Abstract
Graphene, a two dimensional, single-atom thick, periodic structure composed entirely of aromatic carbon atoms, and graphene oxide, a highly oxidized form of graphene, are relatively new materials with incredibly interesting chemical and mechanical properties. These materials have already demonstrated their potential importance in the future of nanotechnology, while their application as catalysts is beginning to emerge. Because of its high surface area and/or tunable electrical properties, graphene and graphene oxide have been widely explored as catalyst supports for metal nanoparticles. Recently, graphene oxide has been shown to be able to function as metal-free catalyst for a variety of chemical transformations that typically are catalyzed with precious metals or under very harsh conditions. Additionally, the variety of oxygenate functional groups on graphene oxide makes it an attractive platform to tether other catalytically active groups (e.g. amines). The development of new heterogeneous metal-free catalysts using graphene oxide could lead to “greener” methods for a variety of chemical transformations of interest to the chemicals and other industries. This review explores some of the recent advances that uncover the potential of graphene and graphene oxide for use in metal-free heterogeneous catalysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In the last quarter century the use of nanomaterials as catalysts and catalyst supports has grown enormously. Particularly interesting is the rapid growth in research on the use of structured carbon nanomaterials for catalysis. Graphene is a two-dimensional carbon material that can be considered the building block for other carbon materials (see Fig. 1.) such as fullerenes (0D), single- and multi-walled carbon nanotubes (1D), and graphite (3D) [1]. These unique materials can be utilized as a support for metals and other catalytic active phases, or as metal-free catalysts. The array of carbon nanomaterials exhibits a variety of unique properties including high specific surface area, chemical and electrochemical stability, as well as the ability for a high degree of functionalization. For a majority of the past quarter century, single-walled carbon nanotubes and multi-walled carbon nanotubes have been studied extensively for their use in catalysis (as well as in electronic and energy devices, as polymer fillers, for drug delivery and other biological applications) [2]. Since the experimental observation of mechanically exfoliated single layer graphene sheets in 2004 [3], there has been an exponential growth in interest to explore its use, as well as that of its derivative the graphene oxide, in various scientific and engineering fields.
Graphene shown as a 2D building block material of (from left to right) buckyballs, 0D; carbon nanotubes, 1D; and graphite, 3D. Reprinted with permission from [1]
Graphene based materials are being investigated for a rapidly expanding variety of applications such as memory devices, energy storage, catalysis, photocatalysis, solar cells, sensing platforms, molecular imaging, drug delivery, and nanocircuitry [4–7]. Pristine graphene possesses unique electronic, optical, thermal, chemical, and mechanical properties that are distinct from other forms of carbon, even carbon nanotubes, and there are a number of excellent reviews covering these topics [1, 8, 9]. However, the difficulties in achieving high throughput preparation and poor processibility of pristine graphene have limited its applications. Graphene oxide (GO), reduced graphene oxide (rGO), and chemically modified graphenes (CMG) are alternatives that can be prepared and processed in large quantities more easily. These alternatives can be synthesized, dispersed, and processed rather easily in water and other organic solvents. However, their electrical and mechanical properties are quite different from graphene, which may become an issue in some applications. Nonetheless, they are suitable as superior starting material for many applications [7, 9].
Pristine graphene is a relatively inert and stable material that has a zero band gap. GO and rGO, however, are insulators or semiconductors. Whereas graphene is made of carbon only, GO and rGO are decorated with a wide variety of organic functionalities that can be further modified. One application where GO and CMGs are advantageous is in the field of catalysis. Until recently, the catalytic application of graphene, GO, and CMGs has been focused primarily on their use as supports for catalytically active transition metals. Supported noble metal or metal oxide catalysts are widely used in industrial processes. However, there is a constant desire to discover more attractive alternatives with respect to cost, long term stability, versatility, and environmental impact. In case of biological grade synthesis processes, toxicity is also a concern, especially regarding metal leaching from their supports. Heterogeneous metal-free catalysts based on carbon [10, 11] and non-carbon [12] materials are alternatives being investigated.
Over the past two decades, the investigation into the use of metal-free carbons as catalysts has progressed, especially with the discovery and development of novel forms of carbon such as fullerenes, nanotubes, nanofibers, and graphene. The potential use of metal-free GO and CMGs as ‘green’ catalysts for various chemical transformations is particularly appealing. To date, a few review articles have appeared that cover the use of graphene based materials in catalysis [13, 14] and these reviews only briefly touch on the use of metal free GO catalysts. Although exploring the use of graphene and GO for catalytic applications is a relatively recent activity, these material have garnered much attention already in fuel cell and battery applications, particularly for the electrocatalytic oxygen reduction reaction (ORR) and for which nitrogen doped graphene materials have shown superior activity. This topic will not be covered here, and interested readers are referred to recent articles and reviews that cover it in great details [15–20]. This short review will focus on recent advances in the development and understanding regarding the synthesis and applications of the inherent catalytic nature of graphene/graphite oxide, functionalized GO catalysts, and heteroatom doped graphene catalysts, topics that have not received as much attention.
2 Preparation of Graphene/Graphene Oxide for Catalysis
2.1 Graphene
The first experimental preparation of a single atomic layer of graphene was achieved by mechanical exfoliation [3]. This synthesis method, while producing high quality graphene, is not easily adaptable to large scale production. Alternatives to mechanical exfoliation to produce graphene sheets from graphite include chemical methods in which a solvent is used that can disrupt the π–π interaction between sheets and stabilize them individually [21], bottom-up methods in which graphene is grown directly from other organic precursors, typically at high temperatures [22], and growth on a substrate by chemical vapor deposition (CVD) onto a catalytic surface [23]. Each of these methods has its limitations and challenges such as poor solubility, which can lead to regraphitization, an increase in the number of unwanted side reactions and defect sites. Even with great advances in these alternative techniques, mechanical exfoliation still produces the highest quality graphene sheets.
2.2 Graphene Oxide and Reduced Graphene Oxide
The most common methods to prepare GO, developed by Brodie [24], Staudenmeier [25], and Hummers [26], involve oxidation of graphite with strong oxidizing acids, and they produce samples with similar degrees of oxidation. In recent years, a modified form of the original Hummers method has become popular, which has a shorter reaction time, does not produce hazardous ClO2 gas, and the pre-oxidation step results in more complete oxidation [27]. In a typical modified Hummers method, flake graphite powder is preoxidized by stirring it into a mixture of potassium persulfate (K2S2O8) and phosphorus pentoxide (P2O5) dissolved in concentrated sulfuric acid (H2SO4). Afterwards, the graphite is further oxidized with a mixture of H2SO4 and potassium permanganate (KMnO4) while chilled to remove the reaction heat. The oxidized graphite is then exfoliated by sonication or mechanically in the presence of surface stabilizing solvents or polymers [28].
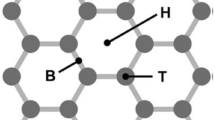
By virtue of its preparation procedure and the harsh oxidizing conditions, the resulting GO has a carbon framework that is highly disrupted by a variety of oxygenate functionalities. It does not have a periodic or uniquely defined structure or well-defined stoichiometry, and the location, distribution, and density of various oxygenate functionalities depend on the details of the preparation procedure and the starting graphite source. The structural model proposed by Lerf and Klinowski [29], shown in Fig. 2, is currently the most widely accepted. It indicates that hydroxyl and epoxy groups decorate the basal plane of a GO sheet, whereas carboxylic acid and carbonyl groups decorate the edge. The presence of these groups disrupts the extensive delocalization of the π electrons and renders GO electrically insulating. Their polarity and ability to participate in hydrogen bonding make GO hydrophilic and easily dispersible in water or polar solvents. The carboxylic acid groups on the edge of the sheets are ionized in aqueous media that are not strongly acidic, and the electrostatic repulsion between these charged groups separates the GO sheets and stabilizes their suspension in aqueous and other polar solvents.
Proposed structure of graphene oxide. GO is thought to be composed of a disrupted honeycomb graphene lattice. The basal plane is decorated with hydroxyl and epoxy groups while the edge and defect sites are populated with carboxylic acid. Adapted from [29]
Reduction of the oxygen functionalities can restore some of the very unique properties of graphene. After reduction, the material is typically referred to as rGO, implying that the reduction is not complete. Indeed, complete reduction has not yet been achieved. The common reduction methods to form rGO include chemical, thermal, or electrochemical means, and they all restore the conjugated π–π network to some extent. GO can be reduced chemically by strong reducing agents, such as lithium aluminum hydride. However, less reactive chemicals, the most common one being hydrazine monohydrate, are preferred because they can avoid dangerous side reactions of lithium aluminum hydride and the reduction can be carried out in aqueous solvents [30]. Other effective chemical reductants include sodium borohydride, hydroquinone, and strong alkaline solutions. The drawback to chemical reduction is that heteroatom impurities can be introduced into the basal plane. It is not fully understood how the nitrogen would incorporate but one of the many possible mechanisms is believed to occur through the reaction of hydrazine with carbonyl groups. These impurities can have a significant effect on the resulting electronic properties. Reduction in a dilute hydrogen atmosphere at elevated temperatures, typically between 500 and 1,000 °C, is also effective. At the high temperatures, the process is most likely a combination of chemical and thermal reduction.
Thermal reduction of GO to rGO is achieved by heating a sample above 500 °C in an inert atmosphere. Higher temperatures, as high as >1,000 °C, would result in more complete reduction. It is due to thermal cracking of C–O and C–C bonds in processes such as decarboxylation, decarbonylation, dehydration, and dehydrogenation. Evolved gas is a byproduct, which includes carbon dioxide, carbon monoxide, water, and possibly hydrogen, and rapid evolution can generate a high pressure between the GO layers which results in exfoliation of the sample. Although this exfoliation process tends to yield a higher surface area sample than those obtained by chemical reduction, it also leads to a large (~30 %) mass loss and formation of a significant density of defects [31]. These large vacancies can affect the electronic and chemical properties of the rGO. The generation of defects can be moderated by a sequential reduction of chemical followed by thermal reduction.
Electrochemical reduction is the third common method. In this method, a GO sample is placed on a substrate that serves as an electrode, which is submerged in an electrolyte. Reduction is achieved by passing a current between the substrate and a counter electrode [32]. The major disadvantage of this method is that it is difficult to scale up for production of large amounts of rGO samples.
Selective conversion of specific oxygenate functional groups is of interest in material design, and techniques such as using hydrazine vapor as the reductant have been developed that removes different groups in a stepwise manner [33]. Such stepwise reduction, coupled with careful tuning of experimental conditions and choice of combination of methods allows for better control of the composition of the final product and the degree of reduction. However, complete restoration of the extensive π–π network of pristine graphene has not yet been achieved to date, and the various reduction methods result in rGO samples that are very heterogeneous with regions of high oxygenate concentrations or high graphitic character [34].
2.3 Functionalized Graphene Oxide
A wide variety of methods have been developed to modify a GO surface through covalent bond transformations to result in a more versatile material that can then be utilized for various applications. Methods that had been explored to functionalize carbon nanotubes have been found to be effective on graphene. For GO, since there is reliable information on the nature of the oxygenate functional groups, well-established chemical reactions that have been developed to transform them into other functionalities have been applied. Of particular interest is to selectively transform one type of oxygenate groups and not another (e.g. transforming only the epoxy groups on the basal plane). Unfortunately, since techniques are not yet available to definitively demonstrate successful selective transformations, it is not possible to establish successful selective transformation. Nonetheless, these types of transformations are widely employed, and the most widely utilized reactions, the transformation of carboxylic acid and epoxy group, will be discussed below, as well as other methods that have proven to be effect for the synthesis of catalysts.
Similar to other organic synthesis, transformation of surface carboxylic acid groups typically requires their activation. Once activated, the carbon of the acid becomes more susceptible to nucleophilic attack to form covalent bonds (couple) with nucleophiles. The common activation or coupling reagents include thionyl chloride (SOCl2) [35–37], 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) or N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDCI) [38–41], and N,N′-dicyclohexylcarbodiimide (DCC) [38]. Common nucleophiles include those that possess an amine or a hydroxyl group are used to form amide or ester bonds with the GO and tether targeted entities onto the GO surface. Typically, modification of the GO surface by reaction with the epoxy groups is believed to occur through a ring opening mechanism [42–44]. However, these reactions might not be highly selective. For example, aminolysis of epoxide may occur simultaneously with the reaction of amine with carboxylic acid.
In addition to using activating or coupling agents, the carboxylic acid and epoxy groups can also be activated using solvothermal methods [45]. In some other procedures, a free radical mechanism might be involved [46]. Other methods to functionalize a GO surface include condensation of ethoxy or methoxy groups with surface hydroxyl groups [47], in a manner similar to that used for grafting molecules onto traditional heterogeneous supports such as SiO2 and Al2O3. Although less common, covalent functionalization of the graphitic region of a rGO with diazonium salts, which proceeds through a radical mechanism, is also utilized in catalyst synthesis [48, 49].
2.4 N-Doped (Heteroatom-Doped) Graphene
Heteroatom-doped graphene catalysts are generally synthesized using two methods, in situ growth or post-treatment of graphene or GO. Thus far, N-doping is the most studied, and the subject has been reviewed in depth recently [15, 50]. The in situ growth method involves incorporation of heteroatoms during the growth process by mixing in a N-containing reagent, such as by CVD. The advantage of the in situ method is that it not only allows the graphene material to retain many of the intrinsic physical and chemical properties of pristine graphene but also provide new catalytic properties. N-doped graphene, for example, can be prepared by CVD by flowing a gaseous mixture of NH3, CH4, and H2 in an Ar carrier over a substrate. The nitrogen can be incorporated into the graphene aromatic structure in a variety of forms such as pyridine and pyrrolic nitrogen (Fig. 3). Other in situ methods include solvothermal synthesis processes. For example, lithium nitride is reacted with tetrachloromethane during its pyrolysis in a stainless steel autoclave.
Schematic illustration of the various forms of ‘N’ atom incorporation into the graphene structure, which include the pyridinic N, pyrrolic N, graphitic N, and amino groups. Adapted from ref [17]
The post-treatment synthesis method incorporates heteroatoms by treating graphene or GO materials with a variety of compounds through chemical or physical means such as by flowing NH3 over GO at high temperatures or mixing GO with compounds that serve as nitrogen sources including melamine, dicyanodiamide, and pyrrole, followed by a post treatment method such as chemical or thermal reduction. Nitrogen atoms could be incorporated in various forms depending on the experimental details.
2.5 Three Dimensional Graphene Frameworks
One of the challenges encountered when utilizing graphene materials for catalytic applications is regraphitization and reduction in surface area when the catalyst is dried to a powder. The graphene layers restack because of strong π-π interaction, and leads to reduction in accessible areas and trapping or enclosure of active sites that makes them inaccessible. A number of methods have been explored to reduce this restacking interaction by creating 3D structures, such as graphene-organic-frameworks (GOFs), graphene aerogels, and graphene hydrogels, and a rather comprehensive look at these methods can be found in recent review articles by Li et al. [51] and Bai et al. [52]. The most common methods utilized to generate such structures for catalysis involve solvothermal self-assembly. These methods seek to balance the hydrophilic–hydrophobic interactions between the attractive interplanar van der Waals forces and the repulsive electrostatic interaction from charged carboxylic acid groups by tuning them with pH of the solution or concentration of the suspension, or by utilizing cross-linking molecules. These self-assembly methods allow the GO sheets to retain a larger d-spacing than a conventionally filtered and dried sample in which these forces are not optimized. This increased d-spacing also results in a larger surface area for the final material and easier access to catalytically active sites. Other techniques to produce 3D graphene samples have been explored, including template-guided approaches and self-assembly at an interface.
3 Catalytic Applications
It is widely known that carbon and carbon-based material such as activated carbon and graphitic carbon are useful catalytic materials, and that functionalized carbons are often more active and/or selective catalysts than their unfunctionalized counterparts. The properties of these materials and their commercial applications have been reviewed [53–55]. In general, however, the heterogeneity of these functionalized carbons makes it difficult to characterize them at the atomic level, or to determine the detailed reaction mechanism or the nature of the specific active site. Functionalized carbon are active for a variety of reactions including selective oxidative dehydrogenation of hydrocarbons [56, 57], oxidation of aromatic compounds [58], and decarboxylation of fatty acids [59]. Here we focus on graphene and GO-based materials.
3.1 Catalysis by Graphite Oxide and Graphene Oxide
As synthesized graphite oxide and the exfoliated version, graphene oxide, is a highly disrupted graphene network. The ease of preparation, low-cost synthesis, ease of recovery by filtration, and high surface-to-volume ratio make GO a very attractive material for a wide variety of catalytic applications, as covered in a review by Dreyer and Bielawski [53]. As mentioned previously the basal plane and edges of GO are decorated with various oxygen functional groups, including epoxy, carbonyl, hydroxyl, and carboxylic acids. These groups impart GO inherent acidity, and oxidative, chemical and catalytic activity. Indeed GO has been shown to be catalytically active for a variety of reactions. In 2010, Bielawski and coworkers [60] demonstrated that GO effects the oxidation of a wide variety of alcohols to ketones and aldehydes, alkenes to diketones, and alkynes to the hydrates in air between 75 and 150 °C. Many of these reactions proceed with high selectivities. For example, the yield in the oxidation of cis-1,2-diphenylethane-1,2-diol to 1,2-diphenylethane-1,2-dione was 96 %, and >98 % for diphenylmethanol to benzophenone. Interestingly, whereas GO oxidized 1,2-diphenylethene to 1,2-diphenylethane-1,2-dione with 56 % conversion, it was either inactive or could only catalyze isomerization of other alkene systems. Thus, it appears that the activity towards cis-1,2-diphenylethene is due to a combination of aromatic substituents and cis geometry. In order to ascertain that GO functions as a catalyst, the authors preformed the experiments in ambient and inert nitrogen environments and observed much less oxidation without the presence of oxygen. In addition, the GO could be separated from the reaction mixture and reused for multiple cycles. However, the activity was low, and carbon to substrate weight ratios as high as 200 % were used to obtain reasonable yields in 24 h. Even taking into consideration the high loading and low activity, a GO catalyst has a higher turnover frequency per dollar compared to a tetrapropylammonium perruthenate oxidation catalyst making it a very attractive alternative [60].
GO was found to catalyze other reactions, including the oxidation of sulfides to sulfoxides and the coupling of thiols to disulfides [61], dehydrogenation of various hydrocarbons and oxidation of methylbenzenes to their respective aldehydes, diarylmethanes to their respective ketones, and other hydrocarbons with sp3-hybridized C–H bonds [62]. It has also shown high activities for the Claisen–Schmidt coupling reactions [63], Friedel‐Crafts reactions [64], Aza‐Michael additions [65], and ring opening polymerization [66]. It is a solid acid catalyst for the preparation of dipyrromethane and calix-4-pyrroles [67], an auto-tandem oxidation–hydration–aldol coupling catalyst performing multiple reactions in a single reaction chamber [63], a photocatalyst for H2 generation from water (but the carbon might be oxidized at the same time) [68], and a catalyst for the aerobic oxidative coupling of amines to imines [69]. Although all of these reactions were carried out under mild conditions, they all require a high weight loading of GO relative to the substrate for reasonable yields. Interestingly, in the study of the oxidative coupling of amines, Loh and coworkers [49] developed a method to reduce the catalyst loading to as low as 5 % and still achieved yields up to 98 % by subjecting the GO to a sequential base and acid treatment, which created defects on the GO basal planes and enhanced the catalytic activity. Table 1 provides a summary of the types of reactions that can be catalyzed by graphene oxide. The advantage of using GO as a catalyst in most of these reactions is that desirable reaction conversions can be achieved without the use of a metal catalyst. GO can also enhance the selectivity of reaction systems; in the preparation of dipyrromethane and calix-4-pyrroles, a GO catalyst had 97 % selectivity to dipyrromethane whereas an Amberlyst-15 catalyst yielded no dipyrromethane and a selectivity of 83 % calix-4-pyrrole and 14 % other products [67].
In addition to typical organic reactions, GO exhibits other interesting catalytic properties. When mixed with polymer monomers, GO can act as a dehydrative polymerization catalyst [70–72]. In these polymerization reaction systems, the GO was partially reduced, thus serving as part oxidant and part catalyst. Usually, it is not separated from the polymer matrix after the reaction, instead serving to enhance the strength and conductivity of the polymer composite. GO porous foams prepared by unidirectional freeze-drying is active in oxidizing SO2 gas to SO3 [73]. In this reaction, the GO also acts as a catalyst and an oxidant, and is partially reduced after coming into contact with the SO2 gas. The catalyst would have to be reoxidized to regain full activity. The fact that the reaction proceeds at room temperature and that GO is a metal free system makes it a very attractive alternative to other traditional systems for the treatment of SO2 gas.
3.2 Functionalized GO Catalysts
Similar to traditional oxide supports, such as SiO2 and Al2O3, GO can serve as a platform for catalytically active species. It also has the potential to exert influence on the catalytic activity through effects such as cooperativity between GO and functional group(s), thus offering another system to investigate a currently active area of research of tuning selectivity and activity of reactions [74, 75]. For graphene-supported metal and semiconductor nanoparticle catalysts, the main influence of graphene is through its ability to donate or withdraw electron density from the nanoparticle [76]. In metal-free catalytic systems, GO acts mainly as a tethering point for the catalytically active moieties.
A graphene-based solid acid catalyst can be prepared by anchoring sulfonic acid-containing aryl radicals to a rGO surface [48], which was active for the hydrolysis of ethyl acetate (64.0 % conversion). It can also be prepared by hydrothermally treating rGO with fuming sulfuric acid [77]. The catalyst was active for the esterification of acetic acid with cyclohexanol (79.5 % conversion), the esterification of acetic acid with 1-butanol (89.1 % conversion), the Peckmann reaction of resorcinol with ethyl acetoacetate (82.1 % conversion), and the hydration of propylene oxide (66.8 % conversion). In both studies, the graphene-based acid catalyst was found to have comparable or superior activity to the unsupported catalyst of concentrated sulfuric acid (~80 % conversion for the hydrolysis of ethyl acetate) and other traditional solid acid catalysts, such as Amberlyst 15 (58.9 % conversion for the esterification of acetic acid with cyclohexanol). Sulfonic acid tethered to amorphous carbon also exhibits excellent catalytic activity, and based on characterization of the solid and reaction rate analysis, the acid groups are presumed to be in regions within the carbon structure where functionalized graphene clusters reside [78–80].
Graphene-based basic catalysts have also been explored as solid base catalysts. Functionalizing the support with basic amino groups has been shown to render activity for the hydrolysis of ethyl acetate with an activity even slightly higher than that observed with a sulfonated graphene catalyst [81]. Table 2 summarizes various reactions catalyzed by functionalized GO.
Other than tethering functional groups, the oxygenated groups inherent in GO can act as catalysts also. In fact, there is the potential that the activity can be tuned by changing the density of a specific group. Depending on pH value, temperature, and reagent concentration in the system, GO containing carboxylic acid groups was found to have a peroxidase-like activity, capable of catalyzing the reaction of peroxidase substrate 3,3,5,5-tetramethylbenzidine in the presence of H2O2 for glucose detection at a rate comparable or superior to a natural horseradish peroxidase [82].
Whereas there is yet an example showing that GO functions as an active participant in cooperative effect, there are examples of using GO to support two different catalytic moieties that can then perform as a cooperative catalyst. One example is GO functionalized with a primary (3-aminopropyltriethoxysilane) and tertiary amine (3-(diethylamino)propyltrimethoxysilane) for the synthesis of trans-β-nitrostyrene. It was hypothesize that the tertiary amine activates the nucleophile and the primary amine activates the carbonyl compound by forming an imine intermediates [47]. Although this example demonstrates one potential role for GO, it is possible that GO could have a broader range of effects by tuning its hydrophobicity, acidity, electrical conductivity, and/or 3-D structure. These have yet to be explored.
3.3 Nitrogen-Doped Graphene Catalysts
As mentioned earlier, N-doped (and heteroatom-doped) graphene are excellent catalysts for the oxygen reduction reaction (ORR) for electrocatalysis in fuel cell and battery applications [15–20]. Nitrogen incorporation into the graphene structure changes the spin density and charge distribution of the surrounding carbon atoms and widens the band gap. There are three forms of N incorporated into the carbon lattice: quaternary (graphitic) N, pyridinic N, and pyrrolic N (Fig. 3). In addition, amino nitrogen groups can also decorate the edges and defects. The distribution of the various forms depends on the synthesis method, which could change the catalytic properties of the material. It is still under debate how to correctly characterize the different forms of nitrogen [50], which makes it difficult to correlate catalytic properties to a specific nitrogen form. Despite this, the impact of the different forms of nitrogen on the ORR has been observed, and it is generally thought that the enhanced activity can be correlated with the amount of pyridinic and/or quaternary nitrogen [17, 83].
Use of N-doped graphene for other reactions has been much less studied. The few studies available include the oxidation of benzene to phenol [84] and of a range of benzylic alcohols [85]. Synthesized by flowing NH3 over GO, quaternary nitrogen atoms were attributed to be responsible for the increased catalytic activity in the oxidation of benzylic alcohols to the corresponding aldehydes. Increasing the temperature at which nitrogen was doped into the GO altered the amount and type of nitrogen incorporation, and flowing NH3 at 900 °C over GO resulted in a catalyst with the highest amount of quaternary nitrogen incorporation. The last entry in Table 2 also depicts the typical oxidation reaction that is catalyzed by this N-doped graphene catalyst.
4 Conclusions
The studies to date demonstrate that metal-free GO is a material of many catalytic opportunities. It has a high surface area, high adsorption capacity for gases, hydrocarbons, and ions, and through reaction with the oxygenate functionalities, offers many avenues for modification and introduction of a wide variety of functional groups. However, even with the rapid increase in knowledge over the past decade on the use of these systems for catalytic applications, there are still many challenges that need to be investigated and resolved. We identify three of them: (1) Since the performance of these catalytic systems is strongly dependent on the structure and composition, methods need to be developed for more precise characterization so that the active sites can be properly identified. (2) Care must be taken to definitively exclude the possibility that the catalytic activity could be ascribed to metal contaminants, even at a trace level. (3) More research and a better understanding is needed to elucidate the potential cooperative effect that the graphene or GO has on catalytic activity. Understanding what advantages a graphene/GO provides over other traditional metal oxide or activated carbon will be essential for future rational design of metal-free catalyst systems. Nonetheless, even with these challenges, there is a great potential to be tapped into to use metal-free structured carbon catalysts to solve industrial problems associated with traditional catalysts. Because graphene/GO catalysts are made of environmentally benign material, their potential use for green processing should be explored vigorously.
References
Geim AK, Novoselov KS (2007) The rise of graphene. Nat Mater 6:183–191. doi:10.1038/nmat1849
Tasis D, Tagmatarchis N, Bianco A, Prato M (2006) Chemistry of carbon nanotubes. Chem Rev 106:1105–1136. doi:10.1021/cr050569o
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Electric field effect in atomically thin carbon films. Science 306:666–669. doi:10.1126/science.1102896
Eda G, Chhowalla M (2010) Chemically derived graphene oxide: towards large-area thin-film electronics and optoelectronics. Adv Mater 22:2392–2415. doi:10.1002/adma.200903689
Georgakilas V, Otyepka M, Bourlinos AB, Chandra V, Kim N, Kemp KC, Hobza P, Zboril R, Kim KS (2012) Functionalization of graphene: covalent and non-covalent approaches derivatives and applications. Chem Rev. doi:10.1021/cr3000412
Brownson DAC, Kampouris DK, Banks CE (2011) An overview of graphene in energy production and storage applications. J Power Sources 196:4873–4885. doi:10.1016/j.jpowsour.2011.02.022
Compton OC, Nguyen ST (2010) Graphene oxide, highly reduced graphene oxide, and graphene: versatile building blocks for carbon-based materials. Small 6:711–723. doi:10.1002/smll.200901934
Wu J, Pisula W, Müllen K (2007) Graphenes as potential material for electronics. Chem Rev 107:718–747. doi:10.1021/cr068010r
Huang X, Yin Z, Wu S, Qi X, He Q, Zhang Q, Yan Q, Boey F, Zhang H (2011) Graphene-based materials: synthesis, characterization, properties, and applications. Small 7:1876–1902. doi:10.1002/smll.201002009
Sun X, Wang R, Su D (2013) Research progress in metal-free carbon-based catalysts. Chin J Catal 34:508–523. doi:10.1016/S1872-2067(11)60515-9
Yu D, Nagelli E, Du F, Dai L (2010) Metal-free carbon nanomaterials become more active than metal catalysts and last longer. J Phys Chem Lett 1:2165–2173. doi:10.1021/jz100533t
Su DS, Zhang J, Frank B, Thomas A, Wang X, Paraknowitsch J, Schlogl R (2010) Metal-free heterogeneous catalysis for sustainable chemistry. ChemSusChem 3:169–180. doi:10.1002/cssc.200900180
Machado BF, Serp P (2012) Graphene-based materials for catalysis. Catal Sci Technol 2:54. doi:10.1039/c1cy00361e
Huang C, Li C, Shi G (2012) Graphene based catalysts. Energy Environ Sci 5:8848. doi:10.1039/c2ee22238h
Liu H, Liu Y, Zhu D (2011) Chemical doping of graphene. J Mater Chem 21:3335. doi:10.1039/c0jm02922j
Xue Y, Yu D, Dai L, Wang R, Li D, Roy A, Lu F, Chen H, Liu Y, Qu J (2013) Three-dimensional B, N-doped graphene foam as a metal-free catalyst for oxygen reduction reaction. Phys Chem Chem Phys. doi:10.1039/C3CP51942B
Zhang C, Hao R, Liao H, Hou Y (2013) Synthesis of amino-functionalized graphene as metal-free catalyst and exploration of the roles of various nitrogen states in oxygen reduction reaction. Nano Energy 2:88–97. doi:10.1016/j.nanoen.2012.07.021
Zheng Y, Jiao Y, Jaroniec M, Jin Y, Qiao SZ (2012) Nanostructured metal-free electrochemical catalysts for highly efficient oxygen reduction. Small 8:3550–3566. doi:10.1002/smll.201200861
Zhu C, Dong S (2013) Recent progress in graphene-based nanomaterials as advanced electrocatalysts towards oxygen reduction reaction. Nanoscale 5:1753–1767. doi:10.1039/c2nr33839d
Wang S, Yu D, Dai L, Chang DW, Baek J-B (2011) Polyelectrolyte-functionalized graphene as metal-free electrocatalysts for oxygen reduction. ACS Nano 5:6202–6209. doi:10.1021/nn200879h
Lotya M, Hernandez Y, King PJ, Smith RJ, Nicolosi V, Karlsson LS, Blighe FM, De S, Wang Z, McGovern IT, Duesberg GS, Coleman JN (2009) Liquid phase production of graphene by exfoliation of graphite in surfactant/water solutions. J Am Chem Soc 131:3611–3620. doi:10.1021/ja807449u
Tyutyulkov N, Madjarova G, Dietz F, Mullen K (1998) Is 2-D graphite an ultimate large hydrocarbon 1. Energy spectra of giant polycyclic aromatic hydrocarbons. J Phys Chem B 102:10183–10189. doi:10.1021/jp982651b
Kim KS, Zhao Y, Jang H, Lee SY, Kim JM, Kim KS, Ahn JH, Kim P, Choi JY, Hong BH (2009) Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 457:706–710. doi:10.1038/nature07719
Brodie BC (1859) On the atomic weight of graphite. Philos Trans 149:249–259. doi:10.1098/rstl 1859.0013
Staudenmaier L (1898) Verfahren zur Darstellung der Graphitsäure. Ber Dtsch Chem Ges 31:1481–1487
Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339
Kovtyukhova NI, Ollivier PJ, Martin BR, Mallouk TE, Sa Chizhik, Buzaneva EV, Gorchinskiy AD (1999) Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations. Chem Mater 11:771–778. doi:10.1021/cm981085u
Stankovich S, Dikin DA, Dommett GH, Kohlhaas KM, Zimney EJ, Stach EA, Piner RD, Nguyen ST, Ruoff RS (2006) Graphene-based composite materials. Nature 442:282–286. doi:10.1038/nature04969
Lerf A, He H, Forster M, Klinowski J (1998) Structure of graphite oxide revisited. J Phys Chem B 102:4477–4482. doi:10.1021/jp9731821
Stankovich S, Dikin DA, Piner RD, Kohlhaas KA, Kleinhammes A, Jia Y, Wu Y, Nguyen ST, Ruoff RS (2007) Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45:1558–1565. doi:10.1016/j.carbon.2007.02.034
Schniepp HC, Li J-L, McAllister MJ, Sai H, Herrera-Alonso M, Adamson DH, Prud’homme RK, Car R, Saville DA, Aksay IA (2006) Functionalized single graphene sheets derived from splitting graphite oxide. J Phys Chem B 110:8535–8539. doi:10.1021/jp060936f
Zhou M, Wang Y, Zhai Y, Zhai J, Ren W, Wang F, Dong S (2009) Controlled synthesis of large-area and patterned electrochemically reduced graphene oxide films. Chem Eur J 15:6116–6120. doi:10.1002/chem.200900596
Mathkar A, Tozier D, Cox P, Ong P, Galande C, Balakrishnan K, Leela Mohana Reddy A, Ajayan PM (2012) Controlled, stepwise reduction and band gap manipulation of graphene OXIDE. J Phys Chem Lett 3:986–991. doi:10.1021/jz300096t
Erickson K, Erni R, Lee Z, Alem N, Gannett W, Zettl A (2010) Determination of the local chemical structure of graphene oxide and reduced graphene oxide. Adv Mater 22:4467–4472. doi:10.1002/adma.201000732
Park JS, Cho SM, Kim WJ, Park J, Yoo PJ (2011) Fabrication of graphene thin films based on layer-by-layer self-assembly of functionalized graphene nanosheets. Appl Mater Interfaces 3:360–368. doi:10.1021/am100977p
Cao H, Wu X, Yin G, Warner JH (2012) Synthesis of adenine-modified reduced graphene oxide nanosheets. Inorg Chem 51:2954–2960. doi:10.1021/ic2022402
Zhang X, Huang Y, Wang Y, Ma Y, Liu Z, Chen Y (2009) Synthesis and characterization of a graphene–C60 hybrid material. Carbon 47:334–337. doi:10.1016/j.carbon.2008.10.018
Liu Q, Shi J, Sun J, Wang T, Zeng L, Jiang G (2011) Graphene and graphene oxide sheets supported on silica as versatile and high-performance adsorbents for solid-phase extraction. Angew Chem Int Ed 50:5913–5917. doi:10.1002/anie.201007138
Sun X, Liu Z, Welsher K, Robinson JT, Goodwin A, Zaric S, Dai H (2008) Nano-graphene oxide for cellular imaging and drug delivery. Nano Res 1:203–212. doi:10.1007/s12274-008-8021-8
Jin L, Yang K, Yao K, Zhang S, Tao H, Lee S-T, Liu Z, Peng R (2012) Functionalized graphene oxide in enzyme engineering: a selective modulator for enzyme activity and thermostability. ACS Nano 6:4864–4875. doi:10.1021/nn300217z
Kou L, He H, Gao C (2010) Click chemistry approach to functionalize two-dimensional macromolecules of graphene oxide nanosheets. Nano-Micro Lett 2:177–183. doi:10.5101/nml.v2i3.p177-183
Lee D, Choi M-C, Ha C-S (2012) Polynorbornene dicarboximide/amine functionalized graphene hybrids for potential oxygen barrier films. J Polym Sci A 50:1611–1621. doi:10.1002/pola.25932
Stankovich S, Piner RD, Nguyen ST, Ruoff RS (2006) Synthesis and exfoliation of isocyanate-treated graphene oxide nanoplatelets. Carbon 44:3342–3347. doi:10.1016/j.carbon.2006.06.004
Yang H, Shan C, Li F, Han D, Zhang Q, Niu L (2009) Covalent functionalization of polydisperse chemically-converted graphene sheets with amine-terminated ionic liquid. Chem Commun. doi:10.1039/b905085j
Wang B, Luo B, Liang M, Wang A, Wang J, Fang Y, Chang Y, Zhi L (2011) Chemical amination of graphene oxides and their extraordinary properties in the detection of lead ions. Nanoscale 3:5059–5066. doi:10.1039/c1nr10901d
Lai L, Chen L, Zhan D, Sun L, Liu J, Lim SH, Poh CK, Shen Z, Lin J (2011) One-step synthesis of NH2-graphene from in situ graphene-oxide reduction and its improved electrochemical properties. Carbon 49:3250–3257. doi:10.1016/j.carbon.2011.03.051
Zhang W, Wang S, Ji J, Li Y, Zhang G, Zhang F, Fan X (2013) Primary and tertiary amines bifunctional graphene oxide for cooperative catalysis. Nanoscale 5:6030–6033. doi:10.1039/c3nr01323e
Ji J, Zhang G, Chen H, Wang S, Zhang G, Zhang F, Fan X (2011) Sulfonated graphene as water-tolerant solid acid catalyst. Chem Sci 2:484. doi:10.1039/c0sc00484g
Su C, Acik M, Takai K, Lu J, Hao SJ, Zheng Y, Wu P, Bao Q, Enoki T, Chabal YJ, Loh KP (2012) Probing the catalytic activity of porous graphene oxide and the origin of this behaviour. Nat Commun 3:1298. doi:10.1038/ncomms2315
Wang H, Maiyalagan T, Wang X (2012) Review on recent progress in nitrogen-doped graphene: synthesis, characterization, and Its potential applications. ACS Catal 2:781–794. doi:10.1021/cs200652y
Li C, Shi G (2012) Three-dimensional graphene architectures. Nanoscale 4:5549–5563. doi:10.1039/c2nr31467c
Bai H, Li C, Wang X, Shi G (2011) On the gelation of graphene oxide. J Phys Chem C 115:5545–5551. doi:10.1021/jp1120299
Dreyer DR, Bielawski CW (2011) Carbocatalysis: heterogeneous carbons finding utility in synthetic chemistry. Chem Sci 2:1233. doi:10.1039/c1sc00035g
Schaetz A, Zeltner M, Stark WJ (2012) Carbon modifications and surfaces for catalytic organic transformations. ACS Catal 2:1267–1284. doi:10.1021/cs300014k
Su DS, Perathoner S, Centi G (2012) Catalysis on nano-carbon materials: going where to? Catal Today 186:1–6. doi:10.1016/j.cattod.2012.04.002
Su DS, Maksimova N, Delgado JJ, Keller N, Mestl G, Ledoux MJ, Schlögl R (2005) Nanocarbons in selective oxidative dehydrogenation reaction. Catal Today 102–103:110–114. doi:10.1016/j.cattod.2005.02.012
Pereira MFR, Orfao JJM, Figueiredo JL (1999) Oxidative dehydrogenation of ethylbenzene on activated carbon catalysts. I. Influence of surface chemical groups. Appl Catal A 184:153–160. doi:10.1016/S0926-860X(99)00124-6
Hayashi M (2008) Oxidation using activated carbon and molecular oxygen system. Chem Rec 8:252–267. doi:10.1002/tcr.20152
Fu J, Shi F, Thompson LT, Lu X, Savage PE (2011) Activated carbons for hydrothermal decarboxylation of fatty acids. ACS Catal 1:227–231. doi:10.1021/cs1001306
Dreyer DR, Jia HP, Bielawski CW (2010) Graphene oxide: a convenient carbocatalyst for facilitating oxidation and hydration reactions. Angew Chem Int Ed 49:6813–6816. doi:10.1002/anie.201002160
Dreyer DR, Jia HP, Todd AD, Geng J, Bielawski CW (2011) Graphite oxide: a selective and highly efficient oxidant of thiols and sulfides. Org Biomol Chem 9:7292–7295. doi:10.1039/c1ob06102j
Jia H-P, Dreyer DR, Bielawski CW (2011) C–H oxidation using graphite oxide. Tetrahedron 67:4431–4434. doi:10.1016/j.tet.2011.02.065
Jia H-P, Dreyer DR, Bielawski CW (2011) Graphite oxide as an auto-tandem oxidation-hydration-aldol coupling catalyst. Adv Synth Catal 353:528–532. doi:10.1002/adsc.201000748
Kumar VA, Rao RK (2011) Recyclable graphite oxide catalyzed Friedel–Crafts addition of indoles to α, β-unsaturated ketones. Tetrahedron Lett 52:5188–5191. doi:10.1016/j.tetlet.2011.08.002
Verma S, Mungse HP, Kumar N, Choudhary S, Jain SL, Sain B, Khatri OP (2011) Graphene oxide: an efficient and reusable carbocatalyst for aza-Michael addition of amines to activated alkenes. Chem Commun 47:12673–12675. doi:10.1039/c1cc15230k
Dreyer DR, Jarvis KA, Ferreira PJ, Bielawski CW (2012) Graphite oxide as a carbocatalyst for the preparation of fullerene-reinforced polyester and polyamide nanocomposites. Polym Chem 3:757. doi:10.1039/c2py00545j
Chauhan SMS, Mishra S (2011) Use of graphite oxide and graphene oxide as catalysts in the synthesis of dipyrromethane and calix[4]pyrrole. Molecules 16:7256–7266. doi:10.3390/molecules16097256
Yeh T-F, Syu J-M, Cheng C, Chang T-H, Teng H (2010) Graphite oxide as a photocatalyst for hydrogen production from water. Adv Funct Mater 20:2255–2262. doi:10.1002/adfm.201000274
Huang H, Huang J, Liu Y-M, He H-Y, Cao Y, Fan K-N (2012) Graphite oxide as an efficient and durable metal-free catalyst for aerobic oxidative coupling of amines to imines. Green Chem 14:930. doi:10.1039/c2gc16681j
Dreyer DR, Jarvis KA, Ferreira PJ, Bielawski CW (2011) Graphite oxide as a dehydrative polymerization catalyst: a one-step synthesis of carbon-reinforced poly(phenylene methylene) composites. Macromolecules 44:7659–7667. doi:10.1021/ma201306x
Lee S, Kim Y-J, Kim D-H, Ku B-C, Joh H-I (2012) Synthesis and properties of thermally reduced graphene oxide/polyacrylonitrile composites. J Phys Chem Solids 73:741–743. doi:10.1016/j.jpcs.2012.01.015
Huang Y, Qin Y, Zhou Y, Niu H, Yu Z–Z, Dong J-Y (2010) Polypropylene/graphene oxide nanocomposites prepared by in situ Ziegler–Natta polymerization. Chem Mater 22:4096–4102. doi:10.1021/cm100998e
Long Y, Zhang C, Wang X, Gao J, Wang W, Liu Y (2011) Oxidation of SO2 to SO3 catalyzed by graphene oxide foams. J Mater Chem 21:13934. doi:10.1039/c1jm12031j
Notestein JM, Katz A (2006) Enhancing heterogeneous catalysis through cooperative hybrid organic-inorganic interfaces. Chem Eur J 12:3954–3965. doi:10.1002/chem.200501152
Lee J-K, Kung MC, Kung HH (2008) Cooperative catalysis: a new development in heterogeneous catalysis. Top Catal 49:136–144. doi:10.1007/s11244-008-9087-y
Kamat PV (2010) Graphene-based nanoarchitectures. Anchoring semiconductor and metal nanoparticles on a two-dimensional carbon support. J Phys Chem Lett 1:520–527. doi:10.1021/jz900265j
Liu F, Sun J, Zhu L, Meng X, Qi C, Xiao F-S (2012) Sulfated graphene as an efficient solid catalyst for acid-catalyzed liquid reactions. J Mater Chem 22:5495. doi:10.1039/c2jm16608a
Kitano M, Yamaguchi D, Suganuma S, Nakajima K, Kato H, Hayashi S, Hara M (2009) Adsorption-enhanced hydrolysis of beta-1,4-glucan on graphene-based amorphous carbon bearing SO3H, COOH, and OH groups. Langmuir 25:5068–5075. doi:10.1021/la8040506
Kang S, Ye J, Chang J (2013) Recent advances in carbon-based sulfonated catalyst: preparation and application. Int Rev Chem Eng 5:133–144
Nakajima K, Hara M (2012) Amorphous carbon with SO3H groups as a solid brønsted acid catalyst. ACS Catal 2:1296–1304. doi:10.1021/cs300103k
Yuan C, Chen W, Yan L (2012) Amino-grafted graphene as a stable and metal-free solid basic catalyst. J Mater Chem 22:7456. doi:10.1039/c2jm30442b
Song Y, Qu K, Zhao C, Ren J, Qu X (2010) Graphene oxide: intrinsic peroxidase catalytic activity and its application to glucose detection. Adv Mater 22:2206–2210. doi:10.1002/adma.200903783
Yang S, Feng X, Wang X, Mullen K (2011) Graphene-based carbon nitride nanosheets as efficient metal-free electrocatalysts for oxygen reduction reactions. Angew Chem Int Ed 50:5339–5343. doi:10.1002/anie.201100170
Yang J-H, Sun G, Gao Y, Zhao H, Tang P, Tan J, Lu A-H, Ma D (2013) Direct catalytic oxidation of benzene to phenol over metal-free graphene-based catalyst. Energy Environ Sci 6:793. doi:10.1039/c3ee23623d
Long J, Xie X, Xu J, Gu Q, Chen L, Wang X (2012) Nitrogen-doped graphene nanosheets as metal-free catalysts for aerobic selective oxidation of benzylic alcohols. ACS Catal 2:622–631. doi:10.1021/cs3000396
Acknowledgments
This work was supported by the Department of Energy, Basic Energy Sciences, grant no. DE-FG02-01ER15184. A fellowship from the 3 M Corporation to DH is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haag, D., Kung, H.H. Metal Free Graphene Based Catalysts: A Review. Top Catal 57, 762–773 (2014). https://doi.org/10.1007/s11244-013-0233-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-013-0233-9