Abstract
The oxidation of 1,2-propanediol to form lactic acid and hydroxyacetone has been investigated using supported metal nanoparticles. A series of supported gold, palladium, platinum and combinations of these metals have been investigated. In the presence of base the major product formed is lactate, often in excellent yields as, under these conditions the terminal hydroxyl group is oxidised. In the absence of base, more vigorous conditions are required to elicit high conversions; surprisingly, a major product from such reactions is a mono-oxidation product hydroxyacetone, along with lactate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
1,2-Propanediol can be formed by the catalytic hydrogenolysis [1] of glycerol which in turn can be obtained as a by-product from biodiesel production. 1,2-Propanediol can be further oxidized to lactic acid, which is a useful intermediate for the production of biodegradable polymers, and food additives. There is therefore interest in developing new catalytic routes for the selective oxidation of 1,2-propanediol. Supported metal nanoparticles are known to be effective for oxidation to the corresponding acids, including monometallic Pd, Pt and Au. Tsujino et al. [2] have shown that 1,2-propanediol oxidation using palladium supported on carbon gave hydroxyacetone, lactic acid and pyruvic acid. Rossi and Prati were the first to report supported gold nanoparticles as being extremely active for oxidation of alcohols to acids under basic conditions [3–6]. Subsequent studies have shown that alloying gold with other metals, for example palladium, gives enhanced activity for the oxidation of various diols, aromatic alcohols and for hydrogen peroxide synthesis [7–14]. The presence of base with supported gold nanoparticles as catalysts is considered to aid the activation of a terminal hydroxyl group. It has also been reported that some diols can be successfully oxidised in the absence of base. Wang et al. [15] reported the oxidation of non-activated alcohols using colloidal platinum nanoparticles and the selective oxidation of glycerol using supported Pt catalysts has been studied by Liang et al. [16]. Villa et al. [17] have shown that glycerol can be oxidised using supported gold–platinum nanoparticles using base-free conditions, and we recently reported that glycerol can be selectively oxidised under base-free conditions over Au–Pd and Au–Pt nanoparticles when supported on MgO [18]. These studies prompted us to extend our previous findings of 1,2-propanediol conversion into lactic acid using supported gold nanoparticles [19] where we used basic conditions to achieve high selectivities to lactate. In this paper we investigate the use of supported gold–platinum and platinum–palladium nanoparticles for 1,2-propanediol oxidation.
2 Experimental
2.1 Catalyst Preparation
A series of Au, Pt, Pd, Au–Pd, Au–Pt and Pd–Pt catalysts supported on activated carbon (KB-B, Aldrich) were prepared using a sol-immobilisation method. Aqueous solutions of PdCl2 (Johnson Matthey) or H2PtCl6 (Johnson Matthey) and HAuCl4.3H2O (Johnson Matthey) of the desired concentration were prepared. To this solution polyvinylalcohol (PVA) (1 wt% solution, Aldrich, weight average molecular weight MW = 9,000–10,000 g mol−1, 80 % hydrolysed) was added [PVA/metal (wt/wt) = 1.2]. Subsequently, a freshly prepared 0.1 M solution of NaBH4 [>96 %, Aldrich, NaBH4/metal (mol/mol) = 5] was added to form a dark-brown sol. After 30 min of sol generation, the colloid was immobilised by adding activated carbon (acidified to pH 1 by sulfuric acid) under vigorous stirring. The amount of support material required was calculated to give a total final metal loading of 1 wt%. After 1 h the slurry was filtered, the catalyst washed thoroughly with distilled water and dried at 110 °C overnight.
2.2 Catalyst Testing
Reactions were carried out using a 100 ml Radleys low pressure reactor. For reactions using basic conditions, NaOH (0.48 or 0.96 g) was dissolved in 1,2-propanediol (20 ml of a 0.6 M aqueous solution). The catalyst was added to the resulting solution to give the required molar ratio of metal to 1,2-propanediol. The reactor was charged with oxygen to the desired pressure, raised to the required temperature and stirring was started. The solution temperature was maintained for the desired reaction time, then the reaction mixture was cooled to room temperature and analyzed by HPLC. Blank reactions of the support alone in the absence of the added metals showed no conversion of the 1,2-propanediol under our reaction conditions.
Reactions were also carried out using a autoclave reactor (100 ml HEL) using magnetic stirring. The catalyst was added to the solution as described above. The vessel was charged with oxygen to the desired pressure (3–10 bar), raised to the required reaction temperature and stirring was started. After the appropriate reaction time, the solution was cooled to room temperature and analyzed by HPLC and NMR spectroscopy.
HPLC analysis was carried out using a Varian 920-LC fitted with a metacarb 67H column with ultraviolet and refractive index detectors. The eluent was a solution H3PO4 (0.01 M) with a flow of 0.3 ml min−1. Samples of the reaction mixture (0.5 ml) were diluted to 5 ml using the eluent. Products were identified by comparison with known pure samples. For quantification of the starting material and products an external calibration method was used and the calibration factor for each was calculated. Carbon mass balances of close to 100 % were observed based on the products observed. Using these reactors it was not feasible to analyse the gaseous products, but as there is a closed mass balance on the reported products we consider that formation of CO2 is minimal, as has been observed previously for glycerol oxidation [8]. Tests for metal leaching were carried out using a Perkin Elmer AA-55B atomic absorption spectrometer equipped with a air/acetylene flame. Loss of gold on catalyst use was insignificant (<0.1 % of total Au) under both basic and base-free reaction conditions.
3 Results and Discussion
3.1 Effect of Temperature on the Oxidation of 1,2-Propanediol Using Basic Conditions
Two significant features emerge from our scan of oxidations of 1,2-propanediol using oxygen and 1 wt% Au–Pt/C as catalyst: firstly, the overall conversion is slightly higher when one rather than two equivalents of sodium hydroxide is used. Secondly, and most significantly, excellent diol conversions of over 75 % have been achieved at remarkably low temperatures (30–60 °C), with excellent selectivity to the lactate (Table 1).
3.2 Effect of Base on Catalyst Performance and Investigation of Base-Free Conditions
To investigate the effect of the counter cation for the hydroxide base, experiments were carried out on the oxidation of 1,2-propanediol with the 1 wt% Au–Pt/carbon catalyst (Au:Pt = 1:1). The bases investigated were LiOH, NaOH, KOH, RbOH and CsOH and the results are presented in Table 2. The conversion of 1,2-propanediol decreased as the size of the alkali metal ion increased, indicating that LiOH, NaOH and KOH are better choices of base for this reaction.
Conversions are considerably lower under these mild conditions in the absence of base (Table 3). Evidently, this is partly compensated for when the catalyst is supported on basic MgO, in which case hydroxyacetone was the major product. We consider that the base activates the primary alcohol group by the removal of a proton thereby enhancing the reactivity for subsequent oxidation. These preliminary observations were further exemplified by a more thorough screening of oxidations of 1,2-propanediol in water using carbon-supported catalysts in the absence of base at the relatively low temperature of 40 °C (Table 4). Again, in all cases, conversions were lower than those obtained in the presence of base but under otherwise very similar conditions (cf. Table 1). Using these conditions the AuPt catalyst was the most active and the trend in activity observed for the carbon-supported nanoparticles was AuPt > PdPt > AuPd (Table 4). To our surprise, and in a complete departure from previous results, the major product was now hydroxyacetone, along with substantial selectivities to lactate, particularly for the supported AuPd catalyst that we have extensively studied previously for the oxidation of other alcohols [20]. This trend was continued when more vigorous conditions were used (Tables 5, 6, 7, 8). Unsurprisingly, at these elevated temperatures, the overall conversions were higher, but notably only when mixed metal nanoparticulate catalysts were used. However, once again, the major or equivalent product to lactate was hydroxyacetone at 100 °C, which was accompanied by an increase in undesired acetate formation; pyruvate was also detected in smaller amounts. In addition trace levels of methyl glyoxal was also detected which was also considered to be an intermediate in the formation of pyruvate, and under our conditions methyl glyoxal would be rapidly transformed to pyruvate. Under even more vigorous conditions and extended reaction times, acetate became the major product (Table 7), clearly demonstrating that, as expected, it is a degradation product. The use of higher oxygen pressures in an autoclave at these higher temperatures did not seem to offer any significant advantage (Tables 8, 6).
3.3 Comments on the Formation of Products
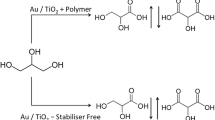
A possible explanation for the observed outcomes of these reactions is presented in Scheme 1. In the presence of base, the oxidation follows a well-established pattern of highly selective oxidation of the primary alcohol group, to the exclusion of any reaction at the secondary hydroxy site. This is in agreement with the relative acidity of the two hydroxyl group, as the primary hydroxyl being about ten times more acidic than the secondary hydroxyl group and hence deprotonation of the primary hydroxyl group is an important step facilitating oxidation. Therefore, it can be expected that in base conditions the primary hydroxyl will be more reactive to oxidation. The initial product should be 2-hydroxypropanal (see Scheme 1); despite many attempts, we have never observed the presence of this aldehyde and therefore assume that it is either very rapidly oxidized to lactate or that it is never released from the catalyst surface prior to the second oxidation step.
We were surprised to find that hydroxyacetone was formed as a major product during the base-free reactions. As a product of a single oxidation, it is perhaps obvious that it originates from selective oxidation at the secondary alcohol site of the propanediol. Such a substantial loss of regioselectivity has not been observed before in this type of catalysed oxidation [20, 21], so we are somewhat dubious about this as the explanation. An alternative explanation is that the initial 2-hydroxypropanal rearranges to the observed hydroxyacetone via an intermediate enediol. This interchange has been inferred previously [22, 23]; ironically, this rearrangement can easily be triggered by an acid but also by a base—exactly the reagent that is missing when the unexpected hydroxyacetone formation takes place in the present work. It would therefore seem that the equilibration must be triggered on the catalyst surface.
The remaining products formed under the base-free conditions are perhaps more easily understood. Hydroxyacetone would be expected to be oxidized readily to pyruvate, as this would involve reaction at a primary alcohol site. Subsequent oxidative cleavage of the sensitive ketoacid group in the pyruvate would then lead to the observed acetic acid and to formic acid, which is noticeable by its absence from most runs. This can be understood by its facile further oxidation to carbon dioxide, especially in experiments run for extended periods.
4 Conclusions
The use of AuPt supported on activated carbon prepared using a sol-immobilisation method is observed to enhance the selective oxidation of 1,2-propanediol significantly under mild reaction conditions. The alloying of gold with platinum leads to a catalyst which is significantly more active than gold alloyed with palladium or palladium alloyed with platinum under the reported conditions. In the presence of base lactate is formed in high selectivity. In the absence of base the reactivity of the catalysts is much lower and now hydroxyacetone is formed as the major product together with lactate. It is considered that the hydroxyacetone arises through a rearrangement of 2-hydroxypropanal via an enediol intermediate.
References
Miyazawa T, Kasunoki Y, Kunimori K, Tomisihige K (2006) J Catal 240:213–221
Tsujino T, Ohigashi T, Sugiyama S, Kawashiro K, Hayashi H (1992) J Mol Catal 71:25–35
Pinxt HHCM, Kuster BFM, Marnin GB (2000) Appl Catal A 191:45–54
Prati L, Rossi M (1998) J Catal 176:552–560
Biella S, Prati L, Rossi M (2002) J Catal 206:242–247
Porta F, Prati L (2004) J Catal 224:397–403
Landon P, Collier PJ, Papworth AJ, Kiely CJ, Hutchings GJ (2002) Chem Commun 18:2058–2059
Landon P, Collier PJ, Carley AF, Chadwick D, Papworth AJ, Burrows A, Kiely CJ, Hutchings GJ (2003) Phys Chem Chem Phys 5:1917–1923
Solsona BE, Edwards JK, Landon P, Carley AF, Herzing A, Kiely CJ, Hutchings GJ (2006) Chem Mater 18:2689–2695
Edwards JK, Solsona B, Landon P, Carley AF, Herzing A, Watanabe M, Kiely CJ, Hutchings GJ (2005) J Mater Chem 15:4595–4600
Edwards JK, Solsona BE, Landon P, Carley AF, Herzing A, Kiely CJ, Hutchings GJ (2005) J Catal 236:69–79
Edwards JK, Carley AF, Herzing AA, Kiely CJ, Hutchings GJ (2008) Faraday Discuss 138:225–229
Dimitratos N, Lopez-Sanchez JA, Morgan D, Carley A, Prati L, Hutchings GJ (2007) Catal Today 122:317–324
Lopez-Sanchez JA, Dimitratos N, Miedziak P, Ntainjua E, Edwards JK, Morgan D, Carley AF, Triuvalam R, Kiely CJ, Hutchings GJ (2008) Phys Chem Chem Phys 10:1921–1930
Wang T, Shou H, Kou Y, Liu H (2009) Green Chem 11:562–568
Liang D, Gao J, Sun H, Chen P, Hou Z, Zheng X (2011) Appl Catal B 106:423–432
Villa A, Veith GM, Prati L (2010) Angew Chem Int Ed 49:4499–4502
Brett GL, He Q, Hammond C, Miedziak PJ, Dimitratos N, Sankar M, Herzing AA, Conte M, Lopez-Sanchez JA, Kiely CJ, Knight DW, Taylor SH, Hutchings GJ (2011) Angew Chem Int Ed. doi:10.1002/anie.201101772
Dimitratos N, Lopez-Sanchez JA, Meenakshisundaram S, Anthonykutty JM, Brett G, Carley AF, Taylor SH, Knight DW, Hutchings GJ (2009) Green Chem 11:1209–1216
Enache DI, Edwards JK, Landon P, Solsona-Espriu B, Carley AF, Herzing AA, Watanabe M, Kiely CJ, Knight DW, Hutchings GJ (2006) Science 311:362–365
Pritchard J, Kesavan L, Piccinini M, He QA, Tiruvalam R, Dimitratos N, Lopez-Sanchez JA, Carley AF, Edwards JK, Kiely CJ, Hutchings GJ (2010) Langmuir 26:16568–16577
Paquette LA, Hofferberth JE (2003) Org React 62:477–567
Hartung RE, Hilmey DG, Paquette LA (2004) Adv Synth Catal 346:713–716
Acknowledgments
This work formed part of the Glycerol Challenge and Sasol Technology (UK) Ltd and the Technology Strategy Board are thanked for their financial support. This project is co-funded by the Technology Strategy Board’s Collaborative Research and Development programme, following an open competition. The Technology Strategy Board is an executive body established by the Government to drive innovation. It promotes and invests in research, development and the exploitation of science, technology and new ideas for the benefit of business—increasing sustainable economic growth in the UK and improving quality of life. For more information visit www.innovateuk.org.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ryabenkova, Y., Miedziak, P.J., Dummer, N.F. et al. The Selective Oxidation of 1,2-Propanediol by Supported Gold-Based Nanoparticulate Catalysts. Top Catal 55, 1283–1288 (2012). https://doi.org/10.1007/s11244-012-9909-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-012-9909-9