Abstract
Transthyretin (TTR) associated amyloidosis is an autosomal dominant disorder characterized by peripheral and autonomic neuropathy. Both genetic and environmental factors are thought to be involved in development of TTR associated amyloidosis. Previously, we demonstrated that amyloid deposition was observed in various tissues of transgenic mouse lines carrying a human mutant TTR (Met30) gene. To analyze the influence of environmental factors on TTR amyloidosis, these amyloidogenic transgenic mouse models were kept under conventional (CV) or specific pathogen free (SPF) conditions. Although the serum levels of Met30 for mice housed in the CV and SPF conditions were similar, amyloid deposition was observed in CV conditions, but not in SPF conditions. In addition, the extent of amyloid deposition in transgenic mice was dependent on duration kept under CV conditions. There were significant differences in proportion of amyloid deposition in several tissues between CV and SPF conditions. Maintenance of these mice at 30°C did not induce amyloid deposition in SPF conditions. These results suggest that the SPF conditions can completely prevent amyloid deposition, and that environmental factors can affect the onset and progression even in a single gene disorder.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amyloidoses are intractable diseases, which are caused by various genetic and environmental factors. Extensive studies have been done on genetic factors, but little is known about environmental factors involved in these disease processes. Identification of environmental factors is important from practical viewpoints to devise effective treatments for diseases. Transthyretin (TTR)-associated amyloidosis (ATTR) is an autosomal dominant form of systemic amyloidosis characterized by the aggregation of variant TTR into stable and insoluble fibrils (Andrade 1952; Glenner 1980a, b). About 110 TTR variants have been identified and more than 90 of which are associated with human amyloidosis. The most common type is caused by the substitution of valine (Val) to methionine (Met) at position 30 of the TTR protein (Met30), which predominates over the other types in Japan (Tawara et al. 1983), Portugal (Saraiva et al. 1983), Sweden (Westermark et al. 1985), and the United States (Benson and Dwulet 1985). Clinical symptoms of these kindreds are characterized by amyloid deposits, which usually occur in peripheral nerves and vital organs such as intestine, kidney, and heart, and result in varied syndromes leading to death (Glenner 1980a, b). It is interesting that the average age at onset differs significantly among Japanese, Portuguese, and Swedish patients. In addition, the age of onset varies greatly even in the same family member. These findings suggest the presence of environmental factor(s), other than a mutation in the TTR gene.
Previously, we produced four different transgenic mouse lines carrying a human mutant TTR gene (Nagata et al. 1995; Takaoka et al. 1997; Wakasugi et al. 1987; Yamamura et al. 1987; Yi et al. 1991) driven by different promoters. First line is termed as the Tg (MT-hTTRMet30) (MT-Met30), because the transgene contains genomic Met30 gene driven by the metallothionein (MT) gene promoter that can express Met30 in various tissues (Wakasugi et al. 1987). Second line is the Tg (0.6-hTTRMet30) (0.6-Met30) that carries genomic Met30 gene with a 0.6 kb upstream region from the hTTR gene. Previous study showed that 0.6 kb upstream region is sufficient for liver-specific expression, but insufficient for expression in choroid plexus (Yamamura et al. 1987). Third line is the Tg (6.0-TTRMet30) (6.0-Met30) that carries the genomic hMet30 gene with a 6.0 kb upstream region from the hTTR gene. In this case, the transgene was expressed not only in the liver, but also in the choroid plexus (Nagata et al. 1995). The fourth line is the Tg (7.2-TTRMet30) (7.2-Met30) that carries a Met30 cDNA with a 7.2 kb upstream region from the hTTR gene (Takaoka et al. 2004). All transgenic mouse lines were proved to develop amyloid deposition in various tissues (Takaoka et al. 2004, 1997; Wakasugi et al. 1987; Yi et al. 1991). Thus, these transgenic mice are good research models to investigate the environmental factors involved in amyloidogenesis of ATTR.
Environmental factors can be classified into intrinsic and extrinsic factors. In the stomach of transgenic mouse line, amyloid deposition was found in the non-glandular side, but not in the glandular side (Takaoka et al. 1997), suggesting that intrinsic factors such as local tissue structure are involved in amyloid deposition. We also demonstrated that the intestinal flora such as weak pathogens, as an extrinsic factor, could affect the amyloid deposition in transgenic mice (Noguchi et al. 2002). In this experiment, we examined whether the extrinsic environmental factor can affect amyloid deposition. Since recent studies demonstrated that environmental factors can affect the gene expression through modification of DNA or chromatin (Jirtle and Skinner 2007), we used four transgenic mouse lines which carry different promoters connected to either genomic Met30 gene or Met30 cDNA as mentioned above. We here showed that amyloid deposition was never found under specific pathogen free (SPF) conditions, and that the extent of amyloid deposition was correlated with duration kept under conventional (CV) conditions.
Materials and methods
Transgenic mouse lines
We used four transgenic mouse lines, MT-Met30, 0.6-Met30, 6.0-Met30 and 7.2-Met30 as described previously (Nagata et al. 1995; Takaoka et al. 2004; Wakasugi et al. 1987; Yamamura et al. 1987). Table 1 shows the characterization of four transgenic mouse lines. In all mouse lines, amyloid deposition occurred in the various organs and tissues, except nervous tissues, under CV conditions. MT-Met30 mouse line was originally produced using fertilized eggs obtained by mating between BDF1 males and females. After establishment of this line, No. 5 line was selected because of the high serum expression of Met30. This mouse was backcrossed to C57BL/6 mouse for more than 10 generations. All other lines were produced using fertilized eggs from C57BL/6. No. 61, No. 15 and No. 2 lines of 0.6-Met30, 6.0-Met30, and 7.2-Met30 lines, respectively, were used in the following studies. Previous studies demonstrated that amyloid deposits during 6–9 months, 12–18, or 21–24 months of age are similar (Yi et al. 1991; Takaoka et al. 1997, 2004), thus we classified our mice into three groups, 6–9, 12–18, and 21–24 months of age and analyzed amyloid deposition. All animal experiments were performed in accordance with the Declaration of Helsinki and were carried out with the approval of the Kumamoto University Ethical Committee for Animal Experiments.
Housing conditions
The transgenic mice were kept in plastic cages under the conventional facility in the Inoue Experimental Animal Center (Kumamoto, Japan). For SPF conditions, we used a SPF area either in the Center for Animal Resources and Development, Kumamoto University (Kumamoto, Japan) or in the Laboratory Animal Unit, Research and Development Division, Takeda Chemical Industries, LTD. These animal rooms were controlled 12-h light-dark cycle at 22 ± 2°C. To analyze the effect of high temperature, transgenic mice were kept under SPF conditions at 30°C in the Laboratory Animal Unit, Research and Development Division, Takeda Chemical Industries. All mice were fed standard rodent diet with water provided ad libitum.
PCR and Western blot analysis
Identification of transgenic mice was performed by polymerase chain reaction (PCR) and Western blot analysis. For PCR, DNA was extracted from tail tissue and amplified using two human TTR primer sets. Western blotting was performed as described (Nagata et al. 1995). Blood samples were collected from the eye artery after ether anesthesia. Mouse sera were analyzed by 17% SDS polyacrylamide gel electrophoresis, and electroblotted onto Immobilon-P transfer membranes (Millipore, USA). The membranes were incubated with rabbit anti-human transthyretin antibody (MBL, Japan), and then treated with horseradish peroxidase-conjugated goat anti-rabbit IgG. Blotted TTR protein was detected with chemiluminescence detection system (ECL, Amersham, USA), according to the manufacturer’s protocol.
Histochemical analysis
All transgenic mice were killed by cervical dislocation. Various tissues including the heart, kidneys, spleen, liver, lungs, pancreas, stomach, small and large intestines, urinary bladder, thyroid gland, lymph nodes, bone marrow, sciatic nerves, autonomic nerves, and brain, were excised and fixed in 10% neutral buffered formalin, and embedded in paraffin (Yi et al. 1991). Paraffin sections were stained with hematoxylin and eosin (HE). For histochemical demonstration of amyloid, paraffin sections were stained with Congo red after potassium permanganate (KMnO4) treatment as described previously (Yi et al. 1991). To detect the emerald green birefringence emitted from amyloid deposits, the Congo red-stained paraffin sections were observed under a polarized microscope. For immunohistochemistry, paraffin sections were stained with the indirect immunoperoxidase methods. The antibodies used were anti-human prealbumin (Beringwerke, Marburg, Germany) and anti-mouse SAP (Behring Diagnostics, La Jolla, USA).
Western blot analysis
Mouse sera (6 μl of mouse serum diluted 1:50 in 0.9% NaCI per lane) were applied to 12% polyacrylamide gel electrophoresis and transferred to an immobilon polyvinylidene difluoride filter (Millipore, Billerica, MA, USA). Primary antibodies were used at the indicated dilutions: rabbit anti-human TTR antibody (diluted 1:1,000) (MBL, Nagoya, Japan). An anti-rabbit immunoglobulin G antibody conjugated with horseradish peroxidase (Amersham Japan, Tokyo, Japan) was used for detection.
Statistical analysis
StatView software (SAS Institute, Cary, NC) was used to compare the data shown in Tables 3 and 4. In Table 3, the statistical significance between groups was estimated using Kruskal–Wallis test and Mann–Whitney U test with Bonferroni correction. P values less than 0.05 were considered significant. In Table 4, the statistical significance between groups was estimated using Fisher’s exact test. P values less than 0.05 were considered significant.
Results
No amyloid deposition under SPF conditions
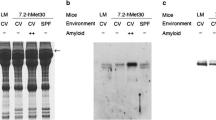
We first investigated the extrinsic environmental factors in amyloid deposition, using three mouse lines, MT-Met30, 0.6-Met30, and 6.0-Met30. The reason is that each line carries the transgene which contains human genomic Met30 gene, but not cDNA. Thus, we can examine the effect of environmental factors on different promoters, and we can avoid any effects caused by structural differences between genomic DNA and cDNA. These mice were kept either in CV or SPF conditions until 24 months of age and were examined for amyloid deposition. Under CV conditions, amyloid deposition was observed in one MT-Met30 mouse during 6–9 months of age. Later on, amyloid deposits were observed in 4 of 8, 7 of 11, and 10 of 13 transgenic mice carrying 0.6-Met30, or 6.0-Met30, or MT-Met30, respectively, during 12–18 months of age. Finally, amyloid deposits were observed in 4 of 6, 6 of 6, and 7 of 7 transgenic mice carrying 0.6-Met30, or 6.0-Met30, or MT-Met30, respectively, during 21–24 months of age. In contrast, no amyloid deposition was observed in all mice under SPF conditions during observation (Fig. 1 and Table 2). Table 3 shows the proportion, location and relative amount of amyloid deposits in the various tissues when housed in CV conditions. There were statistically significant differences in proportion of amyloid deposition in several tissues between 0.6-Met30 and 6.0-Met30 or MT-Met30 mice. But, the pattern of deposition was the same as reported previously (Fig. 1) (Kohno et al. 1997; Takaoka et al. 1997; Yi et al. 1991). We then examined whether CV or SPF conditions affected the serum levels of human TTR in each transgenic mouse line by Western blotting. The serum levels of Val30 for mice housed in the CV and SPF conditions were similar (Fig. 2) as reported previously (Tagoe et al. 2003). These results suggest that SPF conditions can prevent amyloid deposition completely without affecting serum levels of Met30.
Histochemical analysis. Tissues obtained at 24 months of age were stained with Congo red, then viewed under polarizing light. The pattern of amyloid deposition under CV conditions was the same as reported previously. No amyloid deposition was observed in all strains under SPF conditions. Bars = 100 μm
Correlation between CV conditions and amyloid deposition
As CV conditions facilitated amyloid deposition, we examined whether the duration kept under CV conditions is related to the extent of amyloid deposition. In this experiment, we used the 7.2-hMet30 line. This mouse line carries human Met30 cDNA, instead of genomic Met30 gene, but similar amyloid deposition was observed in this line (Takaoka et al. 2004) compared to other mouse lines (Takaoka et al. 1997; Yi et al. 1991). These transgenic mice were divided into four groups. One group, SPF24, was kept under SPF conditions during 24 months. The second group, SPF14/CV10, was kept under SPF for initial 14 months after birth, and then switched to CV for following 10 months. The third group, SPF8/CV16, was kept under SPF conditions for 8 months after birth, then kept under CV conditions for another 16 months. The fourth group, CV24, was kept under CV conditions for 24 months. Amyloid deposition was observed in 0 of 17, 1 of 14, 6 of 19, or 8 of 8 of the SPF24 group, SPF14/CV10 group, SPF8/CV16 group, or CV24 group, respectively. There were statistically significant differences in frequency of amyloid deposition between SPF24 and SPF8/CV16 or CV24 groups (Table 4). Amyloid deposition was most prominent in the small intestine and kidney, less prominent in the heart, thyroid and upper stomach. Thus, tissue distribution of amyloid deposition was similar to that observed in previous studies (Takaoka et al. 2004, 1997; Yi et al. 1991). These results clearly suggest that the extent of amyloid deposition is correlated with the duration kept under CV conditions.
No amyloid deposition under 30°C room temperature
There are many environmental differences between SPF and CV including intestinal flora, room temperature, or moisture. We expected that blood vessels might be dilated under high temperature, and that leakage of tetramers into extra-cellular tissues might occur eventually leading to amyloid deposition. In addition, room temperature is easy to control. To test this possibility, we kept MT-Met30, 0.6-Met30, and 6.0-Met30 mice at 30°C under SPF conditions for 2 years. Although four mice of each strain were sacrificed for pathologic analysis every 6 months, amyloid deposition was observed in none of these transgenic mice.
Discussion
Here we demonstrated that SPF conditions prevented completely amyloid deposition, and that CV conditions as an environmental factor facilitated amyloid deposition in transgenic mice.
Most of human disease is the result of the interaction of genetic factors and environmental factors. Common diseases, such as cancer and diabetes, result from the complex interplay of genes and environment. In addition, environmental factors are also involved in development of monogenic genetic disorders. For example, in Huntington’s disease, the total environmental effect accounts for 63% of the variance at age of onset (Wexler et al. 2004). As environmental factors include stress, physical and mental abuse, diet, exposure to toxins, pathogens, radiation and chemicals, weather and etc., it is difficult to determine environmental factors in human patients. Thus, animal models for human diseases have been used as tools for analyzing environmental factors. Matsuda et al. (1997) demonstrated that skin lesions similar to human atopic dermatitis with IgE hyperproduction spontaneously appeared in inbred NC/Nga mice under CV conditions, but not under SPF conditions. The skin lesion showed increased numbers of mast cells and CD4+ T cells containing IL-4 necessary for IgE synthesis, suggesting that environmental factor(s) triggered IgE hyperproduction. Kuhn et al. (1993) demonstrated that chronic enterocolitis resembling human inflammatory bowel disease (IBD) in IL-10 deficient mice was less severe and delayed in onset under SPF conditions. Later on, Kullberg et al. (1998) revealed that Helicobacter hepaticus triggers colitis in SPF interleukin-10 (IL-10)-deficient mice through an IL-12- and gamma interferon-dependent mechanism. Yoshitomi et al. (2005) demonstrated that rheumatoid arthritis in SKG mice failed to develop under SPF conditions, but that fungal β-glucans triggered severe chronic arthritis in SKG mice under SPF conditions, probably through the IL-4 production (Kobayashi et al. 2006). Taken together, the intestinal flora may promote or be the targets of the dysregulated inflammatory response encountered under CV conditions in animal models of autoimmune diseases.
We showed that amyloid deposition was never found under SPF conditions, and that the extent of amyloid deposition was correlated with duration kept under CV conditions. Shino et al. demonstrated that senile amyloidosis as characterized by deposition of apolipoprotein A-II in senescence-accelerated mouse (SAM) was less severe in mice reared under SPF conditions (Shino et al. 1987). However, impaired immune activity in SAM mice did not affect directly the development of senile amyloidosis or vice versa (Hosono et al. 1997). We also showed that LPS treatment did no affect amyloid deposition in terms of the onset, progression, and tissue distribution (Murakami et al. 1992). Although we demonstrated that intestinal flora from CV conditions facilitated amyloid deposition, this effect was limited to the alimentary tract. Taken together, these results suggest that immune response mediated through intestinal flora may no be involved in ATTR.
Tagoe et al. reported that the frequency, extent or nature of TTR-amyloid deposition did not differ significantly between conventional and specific pathogen-free environments (Tagoe et al. 2003). In their experiment, mice transgenic for the human wild type TTR gene were maintained in a specific pathogen free environment until 12 months of age. Then, half the animals were moved to a CV animal facility. The incidence of both TTR and AA amyloid was the same in both groups at 2 years of age. This is inconsistent with our data that TTR amyloid deposition was never observed in any tissue of transgenic mice under our SPF conditions. Our SPF and CV conditions might be different from those of Tagoe et al. In our CV conditions AA amyloid was indeed observed under CV conditions, but not in SPF conditions. In addition, the extent of amyloid deposition was correlated with duration kept under CV conditions, suggesting that CV conditions are quite different from SPF conditions in our experiment. On the other hand, CV conditions may be similar to SPF conditions in their mouse facility. Another possibility is the difference in the gene used for transgenic production. We used the mutant Met30 gene, while Tagoe et al. used the normal Val30 gene. As Met30 tetramer is more unstable (Ferrao-Gonzales et al. 2003; Nettleton et al. 1998; Niraula et al. 2002; Quintas et al. 1997), dissociation of tetramer may occur more easily under CV conditions in our experiment. As no amyloid deposition was observed in three transgenic lines carrying different transgene constructs, absence of amyloid deposition was not due to altered regulation of Met30 gene expression. In fact, serum levels of Met30 were similar under SPF and CV conditions.
Difference between CV and SPF conditions is not clear yet. Although room temperature is one of environmental factor, at least maintenance of the mice at 30°C did not induce amyloid deposition in SPF conditions. We also previously demonstrated that the thiol residue of Cys10 is involved in the amyloid formation (Takaoka et al. 2004). When we replaced the Cys10 with the serine, amyloid deposition was not observed in transgenic mice in spite of the presence of Met at position 30. Thus, disulfide formation may be involved in amyloidogenesis and thus anti-oxidative agents may be inhibitory for amyloid deposition. This is consistent with the data that severe oxidative stress was observed in ATTR patients (Ando et al. 1997). Furthermore, in the case of an ATTR patient who possessed a mutant extra-cellular superoxide dismutase (SOD) gene, amyloid depositions are much more severe than those in SOD-wild-type ATTR patients (Sakashita et al. 1998). This oxidative damage appears to play a role in other amyloidosis such as Alzheimer’s disease (AD). Increased oxidation of certain specific plasma proteins was reported to occur in AD patients, but not in non-AD controls (Conrad et al. 2000). Taken together, it is likely that the amyloid deposition is facilitated by oxidative stress under CV conditions.
To devise more general and accessible treatment, it is beneficial to find environmental factors involved in amyloidogenesis. As the dissociation of the tetramer into monomers is thought to be a critical step for amyloidogenesis, many efforts were made to find molecules that stabilize the TTR tetramer. So far, small molecules and Cr3+ were shown to mediate kinetic stability of TTR tetramers by binding to the TTR tetramer (Almeida et al. 2004; Ando 2005; Johnson et al. 2005a, b; Nettleton et al. 1998; Sato et al. 2006). Furthermore, immunization of transgenic mice having the Met30 gene using ATTR Y78P resulted in suppression of amyloid deposits (Terazaki et al. 2006). However, liver transplantation (Stangou and Hawkins 2004) is the only reliable therapy at moment. Thus, elucidation of the effect and contribution of environmental factors will facilitate the development of new methods of treatment. The present study represents an important step toward understanding such environmental factors on amyloid deposition.
References
Almeida MR, Macedo B, Cardoso I, Alves I, Valencia G, Arsequell G, Planas A, Saraiva MJ (2004) Selective binding to transthyretin and tetramer stabilization in serum from patients with familial amyloidotic polyneuropathy by an iodinated diflunisal derivative. Biochem J 381:351–356
Ando Y (2005) Liver transplantation and new therapeutic approaches for familial amyloidotic polyneuropathy (FAP). Med Mol Morphol 38:142–154
Ando Y, Nyhlin N, Suhr O, Holmgren G, Uchida K, el Sahly M, Yamashita T, Terasaki H, Nakamura M, Uchino M, Ando M (1997) Oxidative stress is found in amyloid deposits in systemic amyloidosis. Biochem Biophys Res Commun 232:497–502
Andrade C (1952) A peculiar form of peripheral neuropathy; familiar atypical generalized amyloidosis with special involvement of the peripheral nerves. Brain 75:408–427
Benson MD, Dwulet FE (1985) Identification of carriers of a variant plasma prealbumin (transthyretin) associated with familial amyloidotic polyneuropathy type I. J Clin Invest 75:71–75
Conrad CC, Marshall PL, Talent JM, Malakowsky CA, Choi J, Gracy RW (2000) Oxidized proteins in Alzheimer’s plasma. Biochem Biophys Res Commun 275:678–681
Ferrao-Gonzales AD, Palmieri L, Valory M, Silva JL, Lashuel H, Kelly JW, Foguel D (2003) Hydration and packing are crucial to amyloidogenesis as revealed by pressure studies on transthyretin variants that either protect or worsen amyloid disease. J Mol Biol 328:963–974
Glenner GG (1980a) Amyloid deposits and amyloidosis. The beta-fibrilloses (first of two parts). N Engl J Med 302:1283–1292
Glenner GG (1980b) Amyloid deposits and amyloidosis: the beta-fibrilloses (second of two parts). N Engl J Med 302:1333–1343
Hosono M, Hanada K, Toichi E, Naiki H, Higuchi K, Hosokawa T (1997) Immune abnormality in relation to nonimmune diseases in SAM mice. Exp Gerontol 32:181–195
Jirtle RL, Skinner MK (2007) Environmental epigenomics and disease susceptibility. Nat Rev Genet 8:253–262
Johnson SM, Petrassi HM, Palaninathan SK, Mohamedmohaideen NN, Purkey HE, Nichols C, Chiang KP, Walkup T, Sacchettini JC, Sharpless KB, Kelly JW (2005a) Bisaryloxime ethers as potent inhibitors of transthyretin amyloid fibril formation. J Med Chem 48:1576–1587
Johnson SM, Wiseman RL, Sekijima Y, Green NS, Adamski-Werner SL, Kelly JW (2005b) Native state kinetic stabilization as a strategy to ameliorate protein misfolding diseases: a focus on the transthyretin amyloidoses. Acc Chem Res 38:911–921
Kobayashi K, Suda T, Nan-Ya K, Sakaguchi N, Sakaguchi S, Miki I (2006) Cytokine production profile of splenocytes derived from zymosan A-treated SKG mice developing arthritis. Inflamm Res 55:335–341
Kohno K, Palha JA, Miyakawa K, Saraiva MJ, Ito S, Mabuchi T, Blaner WS, Iijima H, Tsukahara S, Episkopou V, Gottesman ME, Shimada K, Takahashi K, Yamamura K, Maeda S (1997) Analysis of amyloid deposition in a transgenic mouse model of homozygous familial amyloidotic polyneuropathy. Am J Pathol 150:1497–1508
Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W (1993) Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75:263–274
Kullberg MC, Ward JM, Gorelick PL, Caspar P, Hieny S, Cheever A, Jankovic D, Sher A (1998) Helicobacter hepaticus triggers colitis in specific-pathogen-free interleukin-10 (IL-10)-deficient mice through an IL-12- and gamma interferon-dependent mechanism. Infect Immun 66:5157–5166
Matsuda H, Watanabe N, Geba GP, Sperl J, Tsudzuki M, Hiroi J, Matsumoto M, Ushio H, Saito S, Askenase PW, Ra C (1997) Development of atopic dermatitis-like skin lesion with IgE hyperproduction in NC/Nga mice. Int Immunol 9:461–466
Murakami T, Yi S, Maeda S, Tashiro F, Yamamura K, Takahashi K, Shimada K, Araki S (1992) Effect of serum amyloid P component level on transthyretin-derived amyloid deposition in a transgenic mouse model of familial amyloidotic polyneuropathy. Am J Pathol 141:451–456
Nagata Y, Tashiro F, Yi S, Murakami T, Maeda S, Takahashi K, Shimada K, Okamura H, Yamamura K (1995) A 6-kb upstream region of the human transthyretin gene can direct developmental, tissue-specific, and quantitatively normal expression in transgenic mouse. J Biochem 117:169–175
Nettleton EJ, Sunde M, Lai Z, Kelly JW, Dobson CM, Robinson CV (1998) Protein subunit interactions and structural integrity of amyloidogenic transthyretins: evidence from electrospray mass spectrometry. J Mol Biol 281:553–564
Niraula TN, Haraoka K, Ando Y, Li H, Yamada H, Akasaka K (2002) Decreased thermodynamic stability as a crucial factor for familial amyloidotic polyneuropathy. J Mol Biol 320:333–342
Noguchi H, Ohta M, Wakasugi S, Noguchi K, Nakamura N, Nakamura O, Miyakawa K, Takeya M, Suzuki M, Nakagata N, Urano T, Ono T, Yamamura K (2002) Effect of the intestinal flora on amyloid deposition in a transgenic mouse model of familial amyloidotic polyneuropathy. Exp Anim 51:309–316
Quintas A, Saraiva MJ, Brito RM (1997) The amyloidogenic potential of transthyretin variants correlates with their tendency to aggregate in solution. FEBS Lett 418:297–300
Sakashita N, Ando Y, Marklund SL, Nilsson P, Tashima K, Yamashita T, Takahashi K (1998) Familial amyloidotic polyneuropathy type I with extracellular superoxide dismutase mutation: a case report. Hum Pathol 29:1169–1172
Saraiva MJ, Costa PP, Goodman DS (1983) Studies on plasma transthyretin (prealbumin) in familial amyloidotic polyneuropathy, Portuguese type. J Lab Clin Med 102:590–603
Sato T, Ando Y, Susuki S, Mikami F, Ikemizu S, Nakamura M, Suhr O, Anraku M, Kai T, Suico MA, Shuto T, Mizuguchi M, Yamagata Y, Kai H (2006) Chromium(III) ion and thyroxine cooperate to stabilize the transthyretin tetramer and suppress in vitro amyloid fibril formation. FEBS Lett 580:491–496
Shino A, Tsukuda R, Omori Y, Matsuo T (1987) Histopathologic observations on the senescence-accelerated mice (SAM) reared under specific pathogen free conditions. Acta Pathol Jpn 37:1465–1475
Stangou AJ, Hawkins PN (2004) Liver transplantation in transthyretin-related familial amyloid polyneuropathy. Curr Opin Neurol 17:615–620
Tagoe CE, Jacobson DR, Gallo G, Buxbaum JN (2003) Mice transgenic for human TTR have the same frequency of renal TTR deposition whether maintained in conventional or specific pathogen free environments. Amyloid 10:262–266
Takaoka Y, Tashiro F, Yi S, Maeda S, Shimada K, Takahashi K, Sakaki Y, Yamamura K (1997) Comparison of amyloid deposition in two lines of transgenic mouse that model familial amyloidotic polyneuropathy, type I. Transgenic Res 6:261–269
Takaoka Y, Ohta M, Miyakawa K, Nakamura O, Suzuki M, Takahashi K, Yamamura K, Sakaki Y (2004) Cysteine 10 is a key residue in amyloidogenesis of human transthyretin Val30Met. Am J Pathol 164:337–345
Tawara S, Nakazato M, Kangawa K, Matsuo H, Araki S (1983) Identification of amyloid prealbumin variant in familial amyloidotic polyneuropathy (Japanese type). Biochem Biophys Res Commun 116:880–888
Terazaki H, Ando Y, Fernandes R, Yamamura K, Maeda S, Saraiva MJ (2006) Immunization in familial amyloidotic polyneuropathy: counteracting deposition by immunization with a Y78F TTR mutant. Lab Invest 86:23–31
Wakasugi S, Inomoto T, Yi S, Naito M, Uehira M, wanaga T, Maeda S, Araki K, Miyazaki J, Takahashi K, Shimada K, Yamamura K (1987) A transgenic mouse model of familial amyloidotic polyneuropathy. Proc Jpn Acad 63:344–347
Westermark P, Pitkanen P, Benson L, Vahlquist A, Olofsson BO, Cornwell GG 3rd (1985) Serum prealbumin and retinol-binding protein in the prealbumin-related senile and familial forms of systemic amyloidosis. Lab Invest 52:314–318
Wexler NS, Lorimer J, Porter J, Gomez F, Moskowitz C, Shackell E, Marder K, Penchaszadeh G, Roberts SA, Gayan J, Brocklebank D, Cherny SS, Cardon LR, Gray J, Dlouhy SR, Wiktorski S, Hodes ME, Conneally PM, Penney JB, Gusella J, Cha JH, Irizarry M, Rosas D, Hersch S, Hollingsworth Z, MacDonald M, Young AB, Andresen JM, Housman DE, De Young MM, Bonilla E, Stillings T, Negrette A, Snodgrass SR, Martinez-Jaurrieta MD, Ramos-Arroyo MA, Bickham J, Ramos JS, Marshall F, Shoulson I, Rey GJ, Feigin A, Arnheim N, Acevedo-Cruz A, Acosta L, Alvir J, Fischbeck K, Thompson LM, Young A, Dure L, O’Brien CJ, Paulsen J, Brickman A, Krch D, Peery S, Hogarth P, Higgins DS Jr, Landwehrmeyer B (2004) Venezuelan kindreds reveal that genetic and environmental factors modulate Huntington’s disease age of onset. Proc Natl Acad Sci USA 101:3498–3503
Yamamura K, Wakasugi S, Maeda S, Inomoto T, Iwanaga T, Uehira M, Araki K, Miyazaki J, Shimada K (1987) Tissue-specific and developmental expression of human transthyretin gene in transgenic mice. Dev Genet 8:195–205
Yi S, Takahashi K, Naito M, Tashiro F, Wakasugi S, Maeda S, Shimada K, Yamamura K, Araki S (1991) Systemic amyloidosis in transgenic mice carrying the human mutant transthyretin (Met30) gene. Pathologic similarity to human familial amyloidotic polyneuropathy, type I. Am J Pathol 138:403–412
Yoshitomi H, Sakaguchi N, Kobayashi K, Brown GD, Tagami T, Sakihama T, Hirota K, Tanaka S, Nomura T, Miki I, Gordon S, Akira S, Nakamura T, Sakaguchi S (2005) A role for fungal {beta}-glucans and their receptor dectin-1 in the induction of autoimmune arthritis in genetically susceptible mice. J Exp Med 201:949–960
Acknowledgements
We thank Mr. O. Nakamura and Ms. Michiyo Nakata for their technical assistance. This work was supported in part by a Grant-in-Aid for Scientific Research (A) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and a grant from the Osaka Foundation of Promotion of Clinical Immunology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Seiya Inoue, Mika Ohta, and Zhenghua Li contributed equally to this work.
Rights and permissions
About this article
Cite this article
Inoue, S., Ohta, M., Li, Z. et al. Specific pathogen free conditions prevent transthyretin amyloidosis in mouse models. Transgenic Res 17, 817–826 (2008). https://doi.org/10.1007/s11248-008-9180-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-008-9180-9