Abstract
Mammalian Heat Shock Proteins (HSP), have potent immune-stimulatory properties due to the natural capability to associate with polypeptides and bind receptors on antigen presenting cells. The present study was aimed to explore whether plant HSP, and in particular HSP70, share similar properties. We wanted in particular to evaluate if HSP70 extracted in association to naturally bound polypeptides from plant tissues expressing a recombinant “reporter” antigen, carry antigen-derived polypeptides and can be used to activate antigen-specific immune responses. This application of HSP70 has been very poorly investigated so far. The analysis started by structurally modeling the plant protein and defining the conditions that ensure maximal expression levels and optimal recovery from plant tissues. Afterwards, HSP70 was purified from Nicotiana benthamiana leaves transiently expressing a heterologous “reporter” protein. The purification was carried out taking care to avoid the release from HSP70 of the polypeptides chaperoned within plant cells. The evaluation of antibody titers in mice sera subsequent to the subcutaneous delivery of the purified HSP70 demonstrated that it is highly effective in priming humoral immune responses specific to the plant expressed “reporter” protein. Overall results indicated that plant-derived HSP70 shares structural and functional properties with the mammalian homologue. This study paves the way to further investigations targeted at determining the properties of HSP70 extracted from plants expressing foreign recombinant antigens as a readily available immunological carrier for the efficient delivery of polypeptides derived from these antigens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heat shock proteins (HSP) are a heterogeneous group of ubiquitous proteins exerting a pivotal function in cell homeostasis by contributing to the folding and unfolding, translocation and stabilization of proteins (Hartl and Hayer-Hartl 2009). Each HSP family includes several highly homologous isoforms, associated to particular cell compartments and/or tissues and expressed in normal or stress conditions (Chang et al. 2007; Tang et al. 2007). Except for small HSP, which are exclusive to the plant kingdom, they are considered among the most phylogenetically conserved proteins (Rutherford 2003).
In recent years the structure of HSP70 from different organisms including Escherichia coli (DnaK) (Zhu et al. 1996), Caenorhabditis elegans (Worrall and Walkinshaw 2007) and mammals (Chang et al. 2008; Jiang et al. 2006) has been resolved. These studies revealed that HSP70 tertiary structure in different organisms is very similar as a consequence of the very high degree of homology in the primary structure. HSP70 is characterized by an N-terminal ATPase domain (nucleotide binding domain, NBD) and by a C-terminal domain involved in client polypeptide-binding (substrate binding domain, SBD) (Mayer and Bukau 2005). The binding and release of a target polypeptide through the SBD induces an ATP-dependent conformational change of the chaperone (Swain et al. 2006). According to this, HSP70 exists in two defined and stable molecular conformations. An “open” state with the NBD occupied by an ATP molecule and the SBD free and a “closed” state with the NBD occupied by an ADP molecule and the SBD non-covalently associated with a polypeptide (Goloubinoff and De Los Rios 2007).
HSP70 does not act on its own in protein quality control but cooperates with cofactors that modulate the ATPase cycle to prevent polypeptide misfolding or mediate targeting to the ubiquitin/proteasome system for degradation (Wickner et al. 1999). It is conceivable that the chaperone is directly involved and required in the degradation process by the proteasome and also in chaperoning peptides exiting the proteasome (Calderwood et al. 2005; Esser et al. 2004; Hohfeld et al. 2001).
Besides the role in controlling protein shaping but as a consequence of this function, HSP70 family members (such as those of HSP90 and HSP110 families) have been demonstrated to possess carrier/adjuvant properties (Srivastava 2002). These properties are related to the ability of HSP70 to associate with peptides and interact with antigen presenting cells (APC) through specific receptors, facilitating uptake and processing (Facciponte et al. 2005; Singh-Jasuja et al. 2001).
HSP70 carrying pathogen or tumor-derived peptides of interest for vaccination can be easily purified by ADP-chromatography from pathogen-infected or tumor tissues (Ménoret 2004). By this method no conformational changes of the HSP70 SBD are induced and naturally chaperoned polypeptides are not released. Alternatively, autologous HSP or HSP of phylogenetically distant organisms purified by ATP-chromatography (therefore free of polypeptides) can be loaded in vitro with synthetic peptides known to trigger immune responses (Blachere et al. 1997; Kumaraguru et al. 2003).
We report here the results of a study aimed at investigating the possibility of using HSP70 extracted from plant tissues expressing recombinant antigens as an easily available immunological carrier for their delivery. Plants have been proven to be suitable expression systems, alternative to classical platforms, for the production of recombinant antigens (Ma et al. 2005). However, despite offering many of the advantages of plant-produced biopharmaceuticals (mainly low production costs, intrinsic biosafety), these antigens by themselves are poorly immunogenic, similarly to those derived from different recombinant host systems (Guy 2007). Data are here presented demonstrating that plant HSP70 (pHSP70) extracted through ADP-chromatography from plant tissues expressing a heterologous “reporter” protein antigen (i.e. the coat protein of the plant virus Potato virus X, PVX), are able to induce antibody responses specific to this protein in mice. These results together with the structural analysis and the identification of the conditions under which pHSP70 expression reach maximal levels in leaf tissues, pave the way to further investigations aimed at evaluating pHSP70 potential in vaccine applications.
Materials and methods
Protein modeling
Homology modeling was performed using the Swiss model PDB View-deepview software v. 4.0 (Guex and Peitsch 1997) and the Expasy modelling server Workspace (http://swissmodel.expasy.org) (Arnold et al. 2006). The alignment of query and templates sequences was obtained and manually refined. The quality of the generated models was assessed using the softwares WHAT CHECK (Hooft et al. 1996), PROCHECK (Laskowski et al. 1993) and verify3D (Lüthy et al. 1992).
Images of the models were rendered using Accelrys DS visualizer (version 2.0; Accelrys software, San Diego, CA, USA).
Plants exposure to stresses
Groups of N. benthamiana plants at 40 days from germination (5–10/group), grown in a containment P2 greenhouse under standard conditions (16 h light/8 h dark, 23°C), were subjected to abiotic or biotic stresses at time 0. Leaves were then harvested at different time intervals (one group of plants at each time interval), frozen in liquid nitrogen and stored at −80°C.
High temperature stress
Well-watered plants were exposed to 42°C into a ventilated heater for 4 h.
Bacterial infiltration (agroinfiltration)
Plants were infiltrated using the vacuum-technique (Tague and Mantis 2006) with buffer or with Agrobacterium tumefaciens GV3101(pSOUP) cells wild-type or carrying the binary vector pGR106 (encoding between T-DNA left and right borders the whole cDNA genome of PVX and inducing in plant tissues the onset of viral infection) (Lu et al. 2003). Agroinfiltration is a method commonly used to drive transient expression of recombinant proteins in plants using A. tumefaciens as vehicle of the encoding sequence (Sparkes et al. 2006). Briefly, a suspension of bacterial cells (grown overnight at 28°C in kanamycin containing Luria Broth to a final optical density (O.D.) at 600 nm of 0.6) was pelleted through centrifugation (5000×g for 15 min) and re-suspended in infiltration buffer (10 mM 2-(N-morpholino) ethanesulphonic acid, 10 mM MgCl2, pH 5.5). N. benthamiana plants were then submerged in a beaker containing the bacterial suspension and subjected to 10 mmHg vacuum for 1 min in a vacuum bell jar (Sigma Chemical Co., St. Louis, MO, USA) to force air out of the stomata. By this approach, when the vacuum was released, the pressure difference forced the bacterial suspension through the stomata into the mesophyll where it transferred the genes to plant cells. All the leaves except the apical ones (previously demonstrated to have a decreased expression efficiency; Lombardi et al. 2009) were sampled.
Viral infection
Plants have been inoculated on the first branches over cotyledons with the viral expression vector pPVX201 (carrying PVX whole genome; kindly provided by D. Baulcombe, University of Cambridge, UK) (Baulcombe et al. 1995), as previously described (Marusic et al. 2001). Only leaves on the first branch under the apical meristem have been sampled.
HSP70 and PVX coat protein (CP) quantification in leaf extracts
Soluble protein extracts were prepared by grinding 200 mg leaf tissue in cold phosphate buffered saline (PBS; 10 mM Na2HPO4, 10 mM NaH2PO4, 150 mM NaCl, pH 7.2) supplemented with 0.5 mM phenylmethanesulphonylfluoride (PMSF; Fluka, St. Louis, Mo, USA) (Buffer A) as described previously (Marusic et al. 2007). After total soluble protein (TSP) quantification using a Bradford colorimetric assay (BioRad, Hercules, CA, USA), ELISA Maxisorp plates (NUNC, Roskilde, Denmark) were coated in triplicate with 50 μg TSP/100 μl/well of each sample. Positive control wells were coated with 200 ng human recombinant HSP70 (huHSP70)/100 μl/well (Sigma) or 500 ng recombinant PVX CP/100 μl/well (Lico et al. 2009). After washing and blocking the plates were incubated with a monoclonal anti-HSP70 antibody (1:800) (clone BRM-22; Sigma) (or, as control, with an isotype-matched antibody of irrelevant specificity) or with a polyclonal horseradish peroxidase (HRP)-conjugated anti-CP antibody (1:200) (Agdia, Elkhart, IN, USA) (or, as control, with an HRP-conjugated polyclonal antibody of irrelevant specificity). Wells receiving anti-HSP70 antibody (or the isotype-matched control antibody) were additionally incubated with an HRP-conjugated anti-mouse IgG antibody (1:2500) (GE Healthcare, Munich, Germany). Bound antibodies were revealed using 2,2′-azino-di-3-ethylbenz-thiazoline sulphonate (ABTS; KPL, Gaithesburg, MD, USA) and the colorimetric reaction measured with an ELISA reader at 405 nm. Wells were considered positive when O.D. values were at least three times above the background (PBS-coated wells).
Extraction and purification of HSP70 from plant or murine tissues and peptide unloading
HSP70 extraction from leaves or murine spleens was performed as described for animal HSP70 (Ménoret 2004) through ADP-chromatography to prevent the release of the naturally chaperoned peptides. Briefly, frozen plant leaf tissues or murine spleens were homogenized in cold PBS supplemented with 0.5 mM PMSF (Buffer A). The extracts were filtered through Miracloth paper (Calbiochem, Darmstadt, Germany) and clarified by centrifugation at 4°C, 20000×g for 30 min, two times. Supernatants were changed to 20 mM Tris–acetate buffer pH 7.5 containing 20 mM NaCl, 15 mM β-mercaptoethanol, and 3 mM MgCl2 (Buffer B), through passage on a G-25 column (GE Healthcare Europe GmbH, Munich, Germany), before loading on an ADP-agarose (Sigma Chemical Co., St. Louis, MO, USA) column pre-swollen in Buffer B. After column washing and elution, those fractions demonstrated to contain highly pure protein by 12.5% (w/v) sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and silver staining, were pooled, changed to PBS (Buffer C) and concentrated using 50000 Molecular Weight Cut-Off (MWCO) polyethersulfone (PES) concentrators (Vivaspin, Sartorius AG, Goettingen, Germany). HSP70 concentration was determined by the Bradford colorimetric assay (BioRad) and confirmed by SDS–PAGE and Coomassie blue staining. Purified plant-HSP70 identity was verified by immunoblotting (performed as previously described) (Donini et al. 2005) using for detection the monoclonal anti-HSP70 antibody (Sigma). A negative control blot was developed with a primary antibody of irrelevant specificity (having the same isotype of the anti-HSP antibody). Protein identity was further confirmed by mass spectrometry, performed by Agilent LC–MS analysis of Coomassie stained bands. The absence of CP in the preparation was verified by ELISA and Western blot (data not shown).
When necessary, peptide unloading from the SBD of the purified pHSP70 was performed, as described by Li (2004), through the addition of 3 mM ATP (Sigma Chemical Co., St. Louis, MO, USA) and incubation for 1 h at 37°C. Peptides were then separated from HSP70 by passage through 10000 MWCO PES concentrators (Sartorius AG, Goettingen, Germany). This treatment caused a 10–15% loss of the originally recovered pHSP70.
Endotoxin removal and evaluation of endotoxin contamination
Endotoxin was removed by a chromatography system using Endo Trap Red column following manufacturer instructions (Hyglos GmbH, Germany). Briefly, the column was washed with 3 volumes of regeneration buffer (RB) and then equilibrated with 3 volumes of equilibration buffer (EB) (both provided by the manufacturer). One ml of pHSP70 (1.18 μg/μl) was added to the column, followed by 0.4 ml of EB. About 1 ml of the flow through was collected, and after column regeneration, the purification was repeated. After three passages on the column the protein concentration in the finally collected flow through (about 1.1 ml), determined by a Bradford colorimetric assay, was about 890 μg/μl.
Plant HSP70 preparations, before and after endotoxin removal, were examined for endotoxin contamination by the classical Limulus Amebocyte Lysate (LAL)-based gel clot assay following manufacturer instructions (Cape Cod, MA, USA).
Immunization experiments
Six weeks old C57BL/6J female mice (Harlan, Udine, Italy) (3–6/group) were maintained under standard housing conditions in the ENEA Animal Care Unit. Mice were immunized subcutaneously (s.c.) into the tail base at day 0 and 7 with 50 μl apyrogen saline alone, peptide-chaperoning pHSP70 (25 μg both at day 0 and 7; 40 μg both at day 0 and 7; 40 μg at day 0 and 80 μg at day 7), “unloaded” pHSP70 (25 μg both at day 0 and 7; 40 μg at day 0 and 80 μg at day 7) or peptide-chaperoning pHSP70 extracted from untreated plants (25 μg both at day 0 and 7) all without adjuvant. In another group of experiments mice were immunized s.c. into the tail base at day 0 and 7 with 50 μl apyrogen saline alone, peptide-chaperoning pHSP70 (25 μg both at day 0 and 7) before or after endotoxin removal or “unloaded” pHSP70 (25 μg both at day 0 and 7).
In all the experiments, blood samples were collected by tail bleeds before immunization and at day 14. All experimental procedures were conducted in accordance with protocols approved by the ENEA ethical committee and by the Italian Ministero del Lavoro, della Salute e delle Politiche Sociali.
Antibody titration in sera
Overall ELISA to determine anti-PVX CP and anti-pHSP70 IgG antibodies titers in individual murine serum samples were performed as described above. Briefly, wells were coated with 50 μl PBS containing 1 μg of recombinant PVX CP (Lico et al. 2009), pHSP70 (tested CP-free), murine spleen-purified HSP70 (mHSP70) or huHSP70 (Sigma). After washing and blocking, 50 μl of 1:10 to 1:400 dilutions of serum samples were added in triplicate to wells. Bound murine IgG antibodies were revealed by adding an HRP-labelled anti-mouse IgG polyclonal antibody (1:2500) (GE Healthcare) followed by ABTS (KPL). The colorimetric reaction was measured at 405 nm.
Endpoint titers were defined as the reciprocal of the highest serum dilution giving an absorbance three standard deviation above the blank (pre-immune sera). Mean titers ± standard deviation were determined for each group. Differences among mean titers were evaluated through the unpaired Student’s t test and considered statistically significant when P < 0.05.
Results
Plant HSP70 structure modeling
The sequences of the cytosolic, constitutively expressed HSC70 and of the stress-induced HSP70 isoforms in Nicotiana species have not yet been clearly distinguished and the two isoforms are generically indicated as HSP70 (Cho and Hong 2004). Moreover, the full amino acid sequence of N. benthamiana HSP70 is not yet available. For these reasons, in order to build a structural model of the protein, we used the sequence of one of the two cytosolic HSP70 of N. tabacum (Protein database accession no. AAR17080.1), phylogenetically very close to N. benthamiana (Goodin et al. 2008). This is in turn the plant sequence closest to the mammalian HSC70 (average identity of 79%) and HSP70 (average identity of 76%) (Table 1). Considering the high degree of identity, the homology modeling method (Chothia and Lesk 1986; Ginalski 2006) was adopted, using as a template Bos taurus HSC70, the only complete mammalian structure available (PDB accession code: 1YUW). To construct the model, a manually refined alignment of template and target sequences was used (Fig. 1). To improve the alignment, sequences of the nucleotide and substrate binding domains of B. taurus, Homo sapiens HSC70 (PDB accession codes: 1BA0, 1KAX, 1NGB, 1S3X, 1UD0, 2E88, 2V7Z), Escherichia coli (PDB accession codes: 1U00, 1Q5L, 3DPP, 2V7Y) and Caenorhabditis elegans (PDB accession codes: 2P32, 3DOB) isoforms were also included. The model with the best validation score was chosen (Fig. 2). Solvent accessibility of the modelled N. tabacum HSP70 and the solved mammalian B. taurus HSC70 structure were very similar (up to 80% identity). A detailed analysis of NBD and SBD demonstrated that the side chains (R groups) of amino acids critical for protein functions have conserved positions. In particular, within the N-terminal NBD, the HLGGED motif in position 234–239, critical for ATP/ADP binding (Wu et al. 2004), is conserved. The same holds true for residues V415, M416, L419, I420, A496 and L498 and the motif I-XX-MV-XX-A-XX-Y521–531, within the C-terminal SBD, critical for peptide binding (Morshauser et al. 1995, 1999).
Alignment between the primary sequence of B. taurus HSC70 and N. tabacum HSP70. Asterisk conserved residue. Dot divergent residue. Hyphen sequence gaps. Continuous line box N-terminal NBD. Dotted line box C-terminal SBD. Black boxes residues critical for protein function. Numbering refers to N. tabacum HSP70 edited sequence
Comparison between mammalian HSC70 crystal structure and pHSP70 model. Panel aB. taurus HSC70. Panel bN. tabacum HSP70. Ribbons with arrowheads β-strands. Coiled ribbons α-helices. Tubes connecting loops. Structures are colored on the basis of solvent accessibility (% maximum Solvent Accessibility Surface, SAS) Black exposed residues (SAS > 30%); white partially exposed residues (10% < SAS < 30%); grey buried residues (SAS < 10%). N-terminal and C-terminal domains are indicated. Conserved residues critical for function are indicated in “ball and stick” mode. 1 HLGEED234–239 motif critical for ATPase activity; 2 I-XX-MV-XX-A-XX-Y521–531 motif and 3 V415, M416, L419, I420, L486 and A490 residues, critical for peptide binding. Figures are obtained with Accelrys DS Visualizer 2.0
Effect of stresses on HSP70 expression in plants
The basal expression level of HSP70 in N. benthamiana leaves and its modification in response to different abiotic and biotic stress conditions was evaluated by ELISA of crude extracts normalized for TSP content. The final aim of these experiments was to identify the conditions inducing the maximal expression of pHSP70. Several groups of plants were exposed to each stress at time 0 and leaves sampled at different time intervals (1 group/time interval). In a preliminary experiment individual variability in the stress response was demonstrated to be not significant through quantification of HSP70 expression in individual plant extracts normalized for TSP (comparing the results with the unpaired Student’s t-test) (data not shown).
For plants exposed to abiotic stresses, pHSP70 expression levels induced by acute (4 h) and prolonged (8 h) cold (4°C) exposure, prolonged heat (42°C) exposure, mechanical injury, drought or prolonged darkness were all reproducibly lower than those induced by acute heat exposure (4 h at 42°C) (inducing an increase of approximately 250% over the basal level at 18 h) (Fig. 3a and Fig. S1 and S2 in Electronic Supplementary material).
Evaluation through ELISA of the modification in the expression levels of pHSP70 (or PVX CP) at different time intervals from stresses in crude leaf extracts normalized for TSP content in a representative experiment. Panel a modification of pHSP70 expression levels induced in plants by high temperature (abiotic stress). Panel b modification of pHSP70 expression levels induced by infiltration with buffer (closed triangles), wild-type A. tumefaciens GV3101(pSOUP) cells (open squares) or GV3101(pSOUP) cells carrying the binary vector pGR106 (open circles). Closed circles PVX CP expression levels induced by infiltration with GV3101(pSOUP) carrying the binary vector pGR106. Panel c modification of pHSP70 (open circles) and CP (closed circles) expression levels induced by PVX infection on systemic leaves in plants inoculated with pPVX201. X axis: hours/days after the stress. X axis: hours/days after the stress. Y axis: O.D. values at 405 nm in triplicate wells (after background subtraction) ± standard deviation
Nevertheless, expression levels induced by abiotic stresses were never as high as those induced by biotic stresses. In fact, leaf infiltration with A. tumefaciens GV3101(pSOUP) strain carrying the binary vector pGR106 reproducibly induced at day 7 an increase in pHSP70 expression of about 600% over the basal level (Fig. 3b). Infiltration of leaves with recombinant A. tumefaciens is a method commonly used to drive transient expression of recombinant proteins in plants. Infiltration with bacteria carrying the vector pGR106 induce the expression in plant tissues of the entire PVX genome and the onset of the viral infection. PVX CP, the most efficiently expressed viral protein (Batten et al. 2003), reached the maximum concentration 7 days after agroinfiltration (in parallel to pHSP70) (about 790% increase) (Fig. 3b).
The effects of a viral infection on HSP70 expression levels were also evaluated. To this aim N. benthamiana plants were inoculated with the viral expression vector pPVX201 and systemic leaves analyzed at different time intervals. As shown in Fig. 3c, the induction of HSP70 expression was similar to that produced by agroinfiltration and reached its maximum at day 8 (about 590% increase) while the CP was best expressed at day 9 (780% increase).
Purification of HSP70 from leaf tissues and evaluation of its immunological properties
Plant HSP70 was extracted through ADP-chromatography (a method for preserving association to naturally chaperoned polypeptides) from N. bethamiana leaves 7 days after infiltration with A. tumefaciens GV3101(pSOUP) strain carrying the binary vector pGR106, testing the purification protocol described for extraction from animal cells (Ménoret 2004). Fractions were verified for purity through silver stained SDS–PAGE (Fig. 4, lanes 1 to 9). By this analysis two major bands of approximate molecular weight of 80 and 70 kDa and several contaminants with lower molecular mass were evidenced. Immunoblotting indicated that all protein species present in the purified sample were recognized by the HSP70-specific monoclonal antibody, suggesting that low molecular weight contaminants could be degradation products (Fig. 4, WB lane). Mass spectrometry analysis of the two major bands indicated that both were corresponding to cytosolic N. tabacum HSP70 (Protein database accession no. 38325815) (see Fig. S3 in Electronic Supplementary material). Yield was approximately of 23 μg pHSP70/g agroinfiltrated fresh leaves.
Purity and identity analysis of a representative pHSP70 preparation. Silver staining of 12.5% SDS–PAGE loaded with 20 ml aliquots of each fraction (total volume: 500 ml) (lanes 1 to 9) eluted from the ADP-agarose column or immunoblotting (WB lane) of an aliquot (500 ng) of the final pooled and concentrated preparation using for detection a monoclonal anti-HSP70 antibody. M: molecular weight marker. Arrows indicate the molecular weight of marker bands
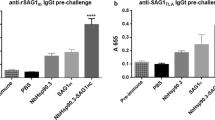
C57BL/6J mice were immunized s.c. at day 0 and 7 with different doses of the purified pHSP70 preparation. A first group of mice (group A) received two times 25 μg pHSP70, a second group (group B) two times 40 μg pHSP70 and a third group received 40 μg at day 0 and 80 μg at day 7 (group C). Control mice were immunized with pHSP70 purified from healthy tissues (two times 25 μg) (group D), pHSP70 extracted from agroinfiltrated tissues and in vitro depleted of the chaperoned peptides through ATP treatment (two times 25 μg, group E; two times 40 μg, group F) or with pyrogen-free saline alone (group G). Animals immunized with the CP alone were not included in these experiments because the purpose of our study was to verify pHSP70 properties as a carrier (i.e. pHSP70 carries polypeptides derived from proteins expressed within plant cells and it is functional in delivering these polypeptides to the mammalian immune system) rather than as an adjuvant. Seven days after the second immunization blood samples were individually collected to quantify antibodies specific to PVX CP (used in our experiments as a “reporter” protein/antigen) or to pHSP70. ELISA results demonstrated that mice immunized with pHSP70 carrying plant-derived peptides produced CP-specific antibodies (groups A, B and C) while these antibodies were absent in all the control groups (groups D, E, F and G) (Fig. 5, left panel). All animals, except those immunized with saline (group F), responded to pHSP70 (Fig. 5, right panel). While the response against pHSP70 increases as a function of the delivered dose (right panel), the response against CP varied inversely to the dose, reaching the highest titer (1:65) in group A (mice immunized two times with 25 μg of peptide-chaperoning pHSP70).
Titration of antibodies specific to PVX CP or to pHSP70. Left Panel CP-specific IgG titers. Right panel pHSP70-specific IgG titers. A mice immunized two times with 25 μg of HSP70 extracted from agroinfiltrated plant tissues in association to naturally chaperoned peptides. B mice immunized two times with 40 μg of HSP70 extracted from agroinfiltrated plant tissues in association to their naturally chaperoned peptides. C mice immunized with 40 μg (day 0) and 80 μg (day 7) of HSP70 extracted from agroinfiltrated plant tissues in association to their naturally chaperoned peptides. D mice immunized two times with 25 μg of HSP70 extracted from healthy plant tissues in association to their naturally chaperoned peptides. E mice immunized two times with 25 μg of HSP70 extracted from agroinfiltrated plant tissues in association to their naturally chaperoned peptides, but in vitro unloaded of their binders. F mice immunized two times with 40 μg of HSP70 extracted from agroinfiltrated plant tissues in association to their naturally chaperoned peptides, but in vitro unloaded of their binders. G mice immunized with apyrogen saline. Titers are expressed as arithmetic mean of individual mice titers obtained in independent experiments ± standard deviation. The differences among each group and controls are significant for P < 0.05
To exclude a possible role of bacterial endotoxin contamination in the activation of the immune response, endotoxin was removed from a purified pHSP70 sample through a chromatography-based method. The classical LAL-based gel clot assay indicated that the sample initially contained about 15.4 endotoxin units (EU; 1 EU = 100 pg)/ml while after column passage the contamination was lower than 1 EU/ml. To test the effects of endotoxin removal on antibody responses, C57BL/6J mice were immunized s.c. at day 0 and 7 with 25 μg pHSP70 before (group A) and after (group B) endotoxin removal. Control mice were immunized with the purified pHSP70 in vitro depleted of the chaperoned peptides through ATP treatment (two times 25 μg; group C) or with pyrogen-free saline alone (group D). As shown in Fig. 6, endotoxin removal do not significantly modify antibody responses to PVX CP (left panel) or to pHSP70 (right panel) in the sera of the immunized mice, 7 days after the second immunization.
Effect of endotoxin removal from pHSP70 preparation on anti-PVX CP and anti-pHSP70 antibody titers. Left Panel CP-specific IgG titers. Right panel pHSP70-specific IgG titers. A mice immunized two times with 25 μg of HSP70 extracted from agroinfiltrated plant tissues in association to naturally chaperoned peptides and not endotoxin-depleted. B mice immunized two times with 25 μg of HSP70 extracted from agroinfiltrated plant tissues in association to naturally chaperoned peptides and endotoxin-depleted. C mice immunized two times with 25 μg of HSP70 extracted from agroinfiltrated plant tissues in association to their naturally chaperoned peptides, but in vitro unloaded of their binders. D mice immunized with apyrogen saline. Titers are expressed as arithmetic mean of individual mice titers obtained in independent experiments ± standard deviation. The differences among each group and controls are significant for P < 0.05
The potential of the antibodies produced against pHSP70 by mice immunized twice with 25 μg of the purified protein to cross-react either with mHSP70 or huHSP70, was investigated and found to be absent despite the slight structural differences among the three isoforms (Fig. 7).
Analysis through ELISA of the ability of a pool of sera of mice immunized two times (day 0 and day 7) with 25 μg pHSP70 (group A), to cross-react with HSP70 extracted from murine spleens or with human recombinant HSP70. Squares wells coated with pHSP70. Circles wells coated with HSP70 extracted and purified from murine spleens. Triangles wells coated with human recombinant HSP70. X axis: mice sera dilution. Y axis: O.D. values at 405 nm in triplicate wells ± standard deviation
Discussion
HSP are ubiquitous proteins expressed in different subcellular compartments in all living organisms and involved in protein folding and unfolding (Ginalski 2006; Hartl and Hayer-Hartl 2009). Besides this housekeeping role, several HSP families, in particular HSP70, possess immune-stimulatory properties (Basu and Matsutake 2004; Calderwood et al. 2007). The activation of adaptive immunity is tightly related to the structure of HSP70 as these proteins possess a C-terminal SBD that can associate non-covalently to peptides against whom the specific immune responses are directed. Several indirect lines of evidence indicate that peptides chaperoned by HSP70 are the ‘fingerprint’ of the proteins expressed within a cell/tissue at any given point (Li et al. 2002). The delivery of HSP carrying peptides to mice is able to elicit antibody and cell-mediated immune responses specific to the carried peptides (Javid et al. 2007).
Because the amino acid sequences of HSP are conserved among phyla, we characterized here pHSP70 to verify whether they could work also as carriers for the delivery of peptides derived from plant-expressed proteins, with a view to potential vaccine applications. Indeed, plants can serve as host for the overproduction of heterologous proteins such as antigens and are considered as a valid alternative to traditional expression systems (Streatfield 2007). This technology offers numerous advantages such as competitive production costs, ease of scale-up and intrinsic safety. Nonetheless the problem still exists as to how to achieve measurable immune responses against the recombinant antigens without the requirement for strong adjuvants and multiple boosts. A problem in common with all subunit vaccines is that they are poorly immunogenic and/or unable to be properly presented to cytotoxic T cells (Sette and Fikes 2003).
Because the immune-stimulatory properties of HSP70 are strictly dependent on the structure, in spite of the high sequence homology (over 79% identity), we verified in silico if pHSP70 structure could indeed be overlapped to its mammalian homologue. A detailed analysis of the SBD showed that, beside the conserved amino acid stretches, residues resident only in the plant isoform were present. These residues may be responsible for differences in the features of the peptides bound to pHSP70. Despite the notion that mammalian HSP70 associate with of peptides 7 to 25 residues, generally characterized by an hydrophobic core mainly flanked by positively charged amino acids (Morshauser et al. 1995), no data are available on residues involved in peptide-HSP70 interaction and rules governing binding. It is therefore highly complex to predict on a structure-based analysis if plant and mammalian HSP70 can bind the same pool of peptides. Similarly, as no knowledge is available regarding HSP70 domain involved in APC receptor interaction, the model cannot anticipate if the plant-derived HSP could bind to these receptors.
To maximize pHSP70 recovery, conditions under which expression reaches the maximum concentration in plant tissues were verified. The most effective induction was obtained 7 days after leaf infiltration with A. tumefaciens GV3101(pSOUP) cells carrying the binary vector pGR106. At this time interval also the expression of the CP of PVX (encoded by the pGR106 binary vector and selected as a “reporter” protein/antigen) reached its maximum. The higher induction of HSP70 expression obtained by this procedure can be explained by the fact that plant tissues undergo concurrent bacterial and viral infection and this results in the massive production of pathogen-associated proteins eliciting a metabolic overloading of the protein biosynthetic pathways within the cells. For extraction the ADP-chromatography protocol previously described to purify HSP70 from animal cells (Ménoret 2004) was tested and demonstrated to work efficiently. SDS–PAGE and silver staining of the purified protein visualized two bands. Mass spectrometry and western blot indicated that both corresponded to pHSP70. A plausible hypothesis is that the highest band corresponds to a post-translationally modified version of pHSP70. Nevertheless, this hypothesis is not supported by the literature.
The highly pure pHSP70 preparations were then used to immunize mice with the aim of verifying if pHSP70 chaperoning polypeptides derived from plant-expressed proteins were able to induce humoral immune responses specific to the “reporter” antigen. As a control, mice were immunized with an aliquot of the same pHSP70 preparation in vitro depleted of peptides through incubation with ATP or with pHSP70 extracted from healthy plant tissues. Immunization with recombinant CP was not carried out as the proof of a possible enhancement of antigen efficacy mediated by pHSP70 was not in the scope of this study. In fact, the main purpose of our work was to provide proof that plant HSP70 shares structural properties with the mammalian homologue, carries polypeptides derived from plant-made proteins and is effective in delivering these polypeptides to the immune system. The results of the immunizations clearly demonstrated the induction of a specific antibody response to CP only in the sera of the mice immunized with pHSP70 extracted from agroinfiltrated tissues and chaperoning peptides. The titers were lower when compared to those developed against the pHSP70 carrier, but it must be considered that only a portion of the delivered pHSP70 was expected to carry CP-derived peptides. The fact that the response against pHSP70 increases in function of the delivered dose while the response against CP varies inversely to the dose may result from the strong stimulation of adaptive responses against the carrier at the highest delivered doses. A similar phenomenon has been previously described for HSP and also for a plant virus-based delivery strategy (Udono and Srivastava 1994; Roman and Moreno 1996; Lico et al. 2009). Because the antibodies raised against pHSP70 were unable to cross-react with the mammalian proteins (both murine and human) the possibility is reduced that immunization with pHSP70 would be able to initiate or propagate autoimmunity due to the high similarity with the mammalian HSP70.
Experiments were also performed immunizing mice with a pHSP70 preparation depleted of endotoxin through a chromatography-based method. Indeed due to the use of Agrobacterium, pHSP70 preparation resulted to be endotoxin contaminated. The results of these experiments indicated that endotoxin removal do not significantly reduce the intensity of the immune response both to the CP and to pHSP70.
Intriguingly and in parallel to mammalian studies, these findings confirm that peptides bound to pHSP70 are a molecular signature of the proteins expressed in a plant cell/tissue at a definite moment of time. Moreover, the results indicate that HSP70 of plant origin are active on the immune system, reinforcing the notion that HSP70 can induce the production of antibodies against the carried polypeptides, a poorly explored aspect so far.
Altogether the results of this multidisciplinary study provide the proof of concept that pHSP70 can indeed function as a vaccine carrier. Moreover they show the possibility of using HSP70 to deliver naturally generated polypeptides derived from a host organisms expressing a recombinant antigen (biofactory), a poorly explored application so far (Heikema et al. 1997). Dedicated experiments will be necessary to verify if pHSP70 are also capable of activating cytotoxic T lymphocytes and, above all, if this immunization strategy is able to guarantee protection against mammalian pathogens. The use of vaccine formulations based on HSP derived from cells expressing recombinant protein antigens (especially plants) could help to overcome the problem that protective epitopes are generally unknown and difficult to identify. Thus plants could be used as biofactories of a definite recombinant antigen and HSP70 derived from these plants used to deliver to the immune system different peptides of this antigen resulting in a naturally formulated multiepitope vaccine.
In this way our approach constitutes a successful attempt to combine the advantages of plant-based production and the HSP70-based delivery strategy.
References
Arnold K, Bordoli L, Kopp J, Schwede T (2006) The SWISS-MODEL workspace: a web-based environment for protein structure homology modeling. Bioinformatics 22:195–201
Basu S, Matsutake T (2004) Heat shock protein-antigen presenting cell interactions. Methods 32:38–41
Batten JS, Yoshinari S, Hemenway C (2003) Potato virus X: a model system for virus replication, movement and gene expression. Mol Plant Pathol 4:125–131
Baulcombe DC, Chapman S, Santa Cruz S (1995) Jellyfish green fluorescent protein as a reporter for virus infections. Plant J 6:1045–1053
Blachere NE, Li Z, Chandawarkar RY, Suto R, Jaikaria NS, Basu S, Udono H, Srivastava PK (1997) Heat shock protein-peptide complexes, reconstituted in vitro, elicit peptide-specific cytotoxic T lymphocyte response and tumor immunity. J Exp Med 186:1315–1323
Calderwood SK, Theriault JR, Gong J (2005) Message in a bottle: role of the 70-kDa heat shock protein family in anti-tumor immunity. Eur J Immunol 35:2518–2527
Calderwood SK, Mambula SS, Gray PJ Jr (2007) Extracellular heat shock proteins in cell signaling and immunity. Ann NY Acad Sci 1113:28–39
Chang HC, Tang YC, Hayer-Hartl M, Hartl FU (2007) Snapshot: molecular chaperones, part I. Cell 128:212
Chang YW, Sun YJ, Wang C, Hsia CD (2008) Crystal structures of the 70-kDa heat shock protein in domain disjoining conformation. J Biol Chem 283:15502–15511
Cho EK, Hong CB (2004) Molecular cloning and expression pattern analysis of heat shock protein 70 genes from Nicotiana tabacum. J Plant Biol 47:149–159
Chothia C, Lesk AM (1986) The relation between the divergence of sequence and structure in proteins. EMBO J 5:823–826
Donini M, Lico C, Baschieri S, Conti S, Magliani W, Polonelli L, Benvenuto E (2005) Production of an engineered killer peptide in Nicotiana benthamiana using a Potato virus X expression system. Appl Environ Microb 71:6360–6367
Esser C, Alberti S, Hofeld J (2004) Cooperation of molecular chaperones with the ubiquitin/proteasome system. Biochim Biophys Acta 1695:171–188
Facciponte JG, MacDonald IJ, Wang XY, Kim H, Manjili MH, Subjeck JR (2005) Heat shock proteins and scavenger receptors: role in adaptive immune responses. Immunol Invest 34:325–342
Ginalski K (2006) Comparative modelling for protein structure prediction. Curr Opin Struct Biol 16:172–177
Goloubinoff P, De Los Rios P (2007) The mechanism of HSP70 chaperones: (entropic) pulling the models together. Trends Biochem Sci 32:372–380
Goodin MM, Zaitlin D, Naidu RA, Lommel SA (2008) Nicotiana benthamiana: its history and future as a model for plant-pathogen interactions. Mol Plant Microbe Interact 21:1015–1026
Guex N, Peitsch MC (1997) SWISS-MODEL and the Swiss-PDBViewer: an environment for comparative protein modelling. Electrophoresis 18:2714–2723
Guy B (2007) The perfect mix: recent progress in adjuvant research. Nat Rev Microbiol 5:505–517
Hartl FU, Hayer-Hartl M (2009) Converging concepts of protein folding in vitro and in vivo. Nat Struct Mol Biol 16:574–581
Heikema A, Agsteribbe E, Wilschut J, Huckriede A (1997) Generation of heat shock protein-based vaccine by intracellular loading of gp96 with antigenic peptides. Immunol Lett 57:69–74
Hohfeld J, Cyr DM, Patterson C (2001) From the cradle to the grave: molecular chaperones that may choose between folding and degradation. EMBO Rep 2:885–890
Hooft RW, Vriend G, Sander C, Abola EE (1996) Errors in protein structures. Nature 381:272
Javid B, MacAry PA, Lehner PJ (2007) Structure and function: heat shock proteins and adaptive immunity. J Immunol 179:2035–2040
Jiang J, Lafer EM, Sousa R (2006) Crystallization of a functionally intact HSC70 chaperone. Acta Crystallogr Sect F Struct Biol Cryst Commun 62:39–43
Kumaraguru U, Gouffon CA, Ivey RA, Barry TR, Barry DB (2003) Antigenic peptides complexed to phylogenically diverse HSP70 s induce differential immune responses. Cell Stress Chap 8:134–143
Laskowski RA, MacArthur MW, Moss DS, Thornton JM (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Cryst 26:283–291
Li Z (2004) In vitro reconstitution of heat shock protein-peptide complexes for generating peptide-specific vaccines against cancers and infectious diseases. Methods 32:25–28
Li Z, Ménoret A, Srivastava P (2002) Roles of heat-shock proteins in antigen presentation and cross-presentation. Curr Op Immunol 14:45–51
Lico C, Mancini C, Italiani P, Betti C, Boraschi D, Benvenuto E, Baschieri S (2009) Plant-produced Potato virus X chimeric particles displaying an influenza virus-derived peptide activate specific CD8+ T cells in mice. Vaccine 27:5069–5076
Lombardi R, Circelli P, Villani ME, Buriani G, Nardi L, Coppola V, Bianco L, Benvenuto E, Donini M, Marusic C (2009) High-level HIV-1 Nef transient expression in Nicotiana benthamiana using the P19 gene silencing suppressor protein of Artichoke Mottled Crinckle virus. BMC Biotech 9:96
Lu R, Malcuit I, Moffet P, Ruiz MT, Peart J, Wu AJ, Rathjen JP, Bendahmane A, Day L, Baulcombe DC (2003) High throughput virus-induced gene silencing implicates heat shock protein 90 in plant disease resistance. EMBO J 22:5690–5699
Lüthy R, Bowie JU, Eisenberg D (1992) Assessment of protein models with three-dimensional profiles. Nature 356:83–85
Ma J, Chikwamba R, Sparrow P, Fischer R, Mahoney R, Twyman R (2005) Plant-derived pharmaceuticals-the road forward. Trends Plant Sci 10:580–585
Marusic C, Rizza P, Lattanzi L, Mancini C, Spada M, Belardelli F, Benvenuto E, Capone I (2001) Chimeric plant virus particles as immunogens for inducing murine and human immune responses against human immunodeficiency virus type 1. J Virol 75:8434–8449
Marusic C, Nuttall J, Buriani G, Lico C, Lombardi R, Baschieri S, Benvenuto E, Frigerio L (2007) Expression, intracellular targeting and purification of HIV Nef variants in tobacco cells. BMC Biotechnol 7:12
Mayer MP, Bukau B (2005) HSP70 chaperones: cellular functions and molecular mechanisms. Cell Mol Life Sci 62:670–684
Ménoret A (2004) Purification of recombinant and endogenous HSP70 s. Methods 32:7–12
Morshauser RC, Wang H, Flynn GC, Zuiderweg ER (1995) The peptide-binding domain of the chaperone protein HSC70 has an unusual secondary structure topology. Biochemistry 34:6261–6266
Morshauser RC, Hu W, Wang H, Pang Y, Flynn GC, Zuiderweg ER (1999) High-resolution solution structure of the 18 kDa substrate-binding domain of the mammalian chaperone protein HSC70. J Mol Biol 289:1387–1403
Roman E, Moreno C (1996) Synthetic peptides non-covalently bound to bacterial hsp 70 elicit peptide-specific T-cell responses in vivo. Immunology 88:487–492
Rutherford SL (2003) Between genotype and phenotype: protein chaperones and evolvability. Nat Rev Genet 4:263–274
Sette A, Fikes J (2003) Epitope-based vaccines: an update on epitope identification, vaccine design and delivery. Curr Opin Immunol 15:461–470
Singh-Jasuja H, Hilf N, Arnol-Schild D, Schild H (2001) The role of heat shock proteins and their receptors in the activation of the immune system. Biol Chem 382:629–636
Sparkes IA, Runions J, Kearns A, Hawes C (2006) Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat Protoc 1:2019–2025
Srivastava P (2002) Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol 2:185–194
Streatfield SJ (2007) Approaches to achieve high-level heterologous protein production in plants. Plant Biotech J 5:2–15
Swain JF, Schulz EG, Gierasch LM (2006) Direct comparison of a stable isolated HSP70 substrate binding domain in the empty and substrate-bound states. J Biol Chem 281:1605–1611
Tague BW, Mantis J (2006) In plantaAgrobacterium-mediated transformation by vacuum infiltration. Methods Mol Biol 323:215–223
Tang YC, Chang HC, Hayer-Hartl M, Hartl FU (2007) Snapshot: molecular chaperones, part II. Cell 128:412
Udono H, Srivastava PK (1994) Comparison of tumor-specific immunogenicities of stress-induced proteins gp96, hsp90, and hsp70. J Immunol 152:5398–5403
Wickner S, Maurizi MR, Gottesman S (1999) Posttranslational quality control: folding, refolding, and degrading proteins. Science 286:1888–1893
Worrall LJ, Walkinshaw MD (2007) Crystal structure of the C-terminal three-helix bundle subdomain of C. elegans HSP70. Biochem Biophys Res Commun 357:105–110
Wu X, Yano M, Washida H, Kido H (2004) The second metal-binding site of 70 kDa heat-shock protein is essential for ADP binding, ATP hydrolysis and ATP synthesis. Biochem J 378:793–799
Zhu X, Zhao X, Burkholder WF, Gragerov A, Ogata CM, Gottesman ME, Hendrickson WA (1996) Structural analysis of substrate binding by the molecular chaperone DnaK. Science 72:1606–1614
Acknowledgements
We thank Linda Bianco and Gaetano Perrotta for mass spectrometry analysis and Veronica Morea, and Chiara Lico for critical reading of the manuscript. The work was partially supported by a Grant of the Italian Foreign Affairs Ministry in the framework of Bilateral Projects of Great Relevance.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Buriani, G., Mancini, C., Benvenuto, E. et al. Plant heat shock protein 70 as carrier for immunization against a plant-expressed reporter antigen. Transgenic Res 20, 331–344 (2011). https://doi.org/10.1007/s11248-010-9418-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-010-9418-1