Abstract
The molecular variability of Plum pox virus (PPV) populations was compared in transgenic European plums (Prunus domestica L.) carrying the coat protein (CP) gene of PPV and non-transgenic plums in an experimental orchard in Valencia, Spain. A major objective of this study was to detect recombination between PPV CP transgene transcripts and infecting PPV RNA. Additionally, we assessed the number and species of PPV aphid vectors that visited transgenic and non-transgenic plum trees. Test trees consisted of five different P. domestica transgenic lines, i.e. the PPV-resistant C5 ‘HoneySweet’ line and the PPV-susceptible C4, C6, PT6 and PT23 lines, and non-transgenic P. domestica and P. salicina Lind trees. No significant difference in the genetic diversity of PPV populations infecting transgenic and conventional plums was detected, in particular no recombinant between transgene transcripts and incoming viral RNA was found at detectable levels. Also, no significant difference was detected in aphid populations, including viruliferous individuals, that visited transgenic and conventional plums. Our data indicate that PPV-CP transgenic European plums exposed to natural PPV infection over an 8 year period caused limited, if any, risk beyond the cultivation of conventional plums under Mediterranean conditions in terms of the emergence of recombinant PPV and diversity of PPV and aphid populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Potential risks have been expressed with the field release of virus-resistant transgenic plants. Some concerns include: (i) transgene flow by pollen from transgenic crops to wild relatives that could express undesirable traits such as weediness, (ii) impact on the diversity and dynamics of arthropods and microbiota, and (iii) recombination events between transgene transcripts and an infecting viral RNA that could lead to the emergence of viable recombinant viruses with novel and potentially harmful characteristics such as increase in pathogenicity (Rubio et al. 1999) or changes in host range and/or vectors.
Plant viruses annually cause severe economic losses in crop production. Plant breeders have focussed their efforts in identifying and deploying natural genes for resistance. However, the successful of genetic engineering in developing virus resistant plants, through the expression of a segment of the virus genome, has made development of transgenic plants a valuable strategy for obtaining virus resistant plants. Although resistance to virus infection is well characterized in transgenic and conventional plants following vector-mediated virus infection, limited information is available on the impact of virus-resistant transgenic crops on the epidemiology of virus diseases. Specifically, no information is available on the impact of transgenic plants on the diversity and dynamics of virus and virus vector populations. For example, it is not known if transgenic phenotype alter the variability of infecting virus populations or if the same number of virus vectors visit transgenic and conventional plants.
Plum pox virus (PPV) is the causal agent of sharka disease, one of the most devastating diseases of Prunus spp. (Cambra et al. 2006b), most seriously affecting apricots, plums and peaches. PPV is transmitted by aphids in a non-circulative, non-persistent manner (Kunze and Krczal 1971; Ng and Perry 2004) and by vegetative propagation. PPV variability has been extensively demonstrated by analysis of the antigenic properties of the coat protein (CP) and on this basis, PPV isolates have been separated into different serogroups in terms of specific epitopes recognized by monoclonal antibodies (Cambra et al. 1994; Boscia et al. 1997; Myrta et al. 1998; 2000). The molecular variability of the PPV genome has also been characterized by RFLP analysis (Wetzel et al. 1991) and sequencing of the entire or partial genome of many isolates (Candresse et al. 1994; Candrese and Cambra 2006). Taken together with serological and biological variability, PPV isolates can be clustered into six different types or strains: PPV-D (Dideron), PPV-M (Marcus), PPV-EA (El Amar), PPV-C (Cherry), PPV-W (Winona) and a recombinant type between D and M, PPV-Rec (Recombinant) (López-Moya et al. 2000; Szemes et al. 2001; Glasa et al. 2004; James and Varga 2005; Candresse and Cambra 2006; James and Glasa 2006). Recombination was not considered a mechanism with any significant role in PPV evolution. The first PPV recombinant was reported by Cervera et al. (1993) but it was considered as an unusual and non-representative isolate. However, the more widely characterization of PPV isolates from several origins, revealed frequent occurrence of recombinant isolates derived from recombination between PPV-D and PPV-M in Central and East Europe (Glasa et al. 2002, 2004). All identified PPV-Rec isolates share the same point of recombination crossover located at the C-terminus of the RNA replicase gene (NIb). Available sequence analyses provided evidence for the existence of a more ancient recombination event at the P3 gene, that makes PPV-D, PPV-M and PPV-Rec to share a common 5′ region (Glasa et al. 2004). Biological characteristics of recombinant PPV isolates have been analysed under both field and experimental conditions and they appear to be as viable and competitive as D and M isolates (Glasa et al. 2002; and 2004).
Due to the economic importance of PPV to the stone fruit industry, efforts have focused on the development of PPV-resistant Prunus cultivars either by conventional breeding (Kegler et al. 1998; Neumüller et al. 2005; Badenes et al. 2006) or by biotechnological approaches. Transgenic European plums carrying the CP gene of PPV (strain D; accession No. D00298, Ravelonandro et al. 1998) were obtained by Agrobacterium-mediated transformation (Scorza et al. 1994). Transgenic lines C2, C3, C4, C6, PT6 and PT23 are susceptible to natural PPV infection, as demonstrated in field trials carried out in Spain and Poland, where 100% of trees were PPV infected over a 7 year experimental period (Malinowski et al. 2006). Behaviour of these transgenic lines was similar to susceptible non-transgenic controls. In the same experiment, transgenic line C5 (cv. HoneySweet) remained free from PPV over the experimental period and demonstrated to be highly resistant to PPV infection by both, graft-inoculation and natural aphid-mediated infection (Malinowski et al. 2006). The resistance mechanism of line C5 is based on gene silencing (Scorza et al. 2001) and the production of siRNA (Hily et al. 2005).
In the present study, the effect of PPV-CP transgenic European plums on the diversity and dynamics of PPV and aphid populations has been evaluated by comparing conventional vs. transgenic plums grown under Mediterranean conditions. To our knowledge, this is the first report of a comparative study of natural virus and aphid populations in a transgenic woody plant grown in the field over an 8 year period.
Material and methods
Transgenic lines and experimental orchards
Five transgenic European plum lines, C4, C5, C6, PT6 and PT23, were established in an experimental orchard located in Liria, Valencia, Spain in 1997 (Malinowski et al. 2006). C4 carries two copies of the PPV CP transgene, transcribes a relatively high level of CP mRNA and accumulates CP. C5 contains at least three copies of the transgene and shows very low levels of CP mRNA and no CP. C6 line carries one copy of the transgene and undetectable levels of CP mRNA and CP. PT6 contains two copies of the CP transgene and produces moderate levels of both CP RNA and CP. As control, PT23 does not harbour the CP gene, and hence, there is no CP mRNA nor CP expression, but only the two marker genes neomycin phosphotransferase nptII and and β-glucoronidase GUS (Scorza et al. 1994; Hily et al. 2004). Ten trees per transgenic line were planted in 5-tree rows separated by rows of non-transgenic plums: P. domestica B70146 and P. salicina (Japanese plum) ‘Black Diamond’. The plot consisted of a total of fifty transgenic plums and fifty non-transgenic plums surrounded by two rows of guard trees (peach x almond hybrids GF677) which are sexually incompatible with plums. An adjacent conventional P. salicina (also sexually incompatible with P. domestica) orchard consisting of 320 trees was used as external control plot.
Variability of PPV populations
PPV isolates
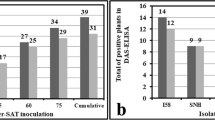
A total of 85 PPV isolates was analysed from three PPV populations: (i) a population infecting transgenic plums from lines C4, C6, PT6 and PT23 from the experimental orchard (32 isolates), (ii) a population infecting non-transgenic plums from the experimental orchard (24 isolates), and (iii) a population infecting conventional Japanese plums from the external control plot (29 isolates). C5 transgenic line was not used in the analyses of variability because this transgenic line is resistant to PPV and, consequently, it can not be infected by the virus. All PPV isolates belonged to the D type as demonstrated by previous serological and molecular studies (Capote and Cambra 2005).
RT-PCR and sequence analysis
Symptomatic leaves from transgenic (except for C5) and non-transgenic trees were collected in May 2004 and ground within individual plastic bags (Bioreba, Reinach, Switzerland) in the presence of 1/20 (w/v) of PBS buffer, pH 7.2 supplemented with 2% (w/v) PVP-10 and 0.2% (w/v) DIECA (Cambra et al. 1994). Total RNA was obtained from these extracts by RNeasy Plant MiniKit (Qiagen, Hilton, Germany) and used as template for RT-PCR reactions.
The most variable region of the Potyvirus genome corresponding to the 3′ end of the NIb gene and the 5′ end of the CP gene (511 bp fragment) was amplified from each PPV isolate by RT-PCR using primers 36 (5′-GAGGCAATTTGTGCWTCAATGG-3′) and 172 (5′-TGCAGGACTGTAATGTGCCAA-3′) (Capote and Cambra 2005), and directly sequenced. The nucleotide sequences from the 85 isolates tested were aligned by Clustal-W analysis (Thompson et al. 1997) using the Align X program from the Vector NTi package. To obtain the percentage of nucleic acid homology among PPV isolates, a pair-wise distance matrix was constructed with the MEGA 2.0 program using the “Kimura-2-parameter” method. The molecular variability within each population and among populations in terms of gene (haplotype) diversity and nucleotide diversity (π) was estimated with MEGA 2.0 and DNAsp programs using the “Kimura-2 parameter” algorithm. To determine the genetic structure of the PPV populations an analysis of molecular variance was carried out by the Arlequin program (AMOVA, Excoffier et al. 1992).
Recombination analysis
Recombination was assessed by comparing the CP gene sequence from 12 PPV isolates (six from C4 transgenic line and six from PT-6 transgenic line; accession Nos. DQ423227–DQ423238) with the CP gene nucleotide sequence from the transgene (accession No. D00298). PPV isolates infecting C6 trees were not included in the analyses because this transgenic line shows undetectable levels of CP transgene transcripts. PT23 and C5 transgenic lines were neither considered for recombination analyses because the first one does not carry the transgene, and the second one is resistant to PPV and, consequently, it can not be infected by the virus. For obtaining the complete sequence of the CP gene, two different RT-PCR reactions were performed that amplify the 5′ and the 3′ regions of the CP gene, respectively. Two sets of primers were used: primers 80 (5′-TTGGGTTCTTGAACAAGC-3′) and 82 (5′-TGGCACTGTAAAAGTTCC-3′) (kindly provided by Dr. JA García) for amplification of the CP 5′ region; and primers 83 (5′-ATGAGGATGCATCACCTAGC-3′) and P1 (Wetzel et al. 1991) for amplification of the CP 3′ region. Comparisons of sequences were performed by the analysis of phylogenetic incongruence using the Bootscan application of the PHYLIP 95 in the Simplot program version 2.5 (Lole et al. 1999).
Aphid species monitoring and statistical analyses
Aphid monitoring was performed by the sticky shoot method (Avinent et al. 1993; Cambra et al. 2000). Selected shoots were sprayed with an adhesive (Souverode aerosol, Scotts, France) and collected after 10 days. Captured winged adult aphids were detached with turpentine, counted, preserved in 70% alcohol and identified under a binocular microscope.
Two shoots per tree were analysed each month from immediately after blossom (February) to leaf fall (end of September) in 2004 in a total of six transgenic European plums from lines C4, C5, C6, PT6 and PT23, and six non-transgenic European and Japanese plums regularly distributed in the experimental plot. Total numbers of aphids and aphid species visiting individual shoots were estimated to determine the alate aphid population dynamics of the experimental plot.
Two sticky shoots per tree in six transgenic European plums from lines C4, C5, C6, PT6 and PT23, six non-transgenic European plums and six non-transgenic Japanese plums from the experimental orchard were analysed by the sticky shoot method in May (the month of maximum populations of winged aphids) 2004 and 2005 to compare the numbers and percentages of aphids species that landed on transgenic vs. non-transgenic plums. Additionally, in May 2005, the same number of sticky shoots were analysed every 10 days to estimate the numbers of aphids landing on a tree in the period of maximum aphid incidence. The average number of shoots per tree was estimated by counting the numbers of shoots (10–15 cm long) in five European and five Japanese plum trees. The total numbers of aphids visiting a single tree was estimated by multiplying the average number of shoots/tree by the numbers of captured aphids/shoot.
Statistical analysis of data was performed by analysis of variance (ANOVA) with two factors (year and species) and their interaction. The normality of the residues was checked by Shapiro–Wilks test and Q–Q plots. Since the interaction was not significant in any analysis, it was deleted from the model. Comparisons between transgenic and non-transgenic trees and between European and Japanese plums were carried out by specific contrasts.
Detection of PPV-RNA targets in individual aphids
The percentage of visiting aphids that carried PPV (viruliferous aphids) was determined by real-time RT-PCR using PPV specific primers: P241 (5′-CGTTTATTTGGCTTGGATGGAA-3′), P316D (5′-GATTAACATCACCAGCGGTGG-3′) and P316M (5′-GATTCACGTCACCAGCGGTGTG-3′) and a PPV universal TaqMan probe: PPV-DM (5′-CGTCGGAACACAAGAAGAGGACACAGA-3′) according to Olmos et al. (2005). Individual aphids were squashed on filter paper with the round bottom of different plastic Eppendorf tubes. Filter papers with squashed aphids were stored in a dry place at room temperature until use. The piece of paper harboring each individual squashed aphid was introduced into an Eppendorf tube and 100 μl of 0.5% Triton X-100 was added, vortexed and incubated for 2 min at room temperature (Olmos et al. 1996). Triton extract (5 μl) was used directly as template in real-time RT-PCR reactions. Forty five to 180 aphids belonging to the two most abundant aphid species (Aphis spiraecola Patch and A. gossypii Glover (Hemiptera: Aphididae)), collected from transgenic and non-transgenic European plums and from conventional Japanese plums from the experimental orchard, were analysed. Data were treated by a generalized linear model (McCullagh and Nelder, 1989) with the tree type as factor. Comparisons between transgenic and non-transgenic trees were carried out by specific contrasts.
Results
Variability of PPV populations
The genetic variability was assessed among 85 PPV isolates from transgenic plum lines C4, C6 , PT6 and PT23 and non-transgenic plums in the 3′ most variable region of the virus genome, i.e. the 3′ end of NIb gene and 5′ end of CP gene. The percentage of nucleic acid homology ranged from 96.9 to 100%. A total of 51 PPV haplotypes were found in the two experimental and control orchards with 18 belonging to isolates from transgenic European plums (transgenic population), 15 to isolates from non-transgenic European and Japanese plums in the experimental orchard (non-transgenic population), and 24 to isolates from the control population (PPV isolates present in conventional Japanese plums from the control plot) (Table 1). The nucleotide and gene (haplotype) diversity within each PPV population was very low and did not significantly differ between transgenic, non-transgenic and control PPV populations (Table 1). The nucleotide diversity between the transgenic and non-transgenic population within the experimental plot was 0.00607 ± 0.00152, and the mean nucleotide diversity among populations was 0.00163 ± 0.00058. These results showed that the molecular variability of the NIb-CP fragment within each population (Table 1) is higher than among populations. These results were confirmed by analysis of molecular variance (AMOVA) with 14.51% variation among populations and 85.49% within populations (Table 2).
Recombination analysis
The emergence of recombinant viruses between PPV CP transgene transcripts and the CP gene of 12 PPV isolates from transgenic plums C4 and PT23 expressing high and moderate CP mRNA levels, respectively, was assessed. Analysis of CP gene sequences indicated that no recombinant virus was found to detectable levels after 8 years of exposure to natural PPV infection in the field.
Comparison of aphid populations visiting transgenic and non-transgenic plums
The dynamics of aphid species that visited the experimental plot in 2004 showed a population peak in May (85% of the total captures) (Fig. 1). No aphid was captured by the sticky shoot method from February to April. Aphid populations drastically decreased (11% of total captures in June and 0% in July and August) in the summer with high temperatures and subsequently slightly increased in September (4%) (Fig. 1).
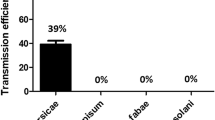
A total of 6,097 individual aphids were captured on sticky shoots in May 2004 and 2005 (Fig. 2). A. spiraecola was the most abundant visitor aphid species (51%) followed by A. gossypii (28%), Hyalopterus pruni (Geoffroy) (16.5%), Brachycaudus helichrysi (Kaltenbach) (1.8%), A. fabae Scopoli (1.0%), A. craccivora Koch (1.0%), Myzus persicae (Sulzer) (0.1%) and other species (0.7%).
The total number of aphids and aphid species that landed on transgenic lines C4, C5, C6, PT6 and PT23 and non-transgenic plums did not significantly differ between May 2004 vs. May 2005 (Table 3). Similarly, no significant difference between the number and percentage of aphid species that visited transgenic and non-transgenic European plums and conventional Japanese plums were found (Table 3). A. spiraecola was the most abundant aphid species that landed in all tree types, constituting 50–53% of all visitor aphids. A. gossypii represented 25–29%, and other aphid species were 18–23% (Fig. 3).
In May 2005 the number of aphids that visited a single tree in the experimental orchard was estimated at about 24,300 individuals. These data were obtained by extrapolating 4,028 aphids caught in 36 shoots to an average of 218 shoots per tree (Table 4).
The two most abundant aphid species (A. spiraecola and A. gossypii) that visited transgenic and non-transgenic plums (European and Japanese) were assayed by real-time RT-PCR for the presence of PPV. Results indicated that PPV-RNA targets were successfully amplified for 26.9% of A. spiraecola and 28.1% of A. gossypii (Table 5). No significant difference between the number of viruliferous aphids that visited transgenic and non-transgenic plum trees was detected (Table 5).
Discussion
No differences between the molecular diversity of PPV populations present in PPV-susceptible transgenic European plums and non-transgenic European and Japanese plums were detected within the most variable region of the PPV genome. Pairwise nucleotide comparisons calculated between all full-length PPV CP gene sequences available in international databases (63 sequences) show between-strain divergence values ranging from 12 to 25% and limited within-strain divergence values (only 5% or less) (Candresse and Cambra 2006). In the PPV population analysed in the present work, the percentage of divergence in the 3′NIb-5′CP region is even lower (3.1%) taking into account that most of the isolates analysed belonged to the same experimental orchard which was surrounded by a barrier of guard trees. These data together with results on the molecular diversity and variance within and among populations clearly suggest that the three artificially defined populations (PPV infecting transgenic, non-transgenic and control plums) constituted a single population or metapopulation.
No recombinant virus was found to detectable level in transgenic plums expressing the CP gene of PPV after 8 years of field exposure to PPV infection. However, the presence of recombinant viruses as minor components of the viral population analysed cannot be ruled out as our experimental approach focused only on the master sequence (the most prevalent). In any event, if recombinant PPV variants emerged during the trial period, they did not prevail in the viral population. If a recombinant has no selective advantage, it is likely to be of no concern because it will not be able to compete with non-recombinant variants. Although recombinant viruses with altered biological properties have been recovered in transgenic plants, these experiments have been done under laboratory or greenhouse conditions with a moderate to high selection pressure in favour of recombinant viruses (Greene and Allison 1994; 1996; Wintermantel 1996; Varrelman et al. 2000). Interestingly, and supporting the low risk of emergence of recombinant viruses in transgenic plants, natural recombinants of Grapevine fanleaf virus (GFLV) were identified in non-transgenic grapevines, but not in transgenic ones expressing the GFLV coat protein gene (Vigne et al. 2004). Additionally, no risk, in terms of emergence of new recombinant viruses with potential harmful characteristics, was detected in transgenic vegetable crops (Fuchs et al. 1998; Thomas et al. 1998). PPV natural recombinants have been reported that actually constitute a new PPV type (PPV-Rec or recombinant between D and M). Isolates from this group have the same recombination crossover site in the C-terminus of the NIb gene, showing D characteristics upstream the recombination breakpoint and M characteristics downstream it (Cervera et al. 1993; Glasa et al. 2001, 2002 and 2005). To date, no recombinant between PPV isolates belonging to the same type (D, M, C, EA or W) has been reported. This data supports the lack of detection of PPV recombinants in the experimental plot where all PPV isolates belonged to the D strain (Capote and Cambra 2005). Recombination is one of the ways that RNA viruses increase variability. Yet, it appears to remain extremely rare. The rarity of recombination is supported by the work of Capote et al. (2006) that showed that no recombination between D and M PPV isolates co-infecting Japanese plums was detected over a 7 year experimental period. Recombination between incoming viruses and virus transgenes is unlikely to provide a substantially increased contribution to virus evolution, particularly when one considers that such events already occur naturally in plants infected with two or more viruses. In the present work, it has been shown that transgenic European plums carrying the CP gene of PPV did not promote the emergence of viable recombinant viruses to detectable levels. Particularly, C5 transgenic plums present a extremely low risk, if any, in terms of the emergence of new recombinant viruses, as this transgenic line produces very low levels of transgene transcript in the cell cytoplasm (Scorza et al. 1994) and is highly resistant to PPV infection (Ravelonandro et al. 1997 and 2002; Malinowski et al. 1998, 2006; Hily et al. 2004; 2005). Actually, C5 was the only transgenic line capable to alter the dynamics of PPV epidemics by preventing PPV infection and secondary plant-to plant spread. Therefore, aphids visiting C5 plums are highly unlikely to acquire and further transmit PPV to susceptible hosts after a probing or feeding period, due to the non-persistent PPV transmission manner. In this way, the use of C5 PPV resistant transgenic line could avoid the use of insecticides, reducing the ecological impact of this common agricultural practice. Transgenic C5 is named ‘HoneySweet’ and considered for deregulation in the USA (Scorza et al. 2007).
Different aphid species landed on transgenic and non-transgenic European plums, and on conventional Japanese plums at the same frequency. This suggests that aphid species have no preference for the transgenic or non-transgenic character of plum trees. Thus, they were probably able to equally feed, acquire and/or transmit PPV to transgenic and non-transgenic plums. The numbers of individuals and aphid species captured vary among years and hosts in the temperate Mediterranean area of Prunus cultivation. In the experimental orchard May had the highest aphid populations, this is in agreement with previous data (Cambra et al. 2006a). A. spiraecola was the most probable significant vector of PPV in European and Japanese plums in the experimental orchard, as has been reported for Japanese plums in other Mediterranean Spanish Prunus growing areas (Cambra et al. 2004; 2006a). Results from other Mediterranean countries showed that A. gossypii is the most abundant aphid in apricot orchards in Spain and Greece (Avinent et al. 1989, 1991, 1993; Varveri et al. 2004) and A. spiraecola is the predominant aphid species in apricot orchards in south-eastern France (Labonne and Dallot 2006 ) In non-Mediterranean climate areas, such as Pennsylvania (USA), the most abundant aphid species caught on peach trees were Rhopalosiphum maidis (Filch) and A. spiraecola. As R. maidis is unable to transmit Pennsylvanian isolates of PPV, A. spiraecola is also the most probable PPV vector in this region (Wallis et al. 2005).
Between 24 and 35% of aphid vectors that landed on plum trees in the experimental orchard were viruliferous, that is, they carried PPV-RNA targets. The high incidence of aphids and the elevated percentage of aphids carrying PPV is consistent with the rapid spread of PPV in the experimental and surrounding plots in which PPV infection has been monitored over an 8 year experimental period (Malinowski et al. 2006). No significant difference was found in viruliferous A. spiraecola and A. gossypii between plum species (European or Japanese) or tree character (transgenic and non-transgenic) suggesting that viruliferous aphids visit plum trees randomly. The dynamics of sharka disease and variability of PPV populations were not altered by transgenic plums, with the possible exception of the C5 line which could potentially interfere with the spread of the disease due to its high level of PPV resistance.
The present study demonstrates that transgenic European plums did not affect the molecular diversity of indigenous PPV populations nor promote the emergence of viable recombinant viruses to detectable levels during the experimental period. Additionally, transgenic plums had a neutral impact on the dynamics of aphid populations that visited the experimental plot and spread the virus. These data support the conclusion that PPV-CP transgenic European plums under Mediterranean conditions present limited, if any, risk beyond the cultivation of conventional plum trees in terms of diversity of PPV and aphid populations and, in fact, the cultivation of the resistant C5 plum (cv. HoneySweet) represents an opportunity to reduce the spread of PPV within and between orchards.
References
Avinent L, Hermoso de Mendoza A, Llácer G (1989) Especies dominantes y curvas de vuelo de pulgones (Homoptera, Aphidinea) en campos de frutales de hueso españoles. Invest Agraria Prod Prot Veget 4:283-298
Avinent L, Hermoso de Mendoza A, Llácer G (1991) Comparison of traps for captures of alate aphids (Homoptera, Aphidinea). Agronomie 11:613–618
Avinent L, Hermoso de Mendoza A, Llácer G (1993) Comparison of sampling methods to evaluate aphid populations (Homoptera, Aphidinea) alighting on apricot trees. Agronomie 13:609–613
Badenes ML, Moustafa TA, Martínez-Calvo J, Llácer G (2006) Resistance to sharka trait in a family from selfpollination of ‘Lito’ apricot cultivar. Acta Hortic 701:381–384
Boscia D, Zeramdini H, Cambra M, Potere O, Gorris MT, Myrta A (1997) Production and characterization of a monoclonal antibody specific to the M serotype of Plum pox potyvirus. Eur J Plant Pathol 103:447–480
Cambra M, Asensio M, Gorris MT, Pérez E, Camarasa E, García JA, López-Moya JJ, López-Abella D, Vela C, Sanz A (1994) Detection of Plum pox potyvirus using monoclonal antibodies to structural and non structural proteins. Bull OEPP/EPPO Bull 24:569–577
Cambra M, Gorris MT, Marroquín C, Román MP, Olmos A, Martínez MC, Hermoso de Mendoza A, López A, Navarro L (2000) Incidence and epidemiology of Citrus tristeza virus in the Valencia Community of Spain. Virus Res 71:85–95
Cambra M, Gorris MT, Capote N, Asensio M, Martínez MC, Bertolini E, Collado C, Hermoso de Mendoza A, Matáix E, López A (2004) Epidemiology of Plum pox virus in Japanese plums in Spain. Acta Hortic 657:195–200
Cambra M, Capote N, Cambra MA, Llácer G, Botella P, López-Quílez A (2006) Epidemiology of sharka disease in Spain. Bull OEPP/EPPO Bull 36:271–275
Cambra M, Capote N, Myrta A, Llácer G (2006) Plum pox virus and the estimated costs associated with sharka disease. Bull OEPP/EPPO Bull 36:202–204
Candresse T, Cambra M (2006) Causal agent of sharka disease: historical perspective and current status of Plum pox virus strains. Bull OEPP/EPPO Bull 36:239–246
Candresse T, Macquaire G, Lanneau M, Bousalem M, Wetzel T, Quiot-Dounie L, Quiot JB, Dunez J (1994) Detection of Plum pox potyvirus and analysis of its molecular variability using immunocapture-PCR. Bull OEPP/EPPO Bull 24:585–594
Capote N, Cambra M (2005) Variability of Plum pox virus populations in PPV-resistant transgenic and non-transgenic plums. Phytopathol Pol 36:107–113
Capote N, Gorris MT, Martínez MC, Asensio M, Olmos A, Cambra M (2006) Interference between D and M types of Plum pox virus in Japanese plums assessed by specific monoclonal antibodies and quantitative real-time RT-PCR. Phytopathology 96:320–325
Cervera MT, Riechmann JL, Martin MT, García JA (1993) 3’-terminal sequence of the Plum pox virus PS and o6 isolates: evidence for RNA recombination within the Potyvirus group. J Gen Virol 74:329–334
Excoffier L, Smouse P, Quattro J (1992) Analysis of molecular variance inferred from metric distances among DNA haplotypes: Application to human DNA restriction data. Genetics 131:479–491
Fuchs M, Klas FE, McFerson JR, Gonsalves D (1998) Transgenic melon and squash expressing coat protein genes of Aphid-borne viruses do not assist the spread of an Aphid non-transmissible strain of Cucumber mosaic virus in the field. Transgenic Res 7:449–462
Glasa M, Kúdela O, Marie-Jeanne V, Quiot JB (2001) Evidence of naturally occurring recombinant Plum pox virus isolate from Slovakia. Plant Dis 85:920
Glasa M, Marie-Jeanne V, Labonne G, Šubr Z, Kúdela O, Quiot JB (2002) Natural population of recombinant Plum pox virus is stable and competitive under field conditions. Eur J Plant Pathol 108:843–853
Glasa M, Palkovics L, Komínek P, Labonne G, Pittnerová S, Kúdela O, Candresse T, Šubr Z (2004) Geographically and temporally distant natural recombinant isolates of Plum pox virus (PPV) are genetically very similar and form a unique PPV subgroup. J Gen Virol 85:2671–2681
Glasa M, Paunovic S, Jevremovic D, Myrta A, Pittnerová S, Candresse T (2005) Analysis of recombinant Plum pox virus (PPV) isolates from Serbia confirms genetic homogeneity and supports a regional origin for the PPV-Rec subgroup. Arch Virol 150:2051–2060
Greene AE, Allison FR (1994) Recombination between viral RNA and transgenic plant transcripts. Science 263:1423–1425
Greene AE, Allison FR (1996) Deletions in the 3’ untranslated region of cowpea chlorotic mottle virus transgene reduce recovery of recombinant viruses in transgenic plants. Virology 225:231–234
Hily JM, Scorza R, Malinowski T, Zawadzka B, Ravelonandro M (2004) Stability of gene silencing-based resistance to Plum pox virus in transgenic plum (Prunus domestica L.) under field conditions. Transgenic Res 13:427–436
Hily JM, Scorza R, Webb K, Ravelonandro M (2005) Accumulation of the long class of siRNA is associated with resistance to Plum pox virus in a transgenic woody perennial plum tree. Mol Plant Microbe Interact 18:794–799
James D, Glasa M (2006) Causal agent of sharka disease: new and emerging events associated with Plum pox virus characterization. Bull OEPP/EPPO Bull 36:247–250
James D, Varga A (2005) Nucleotide sequence analysis of Plum pox virus isolate W3174: Evidence of a new strain. Virus Res 110:143–150
Kegler H, Fuchs E, Gruntzig M, Schwarz S (1998) Some results of 50 years of research on the resistance to Plum pox virus. Acta Virol 42:200–215
Kunze L, Krczal H (1971) Transmisssion of Sharka virus by aphids. Ann Phytopathol HS:255–260
Labonne G, Dallot S (2006) Epidemiology of sharka disease in France. Bull OEPP/EPPO Bull 36:267–270
López-Moya JJ, Fernández-Fernández MR, Cambra M, García JA (2000) Biotechnological aspects of Plum pox virus. J Biotechnol 76:121–136
Lole KS, Bollinger RC, Paranjape RS, Gadkari D, Kulkarni SS, Novak NG, Ingersoll R, Sheppard HW, Ray SC (1999) Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J Virol 73:152–60
Malinowski T, Zawadzka B, Ravelonandro M, Scorza R (1998) Preliminary report on the apparent breaking of resistance of a transgenic plum by chip bud inoculation of Plum pox virus PPV-S. Acta Virol 42:241–243
Malinowski T, Cambra M, Capote N, Zawadzka B, Gorris MT, Scorza R, Ravelonandro M (2006) Field trials of plum clones transformed with the Plum pox virus coat protein (PPV-CP) gene. Plant Dis 90:1012–1018
McCullagh P, Nelder JA (1989) Generalized Linear Models, 2nd edn. Cahpman & Hall, London
Myrta A, Potere O, Boscia D, Candresse T, Cambra M, Savino V (1998) Production of a monoclonal antibody specific to the El Amar strain of Plum pox virus. Acta Virol 42:248–250
Myrta A, Potere O, Crescenzi A, Nuzzaci M, Boscia D (2000) Production of two monoclonal antibodies specific to cherry strain of Plum pox virus (PPV-C). J Plant Pathol 82:95–103
Neumüller M, Hartmann W, Stösser R (2005) The hypersensitivity of European plum against PPV as a promising mechanism of resistance. Phytopathol Pol 36:77–83
Ng JCK, Perry KL (2004) Transmission of plant viruses by aphid vectors. Mol Plant Pathol 5:505–511
Olmos A, Dasí MA, Candresse T, Cambra M (1996) Print-capture PCR: A simple and highly sensitive method for the detection of Plum pox virus (PPV) in plant tissues. Nucleic Acids Res 24:2192–2193
Olmos A, Bertolini E, Gil M, Cambra M (2005) Real-time assay for quantitative detection of non-persistently transmitted Plum pox virus RNA targets in single aphids. J Virol Methods 128:151–155
Ravelonandro M, Scorza R, Bachelier JC, Labonne G, Levy L, Damsteegt V, Callahan AM, Dunez J (1997) Resistance of transgenic Prunus domestica to Plum pox virus infection. Plant Dis 81:1231–1235
Ravelonandro M, Varveri C, Delbos R, Dunez J (1998) Nucleotide sequence of the capsid protein gene of Plum pox potyvirus. J Gen Virol 69:1509–1516
Ravelonandro M, Scorza R, Minoiu N, Zagrai I, Platon I (2002) Field tests of transgenic plums in Romania. Sănătatea Plant 6:16–17
Rubio T, Borja M, Scholthof HB, Jackson AO (1999) Recombination with host transgenes and effects on virus evolution: An overview and opinion. Mol Plant Microbe Interact 12:87–92
Scorza R, Ravelonandro M, Callahan AM, Cordts JM, Fuchs M, Dunez J, Gonsalves D (1994) Transgenic plums (Prunus domestica L.) express the Plum pox virus coat protein gene. Plant Cell Rep 14:18–22
Scorza R, Callahan A, Levy L, Damsteegt V, Webb K, Ravelonandro M (2001) Post-transcriptional gene silencing in Plum pox virus resistant transgenic European plum containing the Plum pox potyvirus coat protein gene. Transgenic Res 10:201–209
Scorza R, Hily JM, Callahan A, Malinowski T, Cambra M, Capote N, Zagrai I, Damsteeg V, Briard P and Ravelonandro M (2007) Deregulation of plum pox resistant transgenic plum “HoneySweet”. Acta Hortic (in press)
Szemes M, Kalman M, Myrta A, Boscia D, Nemeth M, Kölber M, Dorgai L (2001) Integrated RT-PCR/nested PCR diagnosis for differentiating between subgroups of Plum pox virus. J Virol Methods 92:165–175
Thompson DA, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The Clustal-X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 24:4876–4882
Thomas PE, Hassan S, Kaniewski WK, Lawson EC, Zalewski JC (1998) A search for evidence of virus/transgene interactions in potatoes transformed with the Potato leafroll virus replicase and coat protein genes. Mol Breeding 4:407–417
Varrelman M, Palkovics L, Maiss E (2000) Transgenic or plant expression vector-mediated recombination of Plum pox virus. J Virol 74:7462–7469
Varveri C, Zintzaras E, Dimou D, Papapanagiotou A, Di Terlizzi B (2004) Monitoring and spatio-temporal analysis of PPV-M spread in two apricot orchards in Southern Greece. Ann Benaki Phytopathol Inst 20:1–9
Vigne E, Komar V, Fuchs M (2004) Field safety assessment of recombination in transgenic grapevines expressing the coat protein gene of Grapevine fanleaf virus. Transgenic Res 13:165–179
Wallis CM, Fleischer SJ, Luster D, Gildow FE (2005) Aphid (Hemiptera: Aphididae) species composition and potential aphid vectors of Plum pox virus in Pennsylvania peach orchards. J Econ Entomol 98:1441–1450
Wetzel T, Candresse T, Ravelonandro M, Dunez J (1991) A polymerase chain reaction assay adapted to Plum pox potyvirus detection. J Virol Methods 33:355–365
Wintermantel WM, Schoelz JE (1996) Isolation of recombinant viruses between cauliflower mosaic virus and a viral gene in transgenic plants under conditions of moderate selection pressure. Virology 223:156–164
Acknowledgements
This work was supported by the European project TRANSVIR (QLK3-CT-2002-02140), INIA (SC98-060 and RTA03-099) and Ministerio de Educación y Ciencia (RTA05-00190) projects. Authors thank to C. Collado (IVIA) for determination of aphid species, to B. Tamargo and J. Micó (Cooperativa Vinícola de Liria) for technical assistance in the experimental plot, to Dr. S. Elena for assistance in recombination analyses and to Dr. M. Fuchs for critical reading of the manuscript. Permissions for field release of GMO Nos. B/ES/96/16 and B/ES/05/14 were given by the Spanish Ministerio de Medio Ambiente.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Capote, N., Pérez-Panadés, J., Monzó, C. et al. Assessment of the diversity and dynamics of Plum pox virus and aphid populations in transgenic European plums under Mediterranean conditions. Transgenic Res 17, 367–377 (2008). https://doi.org/10.1007/s11248-007-9112-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-007-9112-0