objective of both stratified methods was to determine the crosspollination rate in the conventional field; (3) a diagonal sampling method was applied in order to perform the SSR analysis study. This consisted of a transect traced from the border of A2 and GM plot and along the direction of the prevailing wind (field A1, Fig. 1). Samples were collected at 0, 2, 5, 10, 20, 40, 80 and 120 m from the nearest point to the GM field. Since there was a 10 m path between the two fields, the real distances between the conventional and GM fields were 10, 12, 15, 20, 30 50, 90 and 130 m. A variable number of cobs were collected for each sampling point in order to have enough cross-pollinated kernels from each one of them. After harvesting, all the samples were stored in an empty greenhouse (at 20–35°C) for 3 months.
The assessment of gene flow was carried out by counting the number of yellow kernels in white cobs. As transgenic varieties are homozygous for the co-dominant allele responsible for yellow colour {Kiesselbach, 1999 #164} but hemizygous for the transgene {Pla, 2006 #51} (inserted in a single site and displaying Mendelian inheritance, www.agbios.com), it was expected that all the pollen grains would be yellow, but only half would be transgenic. All of the calculations of the assay were made considering the yellow kernel trait transmission rather than the GM Bt trait. The percentage of GMO was only used when evaluating the field size effects in order to compare the results with similar studies.
DNA isolation and quantification
Yellow kernels in white cobs collected using the Diagonal Sampling method, were grown in the greenhouse. Each resulting plant was frozen in liquid nitrogen when it reached an early vegetative stage (about 15 days after sowing). Maize genomic DNA was subsequently isolated from green ground leaves using a CTAB based protocol (Doyle and Doyle 1990). The extracted DNA was then quantified using the GeneQuant RNA/DNA Calculator spectrophotometer (Amersham Pharmacia Biotech Europe GmbH, Freiburg, Germany) and diluted to a concentration of 50 ngμl−1.
Microsatellite marker analysis
Of the 14 SSRs studied (see Online Resource 1), five were selected to identify polymorphism in the six hybrids (Table 1). Those SSRs were obtained from two main sources (Bonamico et al. 2004; Smith et al. 1997) but also used by other authors (Enoki et al. 2002; Gethi et al. 2002; Kozhukhova and Sivolap 2004; Legesse et al. 2007; Liu et al. 2003; Reif et al. 2006; Salgado et al. 2006). These SSRs were chosen due to the distinctness of the parental polymorphic bands found in the silver stained acrylamide sequencing gels.
Selected primers were used for PCR amplification of the DNA samples. PCR reactions were performed in a 10.5 μl final volume containing 1× LAB buffer, 0.3 units of AmpliTaq DNA polymerase (Roche Diagnostics), 0.15 mM MgCl2, 0.02 mM of each dNTP, 0.002 nM of reverse primer and forward labelled primer, and 100 ng of genomic DNA. The reaction consisted of a 2 min denaturation at 95°C, 35 cycles of 1 min at 95°C, 30 s at 59–65°C (see Online Resource 1 for details of each SSR), 30 s at 72°C, and a final extension step of 7 min at 72°C.
Each amplified PCR product was separated on an Abi Prism 3130 xl DNA Analyzer (Applied Biosystems) and data were collected by Gene Mapper Version 4.0 software (Applied Biosystems).
Methods for differentiating the hybrids
Since it was not possible to find a single primer that could separate all the commercial parental hybrids used here, five primers were selected to identify the five potential pollen donor hybrids: phi015, phi056, phi072, phi085 and phi073. As all the samples came from white cobs, the female parental line was always PR32Y52.
Several identification probes were performed with commercial seeds of each hybrid variety to establish the order of robustness of the markers (Table 1). All possible inheritances of descendants can also be deduced from this table. Some allele sizes were exclusive for a specific parental variety, for example, 89 for PR33P76 when amplified with primer phi015 and 237 for DKC6575 when amplified with phi056. Others had a common origin and it was not possible to discriminate among parental lines.
Statistical analyses
A statistical analysis to evaluate the cross-fertilization rate was carried out using the GLM procedure from SAS/STAT™ software (SAS Institute Inc. Release 8.2 for Windows).
Results and discussion
Since the aim of this field trial was to evaluate gene flow, the good flowering synchrony achieved by all the maize varieties was essential. In the field trial, the flowering of variety PR32Y52 totally coincided with that of PR32P76. Varieties DKC6575 and Aristis Bt flowered 1 day later, while PR33P67 flowered 1 day before (Online Resource 2). Silks were exposed within 5 days of the onset of silking (Carcova et al. 2000) and silks that were not fertilized begin to senesce within about 8 days of emergence from the husk (Anderson et al. 2004; Bassetti and Westgate 1993). Receptive silks were therefore only available for a brief time. The majority of pollen produced by a maize crop is shed within a 5–8 day period but low levels of early and late pollen release may extend for a few days more. In the region where the field trial was performed, and according to its climatic conditions, it is assumed that the period within which fertilization could take place lasts for about 10 days. In consequence, only a 1 day delay in the flowering time corresponded to a high coincidence of flowering among the varieties studied.
The assay design took into account the predominant wind direction in this region for the wind effect and the main diagonal of the assay was oriented along this axis (Fig. 1). The predominant winds blew towards south–south-east (SSE) and were regular and smooth. Their effect was quite important during the 20 days of the flowering period and they typically travelled 300 km; this means that a particle flowing in the wind could have advanced this distance in 20 days (Online Resource 2). The wind direction followed the usual pattern for this zone at this time of year during the most propitious hours for anther dehiscence and pollen emission. This has been shown to favour transgenic pollen movements towards conventional fields in the same region (Melé et al. 2004; Messeguer et al. 2003). During this period, some gusts of wind from the north were also occasionally registered. In 2004, however, the northern wind had a very low frequency and mainly blew at night, when viable pollen was not released (see Online Resource 2). The wind effect in cross-fertilization has also been largely demonstrated in other field trials (see review from Devos et al. 2009).
Several authors have demonstrated the usefulness of the xenia effect for quantifying pollen-mediated gene flow (Jones and Brooks 1950; Klein et al. 2003; Luna et al. 2001; Ma et al. 2004; Palaudelmàs et al. 2008; Pla et al. 2006). Here, by counting the yellow kernels in white cobs, we were able to work with great precision at very low cross-fertilization rates: often at below the quantification (0.1%) or even the detection threshold (0.01%) of quantitative PCR analysis.
The cross-fertilization rates obtained in each zone of the conventional white maize field are shown in Fig. 2. Cross-fertilization rates are expressed as percentages of yellow kernels. In fact, and as previously explained, the number of yellow kernels in white cobs related to the number of GM kernels in a proportion of 2:1. This was in line with the findings of (Pla et al. 2006) who analysed other samples belonging to the same field by means of Q-PCR.
The percentage of yellow kernels found in fields A1 and A2 decreased quickly on entering the conventional field. This percentage was higher in field A2 than in field A1. Both were separated from the transgenic field by a 10 m wide path but A2 was just in front of the GM field. Although A1 was in direction of the prevailing wind, it was farther away and protected by the conventional pollen of field B, which prevented crosspollination with the yellow pollen.
The values for the yellow kernel percentage obtained from the sampling for the SSR study were in accordance with those from the standard sampling. In this SSR study, the number of cobs collected at each distance was variable in order to guarantee a sufficient number of yellow kernels for each SSR analysis point. As a result, few cobs were collected near the GM field (i.e. 15 cobs at 10 m) while a great number of samples had to be taken from greater distances (i.e. 470 cobs at 90 m).
Gene flow values measured as a percentage of yellow kernels were higher in field B than in field C. Taking into account the fact that both fields were immediately next to the GM field, these differences are probably attributable to the wind effect. The fields were not clearly located either in the direction of or against the wind, but the southerly component was clearly favourable to field B.
The same argument could be applied for field D when comparing it to field A2. They were both separated from the GM field by a path, but A2 was in the direction of the wind and D was located in the opposite direction for the East–West component.
In general, in all the fields located around the central GM plot, the percentage of yellow kernels decreased towards the centre of the field, following a very similar pattern in all cases. The values found on the borders were variable (often significantly different) and depended on the distance between the fields and on their orientations relating to the direction of the wind, but in all cases, the diminution curves seem to belong to a family of equilateral hyperboles that could be described by the following expression:
where FX is the percentage of yellow kernels at a distance x; and F0 is the percentage of yellow kernels at the nearest field border to the transgenic field.
To demonstrate this tendency, Fig. 3 shows the transformation of the previous graphs when we divide each yellow kernel percentage by the percentage found on its field border (FX/F0 = 1/x + 1). For each distance, the average of the transformed values and its standard error is represented and compared with the theoretical curve (y = 1/x + 1).
This approximation is not very exact since the theoretical value at a distance of 2 m is a little higher and for greater distances (from 60 m on), it is a little lower. Nevertheless, it fits well for the other values. This uniform diminution suggests the presence of an own effect in the receptor field which uniformly smoothes out the different flow levels which arrive at the field. This effect could be explained by non GM pollen production by the own field which competes efficiently with foreign pollen and rapidly decreases in quantity on approaching the field border. The increase in cross pollination when detasseled plants are used in seed production plots would support this hypothesis (Goggi et al. 2007; Ireland et al. 2006). A complex theoretical curve with more variables and a better fit with the experimental data could probably be found; but F0/(x + 1) is simple and clearly describes the dramatic reduction of adventitious pollination inside the field. It is probable that higher values found at longer distances would also respond to their proximity to the border of the field on the opposite side.
In Fig. 3, the theoretical curve ln(x + 1)/x has also been represented. It shows the variability of the average total gene flow depending on field width. It has been obtained directly from the integral of the function 1/(x + 1) and, under real conditions, it could allow us to estimate the gene flow in a field with a width × (in the preferred direction of the out-coming flow) depending on the average value found at the field border. This consideration is important in terms of the regulations governing the coexistence of GMOs because the established thresholds usually refer to an average value for the whole field instead of a value found at a determined distance.
In order to check the adjustment of this theoretical approach, we compared the theoretical values obtained from neighbouring fields (A1, A2, B, C and D) at different depths (PRE) and those values obtained applying the formula F0ln(x + 1)/x to the percentages of yellow kernels found experimentally in the different considered zones (OBS) (Online Resource 3).
The prediction was very precise and the values obtained by applying the χ2 test were very acceptable. The percentage of adventitious pollination measured at the field border gave a very exact orientation with respect to the total percentage of cross pollination in the field and it depended on the width of the field measured in the direction of flow. We can also confirm that in zones with small fields (like the zone where this study was performed), doubling the width of the receptor field produced a reduction in the average total gene flow that was similar to multiplying by a factor of 0.6. After analysing fields of different sizes and located in different places, several authors (Bannert et al. 2008; Ireland et al. 2006) have suggested that the width of a field may have an effect on its GM content due to cross fertilisation. Here, we have demonstrated how the cross fertilization rate decreases as the width of the field increases.
Although we have considered the adventitious flow of the colour of the kernel, which is homozygous in commercial hybrids, a similar situation can be found when considering the transgene transmitted through pollen, but in this case only 50% of the kernels will carry the event.
In order to generalize these results, two considerations must be taken into account. The first is that it seems that the observed diminution was due to the protective effect of own pollen from receptor field. As there are differences in the amount of pollen produced by different commercial varieties, the adjustment function could produce a different hyperbole.
Secondly, it must be remembered that under real conditions of coexistence between GM and conventional fields, flow can come from different directions simultaneously and the irregular shape of fields and other factors such as physical barriers can interfere with this distribution. It is known, for example, that when a conventional field is surrounded by green fences, gene flow is higher some meters inside the field than on its border (Messeguer et al. 2006).
SSR analysis
The origin of pollen was identified using SSR molecular markers for individual yellow kernels in the different field zones. This technique is usually applied for varietal identification and can be very straightforward when working with fixed genotypes. In our case, as the analyzed samples were F1 generation and collected from white cobs, the maternal origin was well known but there were at least 5 pollen donor candidates (Aristis Bt, DKC 6575, PR33P67, PR32P76 and Eleonora). All these varieties are hybrid lines for commercial use and, as a consequence, selected for the same genetic characteristics, which results in a certain degree of genetic affinity. We found no SSR markers showing heterozygosis for the 5 varieties, so it was necessary to combine several markers to cover all the possibilities. Furthermore, as all the grains had the same maternal origin, the same combination of amplified fragments could be valid for several parents because only one of the alleles defined the pollen donor origin, and a given paternal candidate could be defined by several patterns.
By using the five SSRs selected (see “Materials and methods”), 934 yellow kernels were analyzed and a decision table was designed to identify their parental lines (Online Resource 4). All the samples were tested with SSR marker phi015, which gave seven different possibilities for allele inheritance. These combinations were 83/83, which determined the parent variety as Eleonora and 83/89 and 89/102 which identified the pollen donor as PR32P76. On the other hand, 96/83 and 96/102 discriminated for Aristis Bt, DKC6575 and PR33P67 but could not separate them; and 102/83 and 102/102 could have come from Aristis Bt, PR33P67, PR32P76 or Eleonora. The last two cases required further analysis with more primers. As PR32Y52 presented two alleles that were amplified by this primer (83 and 102), in Online Resource 4 these alleles have been grouped together, because the interpretation of the results was the same regardless which allele was inherited.
The next SSR marker used was phi056. This primer is homozygous for PR32Y52: the maternal line. This reduced the possibilities of allele inheritance to four possible combinations: (1) 237/254, which identified DKC6575 as a parental line; (2) 254/254, which could identify PR33P67 or Eleonora in samples where Eleonora had not been discarded by primer phi015; (3) 246/254 which could not distinguish between Aristis Bt and PR33P67, or between PR32P76, Aristis Bt, PR33P67 and Eleonora depending of phi105 alleles combination; and (4) 248/254, with a possible origin from Aristis Bt, DKC6575 or PR32P76. In the last three cases, the samples had to be analyzed with phi072, phi085 or phi073 primer.
As far as the SSR analyses were concerned, the results show an increasing number of sampled kernels on getting further away from the GM field (Table 2). The aim of this variability was to obtain a significant number of yellow kernels for each distance in order to perform the SSR analysis. It was found that a great number of yellow kernels had come from the Aristis Bt plot, which was the smallest plot, but the nearest to the diagonal established for the purpose of collecting samples for this study. The next two varieties maintained a similar proportion of crosspollination with conventional plants, while PR32P76, which was the furthest away, presented a lower pollination rate.
It was also possible to quantify the small percentage of pollen received from the Eleonora field, which was located more than 300 m from the receptor field (Fig. 1), with respect to other fields in the same property sown with this variety but located further away. This variety is a yellow kernel conventional hybrid which produced some background noise in this study (Table 2). It has been reported that maize pollen can flow large distances (for a review see (Devos et al. 2009)) and produce some cross-pollination (at a very low rate) depending on the specific pollen characteristics and climate conditions. This residual flow, together with the Off type, unidentified (Table 2), can be interpreted as the accumulative effect of all the maize fields being at the flowering stage at the same time and thereby acting as one very large donor field of yellow pollen. It is also possible that the unidentified kernels (off type) were a result of the impurity of the commercial sacks in which 99% of the certified seeds were stored. Another possible explanation is that some of these unidentified kernels could have been produced by volunteer pollen. Even though volunteers had been pulled out by hand during the early developmental stage of the field trial, we cannot be totally sure that none of them remained in the field and produced pollen. This could have contributed at a very low rate (Palaudelmàs et al. 2008) to the total content of yellow grains in the field.
The values shown in Table 2 are not comparable because the size of the sample studied was different in each case. Online Resource 5 therefore shows the relative percentages of yellow kernels according to their origin. The proportion of Aristis Bt was very high at short distances and tended to equilibrate with distance. On the other hand, PR32P76 seemed to play a complementary roll, with its proportion increasing with distance. PR33P67 and DKC6575 exhibited quite constant behaviour over distance.
Combining the information obtained when identifying the origin of the yellow kernels collected at different distances with the % of cross fertilisation obtained at each distance (Table 2), we were able to quantify the contribution of each GM field to the total GMO content recorded in the receptor field. And, knowing the contribution of each variety to the total GM content at this point, we were able to see the effect which corresponded to donor fields of increasing size.
For example, from data from Table 2, it was possible to calculate that at the receptor field border, on the other side of the separation path between field A and the GM field (10 m distance), the percentage of yellow kernels was 9.7% of the total number of kernels sampled. By applying SSR analysis, we subsequently saw that 74.59% of these kernels came from the Aristis Bt plot; 9.9% from DKC; 9.24% from PR33P67 and 4.29% from PR32P76; all of them were GM commercial hybrid varieties produced from only one GM paternal line which carried the transgene in homozygosis and also another conventional line. Thus, only 50% of their pollen carried the transgene. If pollen from Eleonora and Off Type is discarded, the GM content found was:
Figure 4 shows the GM value attributable to the growing surfaces of the GM fields for each distance. Thus, Aristis Bt pollen represents the contribution of a 0.25 ha field, while the addition of Aristis Bt and DKC6575 corresponds to the effect of a 1 ha field and the global content of 4.75% represents a 4 ha field.
Although several distances are represented in Fig. 4, the 10 m distance is very important since it is the separation between the receptor field border and the GM field and, as has already been demonstrated, this gives a good estimation of the total GM content in the receptor field.
The curve seems to show asymptotic behaviour, with smaller increments as the area of the donor field increases. In theory, this would seem to be logical, because when the field width exceeds the maximum distance that viable pollen can fly there will not be any further increase in GM content, as the majority of the pollen would fall within the donor field.
In the case studied, and with a field trial design in which GM fields are located in the main wind direction simulating a variable size donor field, when doubling the donor field size, an approximated increase in the GM content of the receptor field of about 7% is reflected: (4.75/3.62)¼ = 1.07. In fact, 3.62 is the % GM produced by Aristis Bt alone whereas 4.75 is the % GM produced by the whole transgenic field (all four varieties; see Fig. 4). 1.07 is the ratio that allows going from 3.62 to 4.75 in four steps. This implies four doublings in size: from 0.25 to 0.5; from 0.5 to 1; from 1 to 2; and then from 2 to 4 ha. This value indicates that the variations in donor field size have a smaller influence on GM content than the variations in the receptor field size.
Generalising these results may cause some controversy because some of the factors are quite difficult to regulate, such as the amount of emitted pollen and the possible differences in compatibility between the varieties used in this assay.
Even so, in this field trial, all the varieties exhibited similar behaviour since the cross pollination values at the border between the GM field and conventional field B were very similar throughout the entire contact zone. If any of the GM varieties had had fewer options to pollinate the conventional plants, a decrease in adventitious pollination by the variety(s) in question would have been detected in the contiguous zone.
Conclusions
This field trial was specially design to estimate the effect of the relative size of the pollen donor and receptor maize fields in cross fertilization between Bt and conventional maize. Flowering coincidence was of crucial importance to this study. All the varieties flowered simultaneously, except for a 1 day delay between two of them and this did not influence the results of the trial. Other studies (Della Porta et al. 2008) in which little or no reduction in pollen flow was observed when there were only up to 3 days of difference in flowering time between the pollen source and the recipient support this conclusion.
The sizes of the receptor fields should be taken into account, especially when they are elongated in the same direction as the GM pollen flow. Although several authors have previously suggested the importance of this factor (Devos et al. 2005; Hüsken et al. 2007; Messéan et al. 2006), here, we have clearly demonstrated it, based on all experimental data obtained in a field trial.
As has also been demonstrated in other trials (for a review see (2009), under real conditions of coexistence (Ireland et al. 2006; Messeguer et al. 2006) gene flow decreases quickly with the depth of the receptor field. The % of GM found at the borders differs from case to case, according to its relative position with respect to the transgenic field and the direction of the prevailing wind, but in all cases the shape of the diminution curve follows a similar pattern. Based on our experimental results, we validated the function FX = F0/(x + 1) which, at a practical level, may be very useful for estimating the % GM that can be found at any point in the field when we know the % GM at the border. This approach may be also useful for predicting the total GM content of a field due to cross fertilization. Even so, it must be remembered that sampling a field to determine its GM content is a very hard and expensive task. Concentrating sampling along the field borders could be a good way to simplify the collection of samples and to obtain an adjusted value for the whole field.
The results obtained with the SSR analysis to identify the origin of the pollen allowed us to calculate the effect of GMO fields of increasing sizes on a conventional field because by identifying the origin of pollen, cross-fertilization could be attributed to each GM variety. While changes in the size of the donor field clearly influenced the percentage of GMO detected, this effect was moderate. This study demonstrates that doubling the size of the donor field produced an approximate increase in the GM content of the receptor field of 7%. This value suggests that variations in the size of the donor field would have a smaller influence on the GM content than variations in the size of the receptor field.
In a real coexistence scenario, the estimation of the adventitious presence of GM maize in a conventional field would be made just after harvesting and usually in the store silos, where a decision has be taken as to what direction this yield has to go in: transgenic or conventional. However, sometimes it will be necessary to assess the GMO content under field conditions because either the producer will want to know this or because of suspicions that the GMO content will be too high. In the case of lawsuit, this information will be needed, to show where the pollen has come from. In such a case, the data presented here could be very useful to determine how samples should be taken in order to determine the global GM content of fields, starting with measurements taken at the field borders.
It is clear that SSR analysis could give some indications about the varieties located outside the conventional field that have pollinated some of the plants, but it would be extremely difficult and expensive to obtain an accurate enough quantification to be able to declare a particular field responsible for one form of detected gene flow.
It is necessary to establish a coexistence regulation based on distances, flowering synchrony and other factors that participate in cross-fertilization and that are measurable and dependable. This will lead to the adoption of good practices and will minimize the need to make use of costly molecular analyses.
References
Anderson SR, Lauer MJ, Schoper JB, Shibles RM (2004) Pollination timing effects on kernel set and silk receptivity in four maize hybrids. Crop Sci 44:464–473
Bannert M, Vogler A, Stamp P (2008) Short-distance cross-pollination of maize in a small-field landscape as monitored by grain color markers. Eur J Agron 29:29–32
Bassetti P, Westgate ME (1993) Emergence, elongation, and senescence of maize silks. Crop Sci 33:271–275
Bassetti P, Westgate ME (1994) Floral asynchrony and Kernel Set in maize quantified by image-analysis. Agron J 86:699–703
Bonamico N, Aiassa J, Ibanez M, Renzo Md, Diaz D, Salerno J (2004) Characterization and classification of single cross hybrids of maize with SSR markers. RIA, Revista de Investigaciones Agropecuarias 33:129–144
Brookes G, Barfoot P (2004) Coexistence of GM and Non GM crops: case study of maize grown in Spain. In: PEL (ed) Dorchester, UK. http://www.pgeconomics.co.uk
Carcova J, Uribelarrea M, Borras L, Otegui ME, Westgate ME (2000) Synchronous pollination within and between ears improves kernel set in maize. Crop Sci 40:1056–1061
Della Porta G, Ederle D, Bucchini L, Prandi M, Pozzi C, Verderio A (2006) Gene flow between neighbouring maize fields in the Po Valley In: Agrobiotecnologico CD (ed), Milan, Italy
Della Porta G, Ederle D, Bucchini L, Prandi M, Verderio A, Pozzi C (2008) Maize pollen mediated gene flow in the Po valley (Italy): source-recipient distance and effect of flowering time. Eur J Agron 28:255–265
Depeiges A, Goubely C, Lenoir A, Cocherel S, Picard G, Raynal M, Grellet F, Delseny M (1995) Identification of the most represented repeated motifs in Arabidopsis thaliana microsatellite loci. Theor Appl Genet 91:160–168
Devos Y, Reheul D, De Schrijver A (2005) The co-existence between transgenic and non-transgenic maize in the European Union: a focus on pollen flow and cross-fertilization. Environ Biosaf Res 4:71–87
Devos Y, Demont M, Dillen K, Reheul D, Kaiser M, Sanvido O (2009) Coexistence of genetically modified (GM) and non-GM crops in the European Union. A review. Agron Sustain Dev 29:11–30
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Emberlin J, Adams-Groom B, Tidmarsh J (1999) A report on the dispersal of maize pollen. In. Soil association. National Pollen Research Unit. University College Worcester, Worcester
Enoki H, Sato H, Koinuma K (2002) SSR analysis of genetic diversity among maize inbred lines adapted to cold regions of Japan. Theor Appl Genet 104:1270–1277
European-Commission (2003a) Regulation no. 1829/2003 of the European Parliament and of the Council of 22 September 2003 on genetically modified food and feed. Off J Eur Commun 1–23
European-Commission (2003b) Regulation no. 1830/2003 of the European Parliament and of the Council of 22 September 2003 concerning the traceability and labelling of genetically modified organisms and the traceability of food and feed products produced from genetically modified organisms and amending directive 2001/18/EC. Off J Eur Union 24–28
Gethi JG, Labate JA, Lamkey KR, Smith ME, Kresovich S (2002) SSR variation in important US maize inbred lines. Crop Sci 42:951–957
Goggi AS, Lopez-Sanchez H, Caragea P, Westgate M, Arritt R, Clark CA (2007) Gene flow in maize fields with different local pollen densities. Int J Biometeorol 51:493–503
Hamada H, Petrino MG, Kakunaga T (1982) A novel repeated element with Z-DNA-forming potential is widely found in evolutionarily diverse eukaryotic genomes. Proc Nat Acad Sci USA Biol Sci 79:6465–6469
Henry C, Morgan D, Weekes R, Daniels R, Boffey C (2003) Farm scale evaluations of GM crops: monitoring gene flow from GM crops to non GM equivalents in the vicinity: part one forage maize. In: EPG/1/5/138 DR (ed). Department for Environment Food and Rural Affairs, London
Hüsken A, Ammann K, Messeguer J, Papa R, Robson P, Shiemann J, Squire G, Stamp P, Sweet J, Wilhelm R (2007) A major European synthesis of data on pollen and seed mediated gene flow in maize in the SIGMEA project. In: Stein A, Rodríguez-Cerezo E (Eds) Books of abstracts of the third International conference on Coexistence between Genetically Modified (GM) and non-GM-based Agricultural supply Chains European Commission. Stein A., Rodríguez-Cerezo E., pp 53–56
Ireland DS, Wilson DO, Westgate ME, Burris JS, Lauer MJ (2006) Managing reproductive isolation in hybrid seed corn production. Crop Sci 46:1445–1455
Jones M, Brooks J (1950) Effectiveness of distance and border rows in preventing outcrossing in corn. In: Tech Bull T-38. Oklahoma Agric. Exp. Stn., Stillwater
Klein EK, Lavigne C, Foueillassar X, Gouyon PH, Laredo C (2003) Corn pollen dispersal: Quasi-mechanistic models and field experiments. Ecol Monogr 73:131–150
Kozhukhova NE, Sivolap YM (2004) Identification and registration of maize genotypes with the use of molecular markers. Russ J Genetics 40:49–55
Legesse BW, Myburg AA, Pixley KV, Botha AM (2007) Genetic diversity of African maize inbred lines revealed by SSR markers. Hereditas 144:10–17
Liu KJ, Goodman M, Muse S, Smith JS, Buckler E, Doebley J (2003) Genetic structure and diversity among maize inbred lines as inferred from DNA microsatellites. Genetics 165:2117–2128
Luna S, Figueroa J, Baltazar B, Gomez R, Townsend R, Schoper JB (2001) Maize pollen longevity and distance isolation requirements for effective pollen control. Crop Sci 41:1551–1557
Ma BL, Subedi KD, Reid LM (2004) Extent of cross-fertilization in maize by pollen from neighboring transgenic hybrids. Crop Sci 44:1273–1282
Melé E, Ballester J, Peñas G, Folch I, Olivar J, Alcalde E, Messeguer J (2004) First results of co-existence study. Euro/Biotech/News 4
Messéan A, Angevin F, Gómez-Barbero M, Menrad K, Rodríguez-Cerezo E (2006) New case studies on the coexistence of GM and non-GM crops in European agriculture. In: Tech Rep EUR 22102 EN. Institute for Prospective Technological Studies, Joint Research Centre, European Commission, Seville, Spain, p 116
Messeguer J, Melé E (2006) Coexistencia de cultivos tradicionales y genéticamente modificados. In: Organismos modificados genéticamente Editor: Emilio Muñoz Editorial Ephemera, pp 267–278
Messeguer J, Ballester J, Peñas G, Olivar J, Alcade E, Melé E (2003) Evaluation of gene flow in a commercial field of maize. In: Boelt B (ed) 1st European conference on the coexistence of genetically modified crops with conventional and organic crops. Flakkebjerg Research Centre, p 220
Messeguer J, Peñas G, Ballester J, Bas M, Serra J, Salvia J, Palaudelmàs M, Melé E (2006) Pollen-mediated gene flow in maize in real situations of coexistence. Plant Biotechnol J 4:633–645
Palaudelmàs M, Melé E, Peñas G, Pla M, Nadal A, Serra J, Salvia J, Messeguer J (2008) Sowing and flowering delays can be an efficient strategy to improve coexistence of genetically modified and conventional maize. Crop Sci 48:2404–2413
Pla M, La Paz JL, Penas G, Garcia N, Palaudelmàs M, Esteve T, Messeguer J, Melé E (2006) Assessment of real-time PCR based methods for quantification of pollen-mediated gene flow from GM to conventional maize in a field study. Transgenic Res 15:219–228
Plaschke J, Ganal MW, Roder MS (1995) Detection of genetic diversity in closely-related bread wheat using microsatellite markers. Theor Appl Genet 91:1001–1007
Purseglove JW (1972) Tropical crops: monocotyledons 1. Longman Group, London
Reif J, Warburton ML, Xia XC, Hoisington DA, Crossa J, Taba S, Muminovic J, Bohn M, Frisch M, Melchinger AE (2006) Grouping of accessions of Mexican races of maize revisited with SSR markers. Theor Appl Genet 113:177–185
Riesgo L, Areal FJ, Sanvido O, Rodriguez-Cerezo E (2010) Distances needed to limit cross-fertilization between GM and conventional maize in Europe. Nat Biotechnol 28:780–782
Salgado KCPC, Vieira MGGC, Pinho EVR, Guimaraes CT, Pinho RG, Sousa LV (2006) Genetic purity certificate in seeds of hybrid maize using molecular markers. Revista Brasileira de Sementes 28:169–175
Sanvido O, Widmer F, Winzeler M, Streit B, Szerencsits E, Bigler F (2008) Definition and feasibility of isolation distances for transgenic maize cultivation. Transgenic Res 17:317–335
Sargent DJ, Clarke J, Simpson DW, Tobutt KR, Arus P, Monfort A, Vilanova S, Denoyes-Rothan B, Rousseau M, Folta KM, Bassil NV, Battey NH (2006) An enhanced microsatellite map of diploid Fragaria. Theor Appl Genet 112:1349–1359
Senior ML, Heun M (1993) Mapping maize microsatellites and polymerase chain-reaction confirmation of the targeted repeats using a CT primer. Genome 36:884–889
Smith JSC, Chin ECL, Shu H, Smith OS, Wall SJ, Senior ML, Mitchell SE, Kresovich S, Ziegle J (1997) An evaluation of the utility of SSR loci as molecular markers in maize (Zea mays L): comparisons with data from RFLPS and pedigree. Theor Appl Genet 95:163–173
Treu R, Emberlin J (2000) Pollen dispersal in the crops maize (Zea mays), oilseed rape (Brassica napus ssp. oleifera), potatoes (Solanum tuberosum), sugar beet (Beta vulgaris ssp. vulgaris), and wheat (Triticum aestivum). In: Soil association, national pollen research unit. University College Worcester, Worcester
Uribelarrea M, Carcova J, Otegui ME, Westgate ME (2002) Pollen production, pollination dynamics, and kernel set in maize. Crop Sci 42:1910–1918
Van De Wiel CCM, Lotz LAP (2006) Outcrossing and coexistence of genetically modified with (genetically) unmodified crops: a case study of the situation in the Netherlands. Njas-Wageningen J Life Sci 54:17–35
Weber WE, Bringezu T, Broer I, Eder J, Holz F (2007) Coexistence between GM and Non-GM maize crops—tested in 2004 at the field scale level (Erprobungsanbau 2004). J Agron Crop Sci 193:79–92
Westgate ME, Lizaso J, Batchelor W (2003) Quantitative relationships between pollen shed density and grain yield in maize. Crop Sci 43:934–942
Acknowledgments
This work was financially supported by the PORTA Programme (Pol de Recerca de Tecnologia Agroalimentària) and IRTA (Institut de Recerca i Tecnologia Agroalimentària). We would like to thank Mr. Pons for the agronomical management. We also appreciate the collaboration of Vane, Xavi, Gisela, Albert, Sandra, Jose and Pau.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Palaudelmàs, M., Melé, E., Monfort, A. et al. Assessment of the influence of field size on maize gene flow using SSR analysis. Transgenic Res 21, 471–483 (2012). https://doi.org/10.1007/s11248-011-9549-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-011-9549-z