Abstract
Cuprous oxide (Cu2O) microspheres were synthesized through a simple chemical method by using ascorbic acid (AA) as reducing agent at room temperature and short reaction time. The influence of the reducing agent and ammonium hydroxide (NH4OH), as well as reaction time, on the morphology, size, and crystalline phase structure of Cu2O were explored. The obtained materials were characterized by X-ray diffraction, scanning electron microscopy, nitrogen physisorption, and temperature-programmed reduction, whereas the catalytic activity was assessed using the photodegradation of methyl orange (MO). It was found that AA was responsible for the Cu2O nanoparticles self-assembling into Cu2O microspheres after 0.16 h of reaction time. Quantum theoretical calculations revealed that self-assembly is due to the hydrophilic modification of the Cu2O-nanoparticles driven by the parallel-to-surface dehydroascorbic acid chemisorption; likewise, the simulation of a mesoscopic molecular dynamics confirmed that the amphiphilic-like nature of the dehydroascorbic acid-Cu2O complexes leads to nanoparticles’ spherical morphology. The NH4OH concentration affected the porosity and crystalline phase of the microspheres. The porous Cu2O microspheres modified into well-defined Cu0 polyhedrons with the reaction time. The Cu2O microspheres exhibited high photocatalytic activity up to 76% during decolorization of MO. Two catalytic processes were distinguished: the removal of organic compound controlled by the faceting of the particles under dark conditions, and its photodegradation using visible light increased by the porosity of the Cu2O microspheres.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The synthesis of metal oxides with specific control of morphologies, sizes and crystallographic structures has attracted attention in recent years due to their structure-morphology-dependent catalytic, photocatalytic, electrical, and molecular adsorption properties [1,2,3,4,5,6]. Among them, metal-oxide-semiconductors such as TiO2, ZnO and Cu2O [7,8,9] can act as photocatalysts for different applications including degradation of pollutants [10], CO2 reduction [11], H2 production [12], and microbial disinfection [13]. Particularly, cuprous oxide (Cu2O), whose band gap is 2.17 eV, can absorb visible light making it an interesting transition metal oxide with a wide application in the fields of gas sensors [14], solar cells [15], lithium-ion batteries [16], biosensors [17] and catalysis [18]. In fact, it has been reported that metallic copper or copper oxides promoted with ZnO generate active sites at the Cu/ZnO interface, which are able to hydrogenate CO2 into methanol [19].
Therefore, many approaches have been proposed to obtain different Cu2O structures including polyol method [20], hybrid laser processing [21], soft-template method [22], solvo and hydrothermal method [22,23,24,25,26,27]. Different reducing agents, such as hydrazine hydrate (strong reducing agent), glucose and ascorbic acid (mild reducing agent) [2, 28, 29] have been used. Moreover, a variety of surfactants have been employed to control morphologies, such as sodium dodecyl sulfate (SDS) [30], cetyltrimethylammonium bromide [31], polyvinylpyrrolidone [32], and polyethylene glycol [33]. Reaction conditions varied from room temperature to 210 °C with reaction times from 0.16 to 48 h [34]. Through these methods, Cu2O micro/nano structures having varied morphologies such as nanocubes [35], nanowires [36], nanobelts [37], nanospheres [38], and hollow spheres [39] have been obtained. Particularly, hierarchical nanostructures obtained through self-assemblies of smaller units to form microspheres [40] have showed improvements on the control of mass transport and light absorption.
Hierarchical Cu2O microspheres were obtained by a polyol reduction method at 170 °C. They showed enhanced specific capacitance compared with that of cubic and flower-like structures, which was ascribed to the small particles on their rough surface [41]. Zhang et al. synthesized Cu2O using glutamic acid as reducing agent at 160 °C during 48 h. The obtained hierarchical Cu2O microspheres showed large accessibility surface leading to a higher gas sensitivity (1.9) [42]. Besides, Cu2O particles were synthesized by a solvothermal method at 140 °C for 12 h. These Cu2O particles were able to degrade methyl orange not only under visible light irradiation but also under dark conditions; this fact was related to oxygen vacancies remaining on their rough surface [43, 44]. Huang et al. synthesized well faceted Cu2O nanocrystals from cubic to rhombic dodecahedral morphologies using CuCl2 as precursor, SDS as surfactant and hydroxylamine hydrochloride (NH2OH·HCl) as reducing agent. The rhombic dodecahedral structures expose exclusively {110} faces showing the highest photocatalytic activity toward the complete degradation of methyl orange, which was attributed to a high density of surface copper atoms [4]. Whatever the application is, hierarchical Cu2O microspheres have showed higher performance compared with other morphologies. However, high temperatures, long reaction time and different hazardous agents are required to prepare them. Therefore, simple and environmentally friendly methods for the synthesis of well-defined Cu2O microspheres still remain desirable.
Herein, hierarchical Cu2O microspheres and Cu0 crystallites were obtained by a simple chemical method at room temperature in aqueous solution by using ascorbic acid (AA) as reducing agent. The influence of the reducing agent, ammonium hydroxide (NH4OH) concentration and reaction time was examined.
2 Experimental Section
2.1 Synthesis of Cuprous Oxide Particles
All chemical reagents were of analytical grade and used as received without further purification. The synthesis of copper microstructures was carried out at room temperature, by adding an aqueous solution (250 mL) of copper sulfate(II) pentahydrate [0.1 M, CuSO4·5H2O, 99%] and ammonium hydroxide [0.025–0.1 M, NH4OH, 28% vol.) dropwise, in a solution containing phenol (250 mL) upon stirring. The pH was maintained constant at 7.5. A yellowish suspension was obtained, and it was stirred for 0.5 h. Thereafter, a solution of reducing agent (ascorbic acid) [250 mL, 0.1 M, C6H8O6, 99%] was added; the suspension immediately switched to dark brown. The obtained precipitate was stirred for 0.16 to 12 h depending on the reaction time. Thereafter, the obtained solid was filtered and repeatedly washed with abundant distilled water, and finally it was dried at 110 °C.
2.2 Characterization
2.2.1 X-ray Diffraction
The crystalline structure of the prepared samples was identified by X-ray diffraction (XRD) using an X-ray diffractometer (Siemens D-5005 krystalloflex, Cu Kα radiation). The mean size of nanocrystals was measured from the (111) diffraction peaks of the Cu2O and Cu0 by using the Debye–Scherrer equation:
where \(k\) is the Scherrer constant (shape factor, is 0.9), λ is the X-ray wavelength (λ = 0.154 nm), \(\beta\) is the line broadening at half the maximum intensity (FWHM) in radians, θ is the Bragg angle (the position of the diffraction peak maximum) and d is the averaged dimension of crystallites in nanometers.
2.2.2 Scanning Electron Microscopy (SEM)
The morphologies of the samples were examined by SEM in a FEI Nova NanoLab DualBeam scanning electron microscope and the elemental chemical analysis was performed by EDX spectroscopy with EDAX spectrometer attached to the microscope, Nova NanoLab DualBeam.
2.2.3 Textural Properties
The textural properties such as specific surface area (SSA), pore volume and pore size distribution (PSD) measurements were carried out in a Quantachrome NovaTouch equipment by nitrogen physisorption. Prior to adsorption, the samples were degassed (P < 10−1 Pa) at 150 °C for 12 h. SSA was determined from N2 physisorption at − 196 °C by applying BET method. The average pore size was obtained by BJH method from the desorption branch.
2.2.4 Temperature-Programmed Reduction (TPR)
TPR was performed on an ICID equipment model SRyC-2. The experiments were carried out using 0.10 g of catalyst in a quartz tube. First, the sample was dried at 110 °C for 1 h with a 10 °C/min heating rate and 60 mL/min air flow. Then, the sample was cooled at room temperature. Finally, the sample was heated from 20 to 600 °C with 10 °C/min in 10% H2/90% N2 and a gas flow of 30 mL/min. The hydrogen consumption as a function of reduction temperature was continuously monitored with a thermal conductivity detector (TCD).
2.2.5 Theoretical Methodology
The Cu2O crystallographic structure was used to build the atomistic computational model for a cuprous oxide particle. It is experimentally known that the main structures of Cu2O particles in equilibrium are obtained with the combination of the (100), (111), and (110) low-index exposed facets [42, 43, 45]. The stability order of the surfaces was theoretically found to be (111) > (110) > (100) [46]. We used the Material Studio (MS) [47] interface to build the molecular model of the dehydroascorbic acid in horizontal (Fig. 1a) and vertical (Fig. 1b) orientations to be parallel and perpendicularly put on a pristine Cu2O (111) surface (Fig. 1c). Also, in order to have insights about the water affinity of both the pristine and dehydroascorbic acid-containing Cu2O particles, the chemisorption of a water molecule by the uncovered (Fig. 2a) and dehydroascorbic-acid covered (Fig. 2b) Cu2O (111) surface were modeled. The chemisorption’s were studied through periodic-boundary-conditions Density-Functional-Theory (DFT) approach as implemented in the DMol3 module of MS [48].
The dimensions of the unit cell containing the Cu2O (111) surface, which was modeled through a 3-layers slab, are a = b = 6.04 and c = 35.41 Å. From this unit cell, it was built a simulation cell (SC) with extended 2 × 2 × 1 dimensions (Fig. 1c) enough for allocating the dehydroascorbic acid; thus, the SC dimensions are \({a}_{\text{SC}}={b}_{\text{SC}}=\) 12.08 and \({c}_{\text{SC}}=\) 35.41 Å. The periodic images of the dehydroascorbic acid are adequately far away (~ 9 Å) to avoid strong interactions among themselves.
Molecular geometries for all single and interacting models were fully optimized with fixed cell axes through gamma-point DFT energy minimizations using the GGA/PBE functional [49], the double numerical polarization (DNP) basis set, and the Grimme method for DFT dispersion corrections [50]. All predefined run parameters were set at the fine accuracy option.
The interaction energies \(\Delta {E}_{\text{int}}\) among a molecule and the Cu2O surface was calculated as
where ES, EM and ESM are the total electronic energy of the Cu2O surface, the molecule to be chemisorbed, and the molecule containing Cu2O surface, respectively.
2.3 Photodegradation Test
The photocatalytic activity of Cu2O microspheres was measured during the degradation of methyl orange (C14H14N3NaO3S) under dark and visible light irradiation conditions. A 0.1 g sample of the prepared powders was dispersed in 100 mL of MO aqueous solution (15 mg/L) in a quartz bottle. Then, this mixture was magnetically stirred under dark condition over 30 min. Thereafter, it was irradiated with a visible lamp (\(\lambda >400\) nm) at room temperature. 3 mL of the suspensions was fetched from the quartz bottle at regular intervals of time on stream and centrifuged immediately to remove the catalysts particles. The concentration of MO was estimated by the absorbance measurement at 464 nm through UV–Vis spectrophotometer (Thermo Spectronic, Helios).
The MO degradation efficiency (in %) was calculated as follows:
where C0 is the original absorbance of MO solution at 464 nm and C is the absorbance of MO at different degradation time.
3 Results and Discussion
3.1 Effect of Reducing Agent
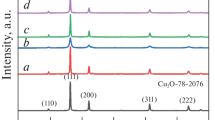
XRD patterns of the samples prepared without and with different reducing agents (glucose, ascorbic acid and hydrazine) during 0.16 h of reaction time are presented in Fig. 3. As expected, the sample prepared without any reducing agent, Fig. 3a, showed diffraction lines characteristic of monoclinic Cu4(SO4)(OH)6H2O phase, according to the JCPDS card No. 83-1410. The CuSO4·5H2O solution precipitated to yield Cu4(SO4)(OH)6H2O pale blue powder. When d-glucose was used as reducing agent, also a pale blue powder was obtained; its diffraction pattern also corresponds to the Cu4(SO4)(OH)6H2O phase, see Fig. 3b. In spite of d-glucose have been used as reducing agent for different copper precursors, the Cu4(SO4)(OH)6H2O was not reduced [24, 49]. According to previous studies, the reducing power of glucose depends on both concentration and reaction temperature (> 60 °C) [51, 52]; in our procedure, the synthesis was given at room temperature and that’s why reduction of Cu4(SO4)(OH)6H2O did not occur. The sample synthesized with ascorbic acid as reducing agent showed reflections at 2θ = 29.6, 36.5, 42.4, 61.6 and 73.6°, corresponding to the planes (110), (111), (200), (220) and (311) of the Cu2O cuprite, according to JCPDS card. No. 005-0667, indicating the formation of cubic cuprous oxide nanocrystals, Fig. 3c. The characteristic peaks of other phases such as metallic Cu or CuO phases were not observed. The reduction of Cu4(SO4)(OH)6H2O occurs due to a well-control in the mixture of weak acid and medium basic entities such as ascorbic acid and NH4OH, respectively [29]. When hydrazine was used as reducing agent, additional to the XRD peaks characteristic of the cubic Cu2O phase, two peaks centered at 2θ = 43.4 and 50.5° were observed corresponding to the cubic phase of metallic copper, according to JCPDS card No. 04-0836, Fig. 3d. Also, a small peak at 2θ = 12.7° appears, corresponding to the Cu4(SO4)(OH)6H2O phase, indicating that the reaction was not completed at very short reaction time. Hydrazine has stronger reduction power than AA, which induces the reduction of Cu4(SO4)(OH)6H2O phase into Cu2O and Cu0. The average crystallite size of Cu2O was estimated by the using Debye–Scherrer equation from the reflection peak at 2θ = 36.5°; it was 7.3 and 18.9 nm for the samples obtained using ascorbic acid and hydrazine, respectively. The AA presented a better control of the reduction process from Cu4(SO4)(OH)6H2O to Cu2O phase than glucose and hydrazine; additionally, AA possesses antioxidant properties and it is not toxic [53].
Particle morphology of synthesized samples with and without different reducing agents are presented in Fig. 4. Samples synthesized without reducing agent and with glucose showed an irregular flake-like morphology (Fig. 4a, b), whereas the Cu2O phase obtained by AA was self-assembled into well-defined microspheres (Fig. 4c) and the sample prepared with hydrazine showed well faceted cubic Cu2O and Cu0 particles (Fig. 4d). From these results, it can be stablished that, when glucose was used as a reducing agent, the particles undergo an irregular shape caused by the OH groups of d-glucose since the polarity of the stabilizing groups leads to higher agglomeration which increases the bulkiness [54]. Likewise, AA was responsible for the self-assembling of the Cu2O nanoparticles into microspheres, probably due to its capping properties [29, 55, 56]. AA molecules reduce the Cu4(SO4)(OH)6H2O phase into Cu2O, then the oxidation products (dehydroascorbic acid) protect the Cu2O nanoparticles avoiding agglomeration. AA has been used for the reduction of different metal nanoparticles; Nene et al. synthesized ultra-small Fe3O4 nanoparticles using AA as chemical reducing agent. They claim that AA acted as a chemical reducing and capping agent in their oxidized form, as dehydroascorbic acid [57]. Then, it can be stablished that AA is responsible of the self-assembling of the Cu2O nanoparticles into microspheres due to its capping properties, as it was shown herein below by quantum and mesoscopic simulations (Sect. 3.4).
N2 adsorption–desorption isotherms are presented in Fig. 5. According to the IUPAC classification [58], the isotherms of the samples correspond to type IV with a H3 hysteresis loop, at relative pressure range between 0.4 and 1.0, indicating the presence of slit-like mesopores. The samples synthesized with AA and hydrazine showed a bimodal shape in the pore size distributions, i.e., having two maxima (Fig. 5b), the first one centered at 3.4 and the second one centered at 9.6 and 40 nm, respectively. In fact, when hydrazine was used as reducing agent, the pore size distribution shows a decrease on first maximum at 3.4 nm, indicating a possible decrease in the porosity. The specific surface area decreased from 36 to 10 m2/g for porous microspheres and cubic particles, respectively. Considering the morphology of porous Cu2O microspheres (Fig. 4c), the first maximum (3.4 nm) can be attributed to the aggregation of the nanoparticles within the intraparticle space of the microspheres, whereas the second one, centered at 9.6 nm, corresponds to the interparticle space between the microspheres.
According to previous studies [59], the basic medium is also crucial for the formation of porous Cu2O spheres and protects them from disproportionation. Without using of basic medium, only a small amount of product is obtained even at high reaction time. Therefore, the influence of NH4OH concentration was also investigated in the following section.
3.2 Effect of NH4OH Concentration
The X-ray diffraction patterns of the samples synthetized by varying the NH4OH concentration from 0.025 to 0.1 M, using ascorbic acid as reducing agent at 0.16 h of reaction time, are shown in Fig. 6. All samples showed the diffraction lines characteristics of cuprite (fcc Cu2O). However, as the molar ratio of AA to NH4OH increases, from 0.31 to 1.26, diffraction lines of metallic copper appeared. The sample obtained with lowest NH4OH concentration, 0.025 M, showed the characteristic lines of metallic Cu phase additional to the Cu2O lines, Fig. 6a. The average Cu2O crystal sizes determined for the samples prepared with 0.025, 0.05 and 0.1 M of NH4OH were 8.2, 20.9 and 7.3 nm, respectively. Likewise, the average crystallite size of Cu0 was 37.5 nm for the sample obtained at 0.025 M of NH4OH. Apparently, low [OH−] concentration promotes crystalline growth and its reduction into Cu0 with larger crystallites, which means that [OH−] in the synthesis media neutralizes the H+ protons donated by the acid; thus, it serves as a control agent of the acid powder of ascorbic acid, i.e., large concentration of OH− produces Cu2O whereas low concentration induces the complete reduction of Cu2O into Cu0. These results agree with that of Vivas et al. [29] who reported that lower concentration of NaOH favors the metallic Cu growing, using ascorbic acid as reducing agent.
Figure 7 shows a high magnification SEM images of Cu2O microspheres prepared with different NH4OH concentrations. All samples showed microspherical morphology varying its faceting and porosity. As already mentioned above, the sample prepared at 0.1 M NH4OH concentration (see Fig. 7a) showed microspheres assembled by ~ 7.3 nm Cu2O cubic nanocrystallites (determined by XRD), where internal porosity can be appreciated. The sample obtained at 0.05 M NH4OH, Fig. 7b, showed the formation of large number of heterogeneous Cu2O nanoparticles having a size ~ 20.9 nm (XRD results), self-assembled and compacted in well-faceted microspheres. Finally, the sample obtained at the lowest NH4OH concentration (0.025 M) revealed that the microparticles are made with a larger number of agglomerated nanocrystals, Fig. 7c, which correspond to ~ 37.5 nm Cu nanoparticles and 8.2 nm Cu2O nanoparticles, according to the XRD analysis. The morphological analysis clearly revealed that the higher NH4OH concentration (0.1 M) promotes the internal porosity within the microspheres. Likewise, at lower NH4OH concentrations, 0.025 and 0.05 M, microspheres with well-faceted and dense particles were exposed on the surface.
The specific surface areas of samples obtained at 0.025, 0.05 and 0.1 M of NH4OH were 20, 25 and 36 m2/g, respectively. The difference in the surface areas should be derived from the compacted particles and the formed pores in the microspheres. Furthermore, the higher NH4OH concentration (0.1 M) resulted in the formation of internal porosity within the microspheres (porous Cu2O microspheres).
According to the Wulff’s rule [60], the morphology is determined by crystal planes exposed on the surface. The preferred growth direction on the surface can be modified by the addition of organic/inorganic additives or surfactants during the crystal growth. Therefore, the high [OH] concentration inhibits the growing of the (111) and (100) crystal planes, i.e., at high NH4OH concentration, the Cu2O surface is saturated by OH groups and non-faceted particles were produced, whereas at lower NH4OH concentration, well-faceted particles were obtained [61, 62]. The low concentration of NH4OH (0.025 M) resulted into agglomerated Cu/Cu2O nanoparticles, whereas 4 times higher concentration, 0.1 M, resulted in small Cu2O nanoparticles self-assembled into microspheres and forming the internal porosity. As reported by Chen et al. [63], morphology of Cu2O or Cu crystal phases can be controlled from nanowires to truncated cuboctahedra by varying the pH of the solution; at higher pH, Cu2O crystals were obtained.
According to previous results, the variation of synthesis parameters such as reducing agent and NH4OH concentration have strong effects on the morphology, pore structure and crystalline phase of Cu2O microspheres. Zang et al. [64] reported that the reaction time can modify the internal porosity of the Cu2O microspheres. Therefore, time-dependent experiments with different reaction time from 0.16 to 12 h were conducted herein.
3.3 Effect of the Reaction Time
X-ray diffraction patterns of the samples synthesized varying the reaction time from 0.16 to 12 h and using ascorbic acid as reducing agent, are presented in Fig. 8. The sample synthesized at 0.16 h showed reflections of pure cuprous oxide (Cu2O). After 2 h of time on stream, additional to the XRD peaks characteristic of the Cu2O cubic phase, diffraction peaks corresponding to metallic copper were observed at 2θ = 43.4 and 50.5°, indicating that after 2 h, Cu2O phase starts to be reduced. And after 5 and 12 h only reflections at 2θ = 43.4, 50.5 and 74.2° were detected corresponding to the planes (111), (200) and (220) of the metallic Cu, suggesting that Cu2O phase has been completely reduced. The average crystallite sizes of Cu2O from the reflection peak at 2θ = 36.5° were 7.3 and 6.4 nm for the samples obtained at 0.16 and 2.0 h. Likewise, the average crystallite sizes of Cu0 calculated from the reflection peak at 2θ = 43.4° were 40.6, 45.8 and 51.0 nm for the samples obtained at 2.0, 5.0, and 12 h, respectively. From latter results, it can be stablished that microspheres composed of Cu2O nanoparticles collapsed between each other and coalesce evolving into cuboctahedra or polyhedral particles of metallic Cu0, as it will be seen later.
From SEM images presented in Fig. 9 it can be observed that microspherical morphology still remains after 2 h, see Fig. 9a and c. However, particles morphology changes after 5 h showing large polyhedral particles. A statistical analysis of particles size distribution is also presented in Fig. 9b, d and f; 150 particles were measured for each sample. The average size of particles was calculated from the Gaussian distribution. The mean particles size of microspheres obtained at 0.16 h was 1.14 µm, whereas after 2 h, Fig. 9d, was 0.79 µm. Finally, at 12 h of time on stream polyhedral particles dimensions grow up to 3.6 µm, Fig. 9f. A detailed analysis of the morphology was done in Fig. 10.
At 0.16 h of time on stream, porous Cu2O microspheres were obtained, which are known as hierarchical microspheres [42], see Fig. 10a. After 2 h, the microspheres were compacted and the internal porosity collapsed, Fig. 10b. In fact, the average size of microspheres decreases from 1.14 to 0.79 µm, respectively. Upon further extending the reaction time to 5 and 12 h, as shown in Fig. 10c, the coalescence of microspheres occurs, changing the morphology from microspheres to truncated cuboctahedra. Finally, at 12 h, the cuboctahedra grew to very large polyhedral particles, losing all the porosity. Reaction time is the main parameter affecting the Cu phase and morphology obtained, i.e., short reaction time yielded hierarchical porous Cu2O microspheres whereas long reaction time induced the evolution of hierarchical microspheres into polyhedral of metallic copper.
Textural properties of samples prepared at 0.16, 2.0 and 5 h, are presented in Fig. 11. As already mentioned, the sample synthesized at 0.16 h (porous Cu2O microspheres) showed isotherm type IV and H3 hysteresis loop at relative pressure range between 0.4 and 1.0, and its SSA was 36 m2/g. The N2 isotherm of sample synthesized at 2 h exhibits a hysteresis loop at high relative pressure range of 0.7–1.0, characteristic of mesoporous material with homogeneous cylindrical pores, see Fig. 11a. The BET surface area decreased from 36 to 24 m2/g; the pore size distribution shows a decrease on intraparticle pores at 3–4 nm, whereas the interparticle pores increased to 9.0 nm. The decrease in SSA must be attributed to the collapsing of internal porosity of Cu2O microspheres, as it is observed by SEM images in Fig. 10b. The sample prepared at 5 h exhibited very low adsorbed volume without any pore size distributions, see Fig. 11a, confirming the absence of porosity on metallic Cu polyhedral particles obtained by reducing with AA at reaction time of synthesis larger than 5 h, showing a very low SSA, 3 m2/g.
Latter results showed that using ascorbic acid as reducing agent, 0.1 M NH4OH concentration and 0.16 h of reaction time, porous Cu2O microspheres were formed. In order to analyze their stability after activation, the TPR analysis was carried out. The TPR curve of porous Cu2O microspheres showed two peaks located at about 200 and 300 °C, which correspond to the reduction of amorphous and nanocrystalline Cu2O, respectively [39]. SEM images of non-reduced and reduced microspheres presented in Fig. 12b and c, show that the spherical and hollow morphology is preserved, despite they have been subjected to a thermal reduction treatment. Porous Cu microspheres were obtained after reduction in H2 atmosphere at 350 °C. In this regard, there are two ways to synthesize metallic copper: either by a chemical method to obtain Cu0 non-porous polyhedrons, or by thermal reduction of the Cu2O at 350 ºC to obtain Cu0 microspheres with internal porosity.
3.4 Theoretical Study of Dehydroascorbic Acid Adsorption on Cu2O (111) Surface
A theoretical methodology for understanding the formation mechanism of porous Cu2O microspheres was used. The most probable chemisorption orientation of the dehydroascorbic acid molecule on the Cu2O surface was investigated (Fig. 13). The chemisorption of the dehydroascorbic acid in a parallel-to-surface orientation of its aromatic ring (Fig. 13a) produces an interaction energy \(\Delta {E}_{\text{int}}\) stronger than the perpendicular orientation (Fig. 13a) by 75.94 kJ/mol (Table 1), i.e., releases 26.6% more energy. At either parallel or perpendicular orientation, dehydroascorbic acid forms three covalent bonds with the Cu2O surface but prefers to arrive parallel since at this way additionally forms a hydrogen bond.
Side (left) and perspective (right) views of the a parallel and b perpendicular chemisorption of the dehydroascorbic acid by the Cu2O (111) surface. In the sake of simplicity, the perspective views show only Cu2O atoms nearest to the dehydroascorbic acid. The lengths (in Å) of the bonds formed among the dehydroascorbic acid and the Cu2O surface are displayed by the black (covalent bonds) and gray (hydrogen bond, shown by the dashed black line) numbers
Covering of Cu2O particles by the dehydroascorbic acid affects its interaction with water, since a water molecule is chemisorbed 3.3 times more intense by a pristine Cu2O surface than by a Cu2O surface having a parallel-adsorbed dehydroascorbic acid (Table 1). This suggests that dehydroascorbic acid regulates the polarity of Cu2O particles conferring them a surfactant-like behavior, i.e., the pristine faces of Cu2O act as polar heads whereas the dehydroascorbic acid-covered faces as non-polar moieties. Therefore, it is expected that Cu2O particles will group in a similar way as surfactant spherical micelles in aqueous solvent, with the pristine faces forming the most outer micelle surface in direct contact with water, and the micelle inside having predominantly Cu2O particles possessing adsorbed dehydroascorbic acid. This behavior is confirmed (Fig. 14) through mesoscopic molecular dynamics (Supporting Information), which reveals that in aqueous solution, particles of dehydroascorbic acid containing Cu2O self-assembled in porous microspheres, as observed in our SEM images.
Molecular simulation of porous Cu2O microspheres in aqueous solution promoted by the chemisorption of dehydroascorbic acid. Note that this image resembles the morphology of Fig. 4c. Blue, gray and orange beads represent water, dehydroascorbic acid and Cu2O, respectively. The pairs of joined gray and orange beads represent Cu2O with adsorbed dehydroascorbic acid
3.5 The Formation Mechanism of Porous Cu2O Microspheres
The formation mechanism of porous Cu2O microspheres can be explained by a self-assembly process of metastable Cu2O nanoparticles, formed since the beginning of the reaction, which is knowing as Ostwald ripening [42, 65]. Similar formation mechanisms have been reported for hollow Cu2O spheres. According to the obtained results, we suggest that formation of the porous Cu2O microspheres was carried out by three levels of structural organization, as described below:
Step 1: Reduction of Cu2+ into Cu1+, using AA as reducing agent. The XRD patterns show that Cu2+ ions were initially transformed to Cu4(SO4)(OH)6H2O as precursor, thereafter the Cu4(SO4)(OH)6H2O was reduced by AA into Cu2O nanoparticles (Cu1+). Also, the dehydroascorbic acid obtained after AA donates two H+ is considered a capping agent molecule which interacts with Cu2O, avoiding the agglomeration of the particles. AA serves as a stable donor (electron + proton) in interactions and is converted into the radical ion called semi-dehydroascorbic acid and then dehydroascorbic acid. AA and dehydroascorbic acid together constitute the redox system (reduction potential ~ 0.060 V) that is enough to reduce Cu(II) particles into Cu(I) nanoparticles (the reduction potential is ~ 0.15 V) [66, 67].
Step 2: Formation of Cu2O nanoparticles. According to previous studies, the Cu2O nanoparticles formed within initial aging time (0.16 h) are not well defined and have low degree of crystallinity; these nanoparticles are found to be thermodynamically disadvantaged and become metastable due to their larger surface energy. The formed metastable Cu2O nanoparticles would remain out of equilibrium with the surrounding polar solution.
However, the non-polar hydrophobic group in the dehydroascorbic acid adsorbed on the polar hydrophilic Cu2O (111) surface decreases the surface energy of nanoparticles, allowing them to remain in equilibrium with the surrounding polar solution. In such a way, the new system formed among dehydroascorbic acid and Cu2O nanoparticles acts as micelles, as it was showed above in the theoretical section (Fig. 14).
Step 3: Relocation and self-assembly of Cu2O nanoparticles to produce microspheres. The non-polar (hydrophobic) part of the metastable nanoparticles begins to attract other non-polar molecules (dehydroascorbic acid), leaving inside them the organic molecules and expositing the hydrophilic part (Cu2O particles). In this regard, the nanoparticles were rearranged and then attracted, allowing the self-assembly of the Cu2O microspheres. This led us to obtain porous microspheres (Fig. 14) with sizes of 1.14 μm formed by Cu2O nanocrystals. The process is similar to the Oswald ripening that is responsible for formation of hollow interiors in colloidal aggregates made up of nanocrystalline. Due to the non-uniform size and distribution of crystallites within aggregates, larger crystallites are almost immobile while the smaller ones undergo mass relocation through dissolution and regrowth [24, 26, 42, 68, 69]. The microspheres controlled by a localized Oswald ripening resulted in an increase in crystal size and a decrease in electron density inside the porous microspheres, Scheme 1.
3.6 Photodegradation Test
The photocatalytic activity of porous and well-compacted Cu2O microspheres was evaluated during MO degradation, see Fig. 15a. The color of the MO solution gradually decreased after the incorporation of the catalyst under dark condition and its subsequent irradiation with visible light. The results showed that well-facet microspheres removed about 64% of the organic compound in the solution, while porous Cu2O microspheres removed about 48% for the first 30 min, under dark condition. The photoactivity under dark condition has been related to oxygen vacancies on the rough Cu2O surface, as reported Zang et al. [43, 44]. Additionally, the high removal efficiency on the well-faceted microspheres, 1.3 times greater than porous ones, could be attributed to the number of terminal copper atoms per unit surface area on the (110) face. The (110) face is positively charged and this should greatly increase the number of adsorbed methyl orange molecules [4]. However, after 30 min under light irradiation, the materials showed opposite behavior. The pseudo-first order kinetics model was used to determine the rate constant of MO photodegradation with respect to the degradation time: ln (C/C0) = − kt, Fig. 15b, where C0 is the initial concentration of MO and C is the concentration at time t, and k is the reaction rate constant. The rate constants of degradation were given by the slopes of linear lines and estimated to be 0.0078 and 0.020 min−1 for well-faceted microspheres and porous Cu2O microspheres, respectively. The degradation rate of porous Cu2O microspheres is two times higher than that of the well-faceted microspheres and can be attributed to the increase in the porous-Cu2O-microspheres’ specific surface (36 m2/g). These results agree with that previously reported by Yu et al. [22], who attributed the higher degradation efficiency of hollow porous Cu2O microspheres under visible light to its peculiar structure with large SSA as well as to its outstanding visible light optical absorption characteristics. Porous structures can provide more surface contact and space for the pollutant molecule and also can make that the excited electrons arrive more easily at the surface [70].
4 Conclusion
Cu2O microspheres were synthesized through a simple method at room temperature by using ascorbic acid as a reducing agent. The analysis of different parameters such as reducing agent, time and concentration of ammonium hydroxide reveals that they have significant effect on the morphology modification, the obtained crystal phase and textural properties of the synthesized materials. A quantum and mesoscopic theoretical study reveal that modification of the Cu2O-nanoparticles hydrophilicity induced by the parallel-to-surface chemisorption of dehydroascorbic acid is responsible for the self-assembling of Cu2O particles and forming of spherical porous agglomerates, which experimentally occur at a short reaction time. In particular, the concentration of NH4OH controlled the internal porosity of the Cu2O microspheres and the crystalline phase. The reaction time modified the morphology and crystalline phase, since through the time it was observed both the loss of porosity in the microspheres and the morphological evolution from Cu2O microspheres to Cu0 polyhedrons. The photocatalytic reaction proceeds through two processes: (i) the efficient removal of the organic compound under dark conditions, related to the surface oxygen vacancies in the well-faceted particles and (ii) the improvement of the MO photodegradation under visible light irradiation by the porosity in the microspheres.
References
Zhang YH, Jiu BB, Gong FL, Chen JL, Zhang HL (2017) Morphology-controllable Cu2O supercrystals: facile synthesis, facet etching mechanism and comparative photocatalytic H2 production. J Alloy Compd 729:563–570
Yeo BE, Cho YS, Huh YD (2017) Evolution of the morphology of Cu2O microcrystals: cube to 50-facet polyhedron through beveled cube and rhombicuboctahedron. CrystEngComm 19:1627–1632
Yang Y, Han J, Ning X, Cao W, Xu W, Guo L (2014) Controllable morphology and conductivity of electrodeposited Cu2O thin film: effect of surfactants. ACS Appl Mater Interfaces 6:22534–22543
Huang WC, Lyu LM, Yang YC, Huang MH (2012) Synthesis of Cu2O nanocrystals from cubic to rhombic dodecahedral structures and their comparative photocatalytic activity. J Am Chem Soc 134:1261–1267
Bi Y, Ouyang S, Umezawa N, Cao J, Ye J (2011) Facet effect of single-crystalline Ag3PO4 sub-microcrystals on photocatalytic properties. J Am Chem Soc 133:6490–6492
Wang J, Gong J, Xiong Y, Yang J, Gao Y, Liu Y, Lu X, Tang Z (2011) Shape-dependent electrocatalytic activity of monodispersed gold nanocrystals toward glucose oxidation. Chem Commun 47:6894–6896
Lee HS, Woo CS, Youn BK, Kim S, Oh ST, Sung Y, Lee HI (2005) Bandgap modulation of TiO2 and its effect on the activity in photocatalytic oxidation of 2-isopropyl-6-methyl-4-pyrimidinol. Top Catal 35:255–260
Liu Y, Wang D, Peng Q, Chu D, Liu X, Li Y (2011) Directly assembling ligand-free ZnO Nanocrystals into three-dimensional mesoporous structures by oriented attachment. Inorg Chem 50:5841–5847
Yao KX, Yin XM, Wang TH, Zeng HC (2010) Synthesis, self-assembly, disassembly, and reassembly of two types of Cu2O nanocrystals unifaceted with 001 or 110 planes. J Am Chem Soc 132:6131–6144
Zeng QX, Xu GC, Zhang L, Lv Y (2019) Porous Cu2O microcubes derived from a metal-formate framework as photocatalyst for degradation of methyl orange. Mater Res Bull 119:110537
Chen D, Zhang X, Lee AF (2015) Synthetic strategies to nanostructured photocatalysts for CO2 reduction to solar fuels and chemicals. J Mater Chem A 3:14487–14516
Hisatomi T, Kubota J, Domen K (2014) Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem Soc Rev 43:7520–7535
Ren J, Wang W, Sun S, Zhang L, Wang L, Chang J (2011) Crystallography facet-dependent antibacterial activity: the case of Cu2O. Ind Eng Chem Res 50:10366–10369
Wan X, Wang J, Zhu L, Tang J (2014) Gas sensing properties of Cu2O and its particle size and morphology-dependent gas-detection sensitivity. J Mater Chem 2:13641–13647
Wijesundera RP (2010) Fabrication of the CuO/Cu2O heterojunction using an electrodeposition technique for solar cell applications. Semicond Sci Technol 25:45015–45019
Kim ES, Kim MC, Moon SH, Shin YK, Lee JE, Choi S, Park W (2019) Surface modified and size-controlled octahedral Cu2O nanostructured electrodes for lithium-ion batteries. J Alloy Compd 794:84–93
Zhu H, Wang J, Xu G (2009) Fast synthesis of Cu2O hollow microspheres and their application in DNA biosensor of hepatitis B. Virus Crystal Growth & Design 9:633–638
Zhang Z, Wu H, Yu Z, Song R, Qian K, Chen X, Tian J, Zhang W, Huang W (2019) Site-resolved Cu2O catalysis in the oxidation of CO. Angew Chem Int Ed 58:4276–4280
Kattel S, Ramírez PJ, Chen JG, Rodriguez JA, Liu P (2017) Active sites for CO2 hydrogenation to methanol on Cu/ZnO catalysts. Science 355:1296–1299
Kim MH, Lim B, Lee EP, Xia Y (2008) Polyol synthesis of Cu2O nanoparticles: use of chloride to promote the formation of a cubic morphology. J Mater Chem 18:4069–4073
Dong C, Zhong M, Huang T, Ma M, Wortmann D, Brajdic M, Kelbassa I (2011) Photodegradation of methyl orange under visible light by micro-nano hierarchical Cu2O structure fabricated by hybrid laser processing and chemical dealloying. ACS Appl Mater Interfaces 3:4332–4338
Yu Y, Zhang L, Wang J, Yang Z, Long M, Hu N, Zhang Y (2012) Preparation of hollow porous Cu2O microspheres and photocatalytic activity under visible light irradiation. Nanoscale Res Lett 7:347–352
Deng X, Zhang Q, Zhao Q, Ma L, Ding M, Xu X (2015) Effects of architectures and H2O2 additions on the photocatalytic performance of hierarchical Cu2O nanostructures. Nanoscale Res Lett 10:8–16
Teo JJ, Chang Y, Zeng HC (2006) Fabrications of hollow nanocubes of Cu2O and Cu via reductive self-assembly of CuO nanocrystals. Langmuir 22:7369–7377
Leng M, Liu M, Zhang Y, Wang Z, Yu C, Yang X, Zhang H, Wang C (2010) Polyhedral 50-facet Cu2O microcrystals partially enclosed by 311 high-index planes: synthesis and enhanced catalytic CO oxidation activity. J Am Chem Soc 132:17084–17087
Yu H, Yu J, Liu S, Mann S (2007) Template-free hydrothermal synthesis of CuO/Cu2O composite hollow microspheres. Chem Mater 17:4327–4334
Zhang Y, Deng B, Zhang T, Gao D, Xu AW (2010) Shape effects of Cu2O polyhedral microcrystals on photocatalytic activity. J Phys Chem A 114:5073–5079
Xu H, Wang W, Zhu W (2006) Shape evolution and size-controllable synthesis of Cu2O octahedra and their morphology-dependent photocatalytic properties. J Phys Chem B 110:13829–13834
Vivas L, Chi-Duran I, Enríquez J, Barraza N, Singh DP (2019) Ascorbic acid based controlled growth of various Cu and Cu2O nanostructures. Mater Res Express 6:065033–065040
Begletsova N, Selifonova E, Chumakov A, Al-Alwani A, Zakharevich A, Chernova R, Glukhovskoy E (2018) Chemical synthesis of copper nanoparticles in aqueous solutions in the presence of anionic surfactant sodium dodecyl sulfate. Colloids Surf A 552:75–80
Chen L, Zhang D, Chen J, Zhou H, Wan H (2006) The use of CTAB to control the size of copper nanoparticles and the concentration of alkylthiols on their surface. Mater Sci Eng A 415:156–161
Mishra AK, Pradhan D (2016) Morphology controlled solution-based synthesis of Cu2O crystals for the facets-dependent catalytic reduction of highly toxic aqueous Cr(VI). Cryst Growth Des 7:3688–3698
Imangaliyeva A, Mastai Y, Seilkhanova G (2019) In situ synthesis and catalytic properties of Cu2O nanoparticles based on clay materials and polyethylene glycol. J Nanopart Res 21:97–107
Sawant S, Bhagwat A, Mahajan C (2016) Synthesis of cuprous oxide (Cu2O) nanoparticles. J Nano- Electron Phys 8:1035–1041
Thoka S, Lee AT, Huang MH (2019) Scalable synthesis of size-tunable small Cu2O nanocubes and octahedra for facet-dependent optical characterization and pseudomorphic conversion to Cu nanocrystals. ACS Sustain Chem Eng 7:10467–10476
Yu C, Shu Y, Zhou X, Ren Y, Liu Z (2018) Multi-branched Cu2O nanowires for photocatalytic degradation of methyl orange. Mater Res Express 5:035046–035054
Dan Z, Yang Y, Qin F, Wang H, Chang H (2018) Facile fabrication of Cu2O nanobelts in ethanol on nanoporous Cu and their photodegradation of methyl orange. Materials 11:446–459
Zhang J, Liu J, Peng Q, Wang X, Li Y (2006) Nearly monodisperse Cu2O and CuO nanospheres: preparation and applications for sensitive gas sensors. Chem Mater 18:867–871
Wei B, Yang N, Pang F, Ge J (2018) Cu2O–CuO hollow nanospheres as a heterogeneous catalyst for synergetic oxidation of CO. J Phys Chem C 122:19524–19531
Kumar S, Parlett CMA, Isaacs MA, Jowett DV, Douthwaite RE, McR C, Lee AF (2016) Facile synthesis of hierarchical Cu2O nanocubes as visible light photocatalysts. Appl Catal B 189:226–232
Chen L, Zhang Y, Zhu P, Zhou F, Zeng W, Lu DD, Sun R, Wong C (2015) Copper salts mediated morphological transformation of Cu2O from cubes to hierarchical flower-like or microspheres and their supercapacitors performances. Sci Rep 5:9672–9978
Zhang H, Zhu Q, Zhang Y, Wang Y, Zhao L, Yu B (2007) One-pot synthesis and hierarchical assembly of hollow Cu2O Microspheres with nanocrystals-composed porous multishell and their gas-sensing properties. Adv Func Mater 17:2766–2771
Guo B, Liu G, Zeng Y, Dong G, Wang C (2018) Rapid mineralization of methyl orange by nanocrystalline-assembled mesoporous Cu2O microspheres. Nanotechnology 29:445701
Zhang F, Dong G, Wang M, Zeng Y, Wang C (2018) Efficient removal of methyl orange using Cu2O as a dual function catalyst. Appl Surf Sci 444:559–568
Hua Q, Shang D, Zhang W, Chen K, Chang S, Ma Y, Jiang Z, Yang J, Huang W (2011) Morphological evolution of Cu2O nanocrystals in an acid solution: stability of different crystal planes. Langmuir 27:665–671
Ferrer MM, Fabris GSL, de Faria BV, Martins JBL, Moreira ML, Sambrano JR (2019) Quantitative evaluation of the surface stability and morphological changes of Cu2O particles. Heliyon 5:e2500
Accelrys.com, Version 7.0, Accelrys Software Inc. (2012)
Delley B (1990) An all-electron numerical method for solving the local density functional for polyatomic molecules. J Chem Phys 92:508–517
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868
Grimme S (2006) Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J Comput Chem 27:1787–1799
Shenoy US, Shetty AN (2014) Simple glucose reduction route for one-step synthesis of copper nanofluids. Appl Nanosci 4:47–54
Panigrahi S, Kundu S, Ghosh SK, Nath S, Praharaj S, Basu S, Pal T (2006) Selective one-pot synthesis of copper nanorods under surfactantless condition. Polyhedron 25:1263–1269
Barrita JLS, del Sánchez MDSS (2013) Antioxidant role of ascorbic acid and his protective effects on chronic diseases. Oxid Stress Chronic Degener Dis 14:45–48
Andal V, Buvaneswari G (2017) Effect of reducing agents in the conversion of Cu2O nanocolloid to Cu nanocolloid. Eng Sci Technol 20:340–344
Kulkarni SR, Saptale SP, Borse DB, Agarwal AD (2011) Green synthesis of Ag nanoparticles using vitamin C (ascorbic acid) in a batch process. Int Conf Nanosci Eng Technol 1:88–90
Jain S, Jain A, Kachhawah P, Devra V (2015) Synthesis and size control of copper nanoparticles and their catalytic application. Trans Nonferrous Metals Soc China 25:3995–4000
Nene A (2016) Fe3O4 and Fe nanoparticles by chemical reduction of Fe3 by ascorbic acid: role of water. World J Nano Sci Eng 6:20–28
Thommes M, Kaneko K, Neimark AV, Olivier JP, Rodriguez-Reinoso F, Rouquerol J, Sing KSW (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution. Pure Appl Chem 87:1051–1069
Liu J, Gao Z, Han H, Wu D, Xu F, Wang H, Jiang K (2012) Mesoporous Cu2O submicro-spheres, facile synthesis and the selective adsorption properties. Chem Eng J 185–186:151–159
Wu Z, Overbury SH (2015) Catalysis by materials with well-defined structures. Elsevier, Amsterdam
Susman MD, Feldman Y, Vaskevich A, Rubinstein I (2014) Chemical deposition of Cu2O nanocrystals with precise morphology control. ACS Nano 8:162–174
Shang Y, Guo L (2015) Facet-controlled synthetic strategy of Cu2O-based crystals for catalysis and sensing. Adv Sci 2:1500140–1500161
Chen K, Xue D (2012) pH-assisted crystallization of Cu2O: chemical reactions control the evolution from nanowires to polyhedra. CrystEngComm 14:8068–8075
Zhang Z, Che H, Wang Y, Gao J, Zhao L, She X, Sun J, Gunawan P, Zhong Z, Su F (2012) Facile synthesis of mesoporous Cu2O microspheres with improved catalytic property for dimethyldichlorosilane. Synth Indus Eng Chem Res 51:1264–1274
Liu B, Hu X (2020) Chapter 1 - Hollow micro and nanomaterials: synthesis and applications. Adv Nanomater Pollut Sensing Environ Catal 1:1–38
Xiong J, Wang Y, Xue Q, Wu X (2011) Synthesis of highly stable dispersions of nanosized copper particles using l-ascorbic acid. Green Chem 13:900–904
Silva N, Ramírez S, Díaz I, Garcia A, Hassan N (2019) Easy, quick, and reproducible sonochemical synthesis of CuO nanoparticles. Materials 12:804–816
Ma ZC, Wang LM, Chu DQ, Sun HM, Wang AX (2015) Template-free synthesis of complicated double-wall Cu2O hollow spheres with enhanced visible photocatalytic activities. RSC Adv 5:8223–8227
Ng CHB, Fan WY (2006) Shape evolution of Cu2O nanostructures via kinetic and thermodynamic controlled growth. J Phys Chem B 110:20801–20807
Duan X, Gao R, Zhang Y, Jian Z (2011) Synthesis of sea urchin-like cuprous oxide with hollow glass microspheres as cores and its preliminary application as a photocatalyst. Mater Lett 65:3625–3628
Acknowledgements
The authors acknowledge financial support from Mexican Institute of Petroleum (IMP), Project-D.61048 and especially to the postgraduate area. Particularly, D. A. Prado-Chay is very grateful to CONACYT for the grant to support his postgraduate studies.
Funding
This research is a product of Project D.61048 “Desarrollo de un proceso catalítico para la obtención de compuestos aromáticos a partir de lignina” funded by Mexican Institute of Petroleum.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest.
Research Involving Human and/or Animals rights
The research involved no human participants and/or animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Prado-Chay, D.A., Cortés-Jácome, M.A., Angeles-Chávez, C. et al. Synthesis and Photocatalytic Activity of Cu2O Microspheres upon Methyl Orange Degradation. Top Catal 63, 586–600 (2020). https://doi.org/10.1007/s11244-020-01256-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-020-01256-5