Abstract
Designing novel cathode materials for a proton exchange membrane fuel cell with high activity for the oxygen reduction reaction, low Pt loading, and enhanced long-term stability is imperative for its sustainability. To date, Pt monolayer based electrocatalysts deposited on a metallic core substrate have shown promising possibilities. In this study, we synthesized bimetallic IrCu nanoparticles and used them as a core for Pt monolayer electrocatalysts. It was found that the de-alloyed IrCu nanoparticle surfaces increased both the mass and specific activities of the resulting Pt monolayer catalyst. In addition, we demonstrated that Pt monolayer electrocatalysts with a de-alloyed IrCu core have a better stability than those using a non-dealloyed core based on a 5,000 potential cycling test. These data describe a new simple synthesis of a high-performance catalyst suitable for practical applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The search for sustainable energy technologies has stimulated great interest in a proton exchange membrane fuel cell (PEMFC) [1]. Regardless of extensive research to develop high activity cathode materials for the oxygen reduction reaction (ORR) (1/2O2 → O2− + 2e−) [2], Pt is still the best electrocatalyst in terms of a pure metal. However, since the ORR kinetics of Pt is considerably slow and irreversible, resulting in overpotentials and the loss of fuel cell efficiencies [3]. This critical problem has obstructed its application to vehicles and power generation. Furthermore, it has been reported that a realistic Pt-based electrocatalyst for commercialization should have at least four times higher mass activity than Pt/C [4]. As Vukmirovic et al. [5] reported, significant advances in developing novel cathode materials in PEMFCs are core–shell types of electrocatalysts, bimetallic cathodes with Pt, and dealloying of bimetallic materials. Regardless of this series of advances in developing of novel cathode materials to replace expensive Pt, the cost-effective cathode design is still a challenge in the fuel cell community. Therefore, rational design of novel high-performance electrocatalysts toward the ORR is crucial to make it more cost-effective for commercialization. In addition to the requirement of the high activity, the novel cathode materials also need low Pt loading and enhanced long-term stability. Furthermore, theoretically, extensive attempts have been carried out to understand the ORR kinetics using density functional theory (DFT) calculations [6, 7], which provide substantial insights into the ORR [8–14]. To date, a novel approach to minimize Pt loading and to maximize its utilization has been introduced by Adzic et al. [5, 15]. By changing the core materials, one can easily modify the electronic and geometric properties of Pt [11, 14, 16]. In particular, Pt monolayer (PtML) based electrocatalysts [15, 17, 18] deposited on a metallic core (i.e., Pd or IrM) greatly enhance both the ORR activity and stability. Recently, IrM (i.e., Fe [19] and Ni [3]) substrates have shown a good activity and stability by the formation of the Ir shell and the M core verified by using scanning transmission electron microscope/electron energy loss spectroscopy (STEM/EELS). Iridium itself is a good candidate to increase the stability by its oxide formation but shows poor ORR activity in acid solution. Based on the previous results [3, 19, 20], in this study, we synthesized a bimetallic IrCu core alloy. The utilization of the non-precious Cu core can reduce the precious metal loading and also change the electronic and geometric structures. Here, we report on a Pt monolayer IrCu core electrocatalyst (denoted as PtMLIrCu/C) tailored to increase the ORR activity and to enhance the stability under ORR conditions.
2 Experimental Details
The preparation of Pt monolayer IrM core–shell nanoparticles can be found in our previous study in detail [3]. Briefly, to prepare the carbon-supported IrCu core materials, an equal molar ratio of (NH4)2IrCl6 (Sigma-Aldrich) and CuSO4·5H2O (Sigma-Aldrich) were mixed and sonicated with Vulcan XC-72 (Cabot Corporation) in Millipore water (18 MΩ cm) under Ar to obtain a 20 wt% metal loading. After an hour, NaBH4 solution was added to reduce the precursors with continued ultrasonication for an hour. The carbon-supported IrCu alloy particles were rinsed with deionized water of ~2 L and dried in vacuum overnight. The dried sample was subjected to an annealing process at 600 °C in 15 % H2/Ar for 2 h.
X-ray diffraction (XRD) analyses of the IrCu core materials were carried out using a Phillips 3100 diffractometer equipped with a CuKα source (1.54056 Å). The elemental compositions of the core materials were determined by energy dispersive X-ray (EDX) analysis using a Quanta scanning electron microscope (SEM). Furthermore, scanning transmission electron microscope (STEM) (JEOL JEM 2100F) was used and operated at 200 kV with an emission current of 200 μA.
Electrochemical measurements were conducted using a computer controlled potentiostat (PGZ402 & VoltaMaster 4, VoltaLab Instruments). All solutions were prepared with deionized water using the MilliQ UV-plus. In this study, potentials (E) were measured using Ag/AgCl (3 M NaCl) reference electrode, but are given relative to a reversible hydrogen electrode (RHE) except the Cu UPD result. In order to deposit Pt monolayer on the core materials, the carbon supported IrCu core materials were dispersed in deionized water (1 mg/mL) and sonicated for ~10 min. Ten microliter of the dispersed IrCu ink was carefully pipetted on a glassy carbon rotating disk electrode (RDE) with the surface area of 0.196 cm2 (Pine instruments). After drying, we pipetted 10 μL of diluted Nafion solution (Sigma-Aldrich; 2 μg/5 μL) to improve its adhesion and dried before immersing the electrode into the electrolyte solution. The Pt monolayer deposition was conducted in a container with two chambers (i.e., one for 50 mM CuSO4 in 50 mM H2SO4 and the other for 1 mM K2PtCl4 (Sigma-Aldrich) in 50 mM H2SO4). We used a leak free Ag/AgCl (3 M KCl) as a reference electrode and Pt foil as a counter electrode. The IrCu electrode was negatively scanned from its open-circuit potential in the CuSO4 solution and before reaching its mass deposition value, the potential was fixed for 1 min for Cu to deposit on the IrCu surface. Then, the electrode with the copper deposition was carefully immersed into the Pt solution. To ensure the total replacement of Cu to Pt, the electrode was immersed for around 5 min. The schematic of the process is described in Fig. 1. For the ORR analyses, the Koutecky–Levich equation was used [21]. Furthermore, we carried out RDE cycling stability experiments for the electrocatalysts in air-saturated electrolyte in the potential range from 0.60 to 1.00 V/RHE at the scan rate of 50 mV/sec. After 5,000 potential cycles each Pt monolayer electrode was scanned for cyclic voltammetry in Ar saturated and ORR polarization curves in O2 saturated 0.1 M HClO4 acid. To study the activity enhancement after the dealloying process the IrCu electrode was subjected to 50 potential cycles between 0.6 V and 1.0 V/RHE at a scan rate of 20 mV/s in an Ar-saturated 0.1 M HClO4 solution. After potential cycling the dealloyed IrCu electrode was covered with a Pt monolayer using the Cu UPD method as described above. In this study, after Pt monolayer deposition, we designate the IrCu and the dealloyed IrCu electrodes by PtMLIrCu/C and PtML,dIrCu/C, respectively.
3 Results and Discussion
Shown in Fig. 2a is an XRD spectrum of as-synthesized IrCu nanoparticles. It clearly shows that it is an alloy of Ir and Cu. As discussed, the relative atomic ratio of Ir versus Cu of the nanoparticles was analyzed by EDX, proving it is Ir0.51Cu0.49 (nominal composition: Ir0.50Cu0.50). It is known that Ir and Cu (Ir0.50Cu0.50) forms a solid solution according to a determination of phase equilibrium [22]. In this study, one of our objectives is to understand the effect of dealloying using potential cycling applied to the RDE. So similar to our previous study [19], we washed the carbon-supported IrCu nanoparticles with 0.5 M H2SO4 solution (Optima, Fisher-Scientific). Figure 2b shows a XRD spectrum after acid washing, in which Cu peaks disappear and the peaks shift towards Ir phases, manifesting that Cu precipitates are dissolved away. However, to verify the existence of Cu in the nanoparticles, we carried out EDX again. EDX detected Cu in the nanoparticles, and the relative ratio of Ir versus Cu became Ir0.82Cu0.18. However, because this EDX technique provides bulk composition not the surface composition, in order to examine the potential surface segregation similar to the previous studies [3, 19, 20], we carried out STEM/EDS analyses which is discussed in the following section. The average size of the IrCu nanoparticles is ~12 nm using Scherrer’s equation [23]. Based on the careful examination of IrCu nanoparticles with and without the acid washing, we found that the surface composition of the prepared IrCu core materials could be changed, such as due to Cu dissolution. Figure 3a shows a low-resolution TEM image of as-synthesized IrCu/C, while Fig. 3b depicts a high-resolution TEM image. In this section we show only the images of non-dealloyed particles. Most of particles are round and have sphere-like shapes, while we found semi-rounded ones. The TEM image clearly shows well dispersed nanoparticles on the carbon substrate. The lattice spacing is 1.932 Å, which is in good agreement with pure Ir (1.922 Å). In addition, the atomic and component distributions of the IrCu particles were examined using aberration-corrected STEM/EDS, equipped with a cold-field emission source. We could easily image the IrCu nanoparticles and obtain the Ir and Cu distribution using the M- and L-edge, respectively. Figure 3c is the bright field (BF) image of a sphere-like IrCu nanoparticle, while its line scanned spectra are shown in Fig. 3d. For clarity, the EDS spectra were smoothed. The X-ray line scan profile along the line in a single particle shown in the BF-STEM image suggests that the IrCu nanoparticle (as-prepared) is Ir-rich in the whole range of the particle except at the edges. The surface becomes slightly Cu-rich as shown in the STEM image. Also, it clearly shows that the IrCu nanoparticle forms a random alloy. This is in good agreement with the XRD data, showing that IrCu is in an alloy phase.
(a) LRTEM and (b) HRTEM images of carbon supported IrCu core materials (before dealloying). (c) A BF-STEM image of an IrCu nanoparticle. (d) Line scan profile of the IrCu nanoparticle along the dashed line shown in Fig. 3c
Shown in Fig. 4a are the cyclic voltammetry (CV) curves for the IrCu core materials and PtMLIrCu (without dealloying) in de-aerated 0.1 M HClO4 solution, in which IrCu/C shows a small H UPD, while after Pt monolayer deposition using Cu upd, the H upd regions is bigger and a new peak for PtOH reduction appears. After the correction for the double layer, the hydrogen adsorption/desorption charge is 152 μC. Similar to the IrNi core [3], no anodic currents attribute to the oxidation/dissolution of Cu are observed. In order to examine the de-alloying effect similar to the work reported by Shao et al. [24], as ascribed, we compared non- and dealloyed IrCu/C particles. Figure 4b displays voltammetry curves for the IrCu core materials and PtMLIrCu after dealloying (PtML,dIrCu). It apparently shows the change of the voltammetry curve of IrCu/C by dealloying, leading to an increase in charge from 152 to 173 μC. It is not shown here, but our STEM/EDS attempt of dealloyed IrCu/C was indistinguishable with the as-synthesized one. This may mean that the cycling up to 50 potential cycles does not affect the alloy bulk properties but removing Cu atoms from the alloy do affect their surface properties, which in return change the Pt monolayer deposition charge.
Voltammetry curves using (a) non- and (b) dealloyed IrCu/C core materials in de-aerated 0.1 M HClO4 solution at 20 mV/s. In Fig. 4a, the curves in blue and in red are for IrCu/C and Pt monolayer IrCu/C, respectively. For a clear comparison, the voltammetry curve for non-dealloyed IrCu/C is added in Fig. 4b in blue
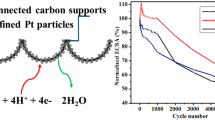
Figure 5 illustrates polarization curves for the ORR at 1,600 rpm in oxygenated 0.1 M HClO4 solution of PtMLIrCu/C and PtML,dIrCu/C. Although the H upd charge difference is only 21 μC, we observed a 36 % increase in current density from 1.108 mA/cm2 to 1.504 mA/cm2. Thus it clearly manifests that the change of the IrCu/C by dealloying before Pt monolayer deposition affects its surface, resulting in Cu dissolution from the surface and keeping its core as a mixture of Cu and Ir. We found that the dealloying process improved the bimetallic interaction between Pt and the Ir shell, enhancing the kinetic current. This interaction is likely due to geometric, electronic, and segregation effects which have a key role in rationalizing the increase in the ORR activity of PtML,dIrCu/C compared to that of PtMLIrCu/C and Pt/C [3].
As discussed in the introduction, the stability of ORR electrocatalysts plays a crucial role in the application of PEMFCs to vehicles and power generation. In this study, we carried out RDE based long-term stability tests with both PtMLIrCu/C and PtML,dIrCu/C as shown in Fig. 5. For the experiments, we carried out an accelerated potential cycling from 0.6 to 1.0 V in air-saturated 0.1 M HClO4 solution at 50 mV/s. As shown in Fig. 5, after 5,000 cycles, the non-dealloyed sample significantly decrease in the current density from 1.108 to 0.754 mA/cm2, the dealloyed one shows no substantial decrease. No improved ORR kinetics after 5,000 cycles of PtMLIrCu/C (Fig. 5a) may suggest that the initial proper deposition of Pt on the core material plays a significant role in enhancing activities.
Using the polarization curves of the electrocatalyst in 0.1 M HClO4 solution purged by O2 at different rotation speeds at a scan rate of 10 mV/s, the Koutecky–Levich plots can be plotted. Figure 6 shows Koutecky–Levich plots for PtMLIrCu/C and PtML,dIrCu/C at 0.9 V. As clearly shown, we plotted the inverse current (1/j) as a function of the inverse of the square root of the rotation rate (ω−1/2). The kinetic currents for the ORR are determined from the intercepts of the 1/j axis at ω−1/2 = 0.
As compiled in Table 1, the Pt loadings for non- and dealloyed IrCu/C using the Cu upd are 2.21 and 2.20 nmol, respectively, corresponding to 0.43 μg. The charges associated with the Cu upd are 214 and 213 μC/cm2, respectively [15]. Using the Pt loading, we calculated their mass activities are 0.64 and 1.35 mA/μg, Pt, respectively, while their specific activities are 0.38 and 0.71 mA/cm2, respectively. The electrochemical areas are calculated from the H charge (see Fig. 4, 152 and 173 μC, respectively) by assuming that the monolayer charge density for hydrogen adsorption/desorption is 210 μC/cm2 [25]. To understand how much Pt is on the surface of both the IrCu substrates the specific activity was calculated using the total Pt loading and Pt surface area from the CV. As shown in Table 1, only a small change in the specific activity is observed indicating that about 75 % of the Pt atoms are on the surface, demonstrating a better Pt utilization. As shown in Fig. 7, the Pt monolayer on non-dealloyed IrCu/C has a comparative specific activity than pure Pt/C, while its mass specific activity is higher. However, the de-alloyed one (PtML,dIrCu/C) shows enhanced specific and mass activities than both pure Pt/C and PtMLIrCu/C.
In summary, upon dealloying the IrCu alloy substrate, we made a PtMLIrCu core–shell structure, and it enhanced the ORR activities and stability of the catalyst compared to pure Pt/C. Also, Cu in the core of the alloy is playing an important role as the electrocatalyst shows better activities than Pt monolayer Ir/C [3]. Theoretical understanding of the improvement of the ORR activity is beyond the scope of this manuscript. However, according to our previous study [3] using DFT calculations, the IrCu core may change the electronic and geometric properties of Pt monolayer, resulting in reducing the oxygen adsorption energy and making the d-band center of Pt in the shell away from the Fermi level.
It was difficult to distinguish the surface structures, composition, and segregation properties of the IrCu bimetallic core materials. However, based on STEM/EDS results, it clearly shows that Cu forms a random alloy with Ir. Also by means of cyclic voltammetry, we observed the change of the IrCu surface after dealloying, resulting in the dissolution of Cu from the IrCu surface. In this study, we demonstrated that the dealloying helps increase the activities of PtML,dIrCu/C by improving the interaction between the IrCu surface and Pt.
4 Conclusions
We demonstrated the high activity and stability of PtMLIrCu/C core–shell electrocatalyst by using an IrCu core to alter the electronic and geometrical properties of Pt monolayer. After dealloying, the Cu on the IrCu alloy is dissolved, resulting in a relatively Ir-rich shell. Partial removal of the surface Cu by dealloying greatly increased the mass activity and the specific activity of the catalyst. This IrCu particles may contract the Pt monolayer like IrNi [3], leading to a downshift of the d-band center with respect to the Fermi level. The altered electronic and geometrics effects due to the IrCu core compared to pure Ir, enables enhanced ORR activities and an augmented stability for the PtMLIrCu core–shell electrocatalyst. In addition, we demonstrated that Pt monolayer electrocatalysts ORR activity can be increased using the de-alloying method. The dealloyed PtMLIrCu core has a better activity and stability than that using a non-dealloyed core; emphasizing Pt monolayer activity is totally substrate dependent.
References
Steele BCH, Heinzel A (2001) Nature 414:345
Adzic RR (1998) In: Lipkowski J, Ross P (eds) Electrocatalysis. Wiley, New York, p 197
Kuttiyiel KA, Sasaki K, Choi Y, Su D, Liu P, Adzic RR (2012) Energy Environ Sci 5:5297
Gasteiger HA, Kocha SS, Sompalli B, Wagner FT (2005) Appl Catal B Environ 56:9
Vukmirovic MB, Bliznakov ST, Sasaki K, Wang JX, Adzic RR (2011) Electrochem Soc Interface 20:33
Kohn W, Sham LJ (1965) Phys Rev B 140:A1133
Kresse G, Hafner J (1993) Phys Rev. B 47:558
Nørskov JK, Bligaard T, Rossmeisl J, Christensen CH (2009) Nat Chem 1:37
Nørskov JK, Rossmeisl J, Logadottir A, Lindqvist L, Kitchin JR, Bligaard T, Jónsson H (2004) J Phys Chem B 108:17886
Strasser P, Koh S, Anniyev T, Greeley J, More K, Yu C, Liu Z, Kaya S, Nordlund D, Ogasawara H, Toney MF, Nilsson A (2010) Nat Chem 2:454
Wang JX, Inada H, Wu L, Zhu Y, Choi Y, Liu P, Zhou W-P, Adzic RR (2009) J Am Chem Soc 131:17298
Wang JX, Ma C, Choi Y, Su D, Zhu Y, Liu P, Si R, Vukmirovic MB, Zhang Y, Adzic RR (2011) J Am Chem Soc 133:13551
Zhang J, Vukmirovic MB, Xu Y, Mavrikakis M, Adzic RR (2005) Angew Chem Int Ed 44:2132
Kuttiyiel KA, Sasaki K, Choi Y, Su D, Liu P, Adzic RR (2012) Nano Lett 12:6266
Adzic R, Zhang J, Sasaki K, Vukmirovic M, Shao M, Wang J, Nilekar A, Mavrikakis M, Valerio J, Uribe F (2007) Top Catal 46:249
Sasaki K, Naohara H, Cai Y, Choi YM, Liu P, Vukmirovic MB, Wang JX, Adzic RR (2010) Angew Chem. Int. Ed. 49:8602
Gong K, Choi Y, Vukmirovic MB, Liu P, Ma C, Su D, Adzic RR (2012) Z Phys Chem 226:1025
Sasaki K, Naohara H, Choi Y, Cai Y, Chen W-F, Liu P, Adzic RR (2012) Nat Commun 3:1115
Sasaki K, Kuttiyiel K, Su D, Adzic R (2011) Electrocatalysis 2:134
Sasaki K, Kuttiyiel KA, Barrio L, Su D, Frenkel AI, Marinkovic N, Mahajan D, Adzic RR (2011) J Phys Chem C 115:9894
Bard AJ, Faulkner LR (2001) Electrochemical methods: fundamentals and applications, 2nd edn. Wiley, New York, p 114–115
Scientific Group Thermodata Europe (SGTE), Franke P, Neuschütz D, Cu–Ir (Copper–Iridium). Franke P, Neuschütz D (eds). Springer Materials: the Landolt-Börnstein Database. doi:10.1007/978-3-540-45280-5_55, http://www.springermaterials.com. Accessed 2 Jan 2012
Cullity BD, Stock SR (2001) Elements of X-Ray Diffraction Prentice-Hall Inc., New York
Shao M, Shoemaker K, Peles A, Kaneko K, Protsailo L (2010) J Am Chem Soc 132:9253
Miki A, Ye S, Osawa M (2002) Chem Commun 1500
Acknowledgments
This research was performed at Brookhaven National laboratory under contract DE-AC02-98CH10886 with the US Department of Energy, Division of Chemical Sciences, Geosciences and Biosciences Division. This work was conducted under the framework of Research and Development Program of the Korea Institute of Energy Research (KIER) (B3-2415). Y.C. truly acknowledges the kind support by Drs. Hicham Idriss and Essam H. Jamea to carry out this project in Brookhaven National Laboratory. Also, Y.C. thanks Dr. Toseef N. Ahmed and Hugh Issacs and Wei-Fu Chen for SEM/EDX measurements and fruitful discussion on electrochemistry, respectively.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Choi, Y., Kuttiyiel, K.A., Labis, J.P. et al. Enhanced Oxygen Reduction Activity of IrCu Core Platinum Monolayer Shell Nano-electrocatalysts. Top Catal 56, 1059–1064 (2013). https://doi.org/10.1007/s11244-013-0070-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-013-0070-x