Abstract
The effect of the binders, such as silica, alumina, and aluminum phosphate solution (APS), was studied on the acidity and catalytic performance of HZSM-5 zeolite (SiO2/Al2O3 = 80) in methanol-to-propylene (MTP) process. The strong acidity of catalyst increased slightly with alumina binder but decreased with silica and APS binders. It is noted that the catalyst with APS binder showed the highest bulk crush strength. Catalytic performance of the alumina or silica bound catalyst was comparable to that of pure HZSM-5 catalyst while that of APS bound catalyst was completely different depending on the binder content. Low content (10 wt%) of APS resulted in a dramatically enhanced propylene selectivity (>40 C mol%) due to the decrease in strong acidity. On the other hand with high content (>20 wt%) of APS, methanol was mostly dehydrated into dimethylether without further transformation into hydrocarbons even if the mechanical strength was significantly improved. The binary binder system was proposed not only to improve the mechanical strength of catalyst with small amount of APS binder but also to keep a high propylene selectivity. The catalyst bound with APS-and-alumina or APS-and-silica showed a comparable propylene selectivity to that of HZSM-5 with the 10 wt% of APS single binder while demonstrated much higher bulk crush strengths.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Propylene has been used as feedstock for the production of polypropylene and its derivatives such as acrylonitrile, oxo alcohols, propylene oxide and cumene in the petrochemical industry. Most of the propylene, however, is produced as a by-product of ethylene production by the naphtha steam cracking and of gasoline production by fluid catalytic cracking (FCC). Due to the growing demand for propylene and the shortage of petroleum resources in the future, new processes with high yield of propylene are required [1].
The methanol-to-propylene (MTP) process has been highlighted in the past few years as an attractive alternative method for propylene production, since methanol could be simply produced from natural gas, coal, and biomass. The zeotypes or acidic zeolites, such as HSAPO-34 and HZSM-5, have been used as catalysts in the MTO and/or MTP processes [1–8]. The important factors influencing olefin selectivity in methanol conversion are the temperature of the reaction and the acidic property of catalyst. It was reported that the propylene selectivity was enhanced by a cooperative effect of increased reaction temperature and increased SiO2/Al2O3 ratio of HZSM-5 [3], and improved with decreasing HZSM-5 crystal size [4]. Propylene selectivity and the propylene/ethylene ratio (P/E) could be improved by modifying HZSM-5 catalyst through the ZrO2 and H3PO4 addition [5] and alkaline treatment [1]. The decrease of the partial pressure of methanol by use of excessive carrier gas or feed with low methanol/water ratio leads to a higher yield of light olefins [2, 6].
Zeotype materials and acidic zeolites need to be bound with binder to produce a desired physical shape and mechanical strength for industrial applications because of their poor self-binding property. However, binders can affect the physicochemical properties of catalyst and these changes can have a strong influence on the catalytic activity and selectivity [9–14].
In this work, silica, alumina and aluminum phosphate solution (APS) were selected as binders and their effects on the acidic property, the mechanical strength and catalytic performance of HZSM-5 zeolite for MTP reaction were investigated in the single and also binary binder system.
2 Experimental
2.1 Preparation of Catalysts
2.1.1 Single Binder
Each of Ludox HS-40 (silica source), pseudo-boehmite(alumina source) or aluminum phosphate solution (APS) was mixed with distilled water. After vigorous stirring for 1 h, HZSM-5 (Zeolyst, SiO2/Al2O3 = 80) was added into the mixture of the single binder and distilled water. These samples were dried at 100 °C in a static air oven overnight and then calcined at 550 °C for 3 h.
2.1.2 Binary Binder
Binary binder system consists of two different binders among the three binders (Ludox HS-40, pseudo-boehmite and APS). Two different binders were mixed together with distilled water. After vigorous stirring for 1 h, HZSM-5 (Zeolyst, SiO2/Al2O3 = 80) was added into the mixture. These mixtures were dried at 100 °C for 10 h and then calcined at 550 °C for 3 h.
2.1.3 Extrudates
HZSM-5, distilled water, and binder were mixed with hydroxyethylcellulose (HEC), and the mixture was kneaded. Then, the dough was extruded into cylindrical shape using a hand extruder for the mechanical strength test. The extrudates were dried at 100 °C in convection oven overnight and then calcined at 550 °C for 3 h.
The prepared catalysts are designated as Z(XY) in the single binder system, and Z(XY, XY) in the binary binder system. X is the kind of binder, where A, S and P represent alumina, silica and APS, respectively, and Y is the wt% of the binder.
2.2 Catalysts Characterization
Powder X-ray diffraction (XRD) patterns were obtained with a Rigaku D/MAX-III diffractometer using CuKα radiation (λ = 1.54173 Å). Data were collected in a continuous scan mode from 5° to 50° of 2θ with a 0.01° sampling and 3°/min scan rate for the confirmation of crystal structure of the prepared catalysts.
BET surface area and pore volume of the prepared catalysts were measured by N2 adsorption/desorption using ASAP2010 (Micromeritics Inc.). The samples were degassed at 150 °C, and N2 adsorption was carried out at −196 °C.
Temperature-programmed desorption of NH3 (TPD) for the prepared catalysts was carried out with a conventional flow apparatus (Pulsechemisorb 2705, Micromeritics Inc.). A 0.05 g of sample was loaded in the U-type tube. Before adsorption the samples were degassed at 500 °C with He flow of 20 mL/min for 2 h. After cooling to 100 °C, ammonia was adsorbed by pulse injection. The temperature of the samples was raised by the rate of 5 °C/min from 100 °C to 600 °C, and the TCD signal was recorded.
The bulk crush strength of the prepared catalysts was measured with a universal testing machine (INSTRON 4206, Instron Corporation) by following the standard test method of ASTM international [15].
2.3 Catalytic Conversion of Methanol
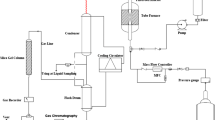
The methanol conversion was carried out in a fixed bed reactor at 450 °C under atmospheric pressure. Prior to each reaction, the samples (0.5 g) were pretreated in He flow at 550 °C for 2 h and cooled to a reaction temperature. Methanol (Sigma-Aldrich, ≥99.9%) was fed into a reactor by a liquid mass flow controller (Bronkhorst High-Tech, LIQUID-FLOW series L1) and the weight hourly space velocity (WHSV) was 2.55 h−1. A homogeneous mixture of MeOH (10%) and He (90%) was achieved by using a pre-heater to vaporize the methanol. All products were passed through a heated transfer line to a gas chromatograph with a thermal conductivity detector and a flame ionization detector (column: HP-PLOT Q, Agilent) in series.
3 Results and Discussion
3.1 Single Binder System
The XRD patterns of the catalysts bound with a single binder are shown in Fig. 1, being similar to that of the typical HZSM-5 catalyst. It is also confirmed with binary binders that the HZSM-5 crystal structure was maintained with various binders introduced.
The physical and acidic properties of the prepared catalysts were affected by binders. Table 1 summarizes the physical properties of the catalysts bound with various binders. All of the prepared catalysts have a smaller BET surface area and micropore volume than pure HZSM-5, and BET surface area and micropore volume of the prepared catalyst decreased with increasing binder content. Compared with HZSM-5, mesopore volume of alumina or silica bound catalysts is larger while that of APS bound catalysts is smaller. This result is well matched with the previous reports [16–18]. Z(P20) catalyst showed the smallest BET surface area, micropore volume and mesopore volume with acid sites sacrificed, but the highest bulk crush strength (471 KPa). The prepared catalysts showed significant difference in bulk crush strength due to the nature of the binder. After agglomeration with a single binder, catalyst bound with APS showed the highest bulk crush strength while catalyst bound with silica showed the lowest mechanical strength. The mechanical strength of catalysts with conventional binders like alumina or silica evolves through adhesive forces and cross-linking of terminal hydroxyl groups between neighboring binder particles. The matrix formed by conventional binders represents a network of somewhat strongly interconnected particles with void in-between. However, it was reported that the dense and continuous matrix is formed due to the viscous sintering when APS is used as a binder [17].
NH3-TPD profiles and results of the catalysts bound with a single binder are shown in Fig. 2 and summarized in Table 2. Generally, the NH3-TPD profile of HZSM-5 zeolite shows two peaks: a low temperature peak at around 150 °C (weak acid sites) and a high temperature peak at around 350 °C (strong acid sites). Even if the NH3-TPD spectra can not distinguish between Brønsted and Lewis acidity, the low temperature peak is assigned to ammonia desorbed from weak acid Lewis sites (non-framework Al species, 3-fold bound Al in the framework, and ammonia additionally co-ordinated to ammonium ions located on Brønsted sites) and the high temperature peak is ascribed to desorption from the more strongly acidic Brønsted sites of the bridged OH groups [19]. The strong acidity of catalyst with alumina binder increased slightly due to acidic nature of alumina but decreased in the case of silica and APS in single binder system. The decrease in the strong acidity of the silica bound catalyst was induced by neutralization with sodium cations in Ludox HS-40 (40 wt% SiO2, 0.41 wt% Na2O and 59.5 wt% H2O) [10]. Especially, APS binder showed more drastic decrease in strong acidity than silica due to the phosphorous compounds (H2PO4 −) in the APS [17, 20]. The strength of strong acids is reduced for all the prepared catalysts.
Methanol conversions as well as selectivities (product distribution) of the catalysts bound with a single binder are presented in Table 3. For the pure HZSM-5 (SiO2/Al2O3 = 80), selectivity to C1–C4 saturated hydrocarbon, ethylene, propylene and butylene was 17.9, 10.5, 23.8 and 19.6 C mol%, respectively. Compared with pure HZSM-5, the propylene selectivity of the Z(A20) catalyst remained almost the same while the C1–C4 saturated hydrocarbon selectivity increased and the butylene selectivity decreased. The changes in product distribution must be due to the increased acidity of the Z(A20) catalysts. The product distribution in methanol conversion of the silica bound catalysts (Z(S10), Z(S20)) was not changed significantly compared with pure HZSM-5. For the Z(P10) catalyst, the propylene and butylene selectivity dramatically increased to 41.9 and 30.6 C mol%, respectively, while the C1–C4 saturated hydrocarbon decreased to 9.5 C mol% due to the decrease in strong acidity as well as the weakening of strong acid strength. Moreover, mechanical strength of the Z(P10) catalyst showed the highest bulk crush strength among the 10 wt% of binder bound catalysts. The Z(P20) catalyst showed even much higher bulk crush strength of 471 KPa, but it showed the lowest methanol conversion of 82.5% where methanol was converted only into dimethylether (DME) obviously due to elimination of the strong acid sites of Z(P20) catalyst by the excess amount of phosphorous compounds in APS.
The well-known methanol conversion consists of three main reaction steps. Methanol is dehydrated to dimethylether and the equilibrium mixture formed, consisting of methanol, dimethylether and water, undergoes further dehydration to produce light olefins. The subsequent conversion of light olefins to paraffins, aromatics, naphthenes and higher olefins occurs. The propylene selectivity is affected by the Brønsted acidity of catalyst and there is an optimum Brønsted acidity of catalyst for the high propylene selectivity. In the case of HZSM-5 without Brønsted acidity, methanol was not converted to hydrocarbon and main product was dimethylether [16]. The HZSM-5 bound with the proper amount of APS (around 10 wt%) must show the improved propylene selectivity compared with the pure HZSM-5, but HZSM-5 bound with more than 20 wt% APS showed only the dehydration activity of methanol to DME without further transformation into hydrocarbons.
The binary binder system can be proposed not only to improve the mechanical strength of catalyst which was bound with small amount of APS binder but also to keep a high propylene selectivity. To clarify the influence of the amount of the APS on acidity, catalysts bound with different amount of APS are prepared and NH3-TPD results are shown in Fig. 3 and summarized in Table 4. The acidity and strength of strong acids decreased with increasing APS content which was caused by the increased amount of phosphorous compounds in APS.
3.2 Binary Binder System
The catalysts bound with binary binders were prepared and XRD patterns are shown in Fig. 4. The X-ray diffraction patterns of the prepared catalysts show that the HZSM-5 structure was not changed after binding with binary binders.
The physical properties of the catalysts bound with binary binders are summarized in Table 5. The Z(A10, S10) catalyst showed the BET surface area of 426 m2/g and the micropore volume of 0.10 cm3/g. The catalysts bound with APS-and-alumina and APS-and-silica have the BET surface area of around 340 m2/g and the micropore volume of about 0.09 cm3/g which is similar to those of Z(P10) catalyst. As mentioned before, the catalysts with APS binder showed larger decrease in surface area, micropore volume and mesopore volume than others due to viscous sintering. The catalysts bound with APS-and-alumina and APS-and-silica showed the bulk crush strength of more than 300 KPa while the Z(A10, S10) catalyst presented the lowest bulk crush strength of 276 KPa. These results can be attributed to the dense and continuous matrix formed by viscous sintering when APS is used and Z(P15, A5) catalyst showed the highest bulk crush strength of 434 KPa.
NH3-TPD profiles and results of the catalysts bound with binary binders are shown in Fig. 5 and summarized in Table 6. In the case of all samples, the acidity and strength of strong acid decreased. The catalysts bound with APS-and-alumina or APS-and-silica (containing APS binder) showed a larger decrease in the number and strength of strong acid sites than catalyst bound with alumina-and-silica because APS have a greater impact on the decrease in strong acidity than alumina or silica (Table 2). The catalyst bound with APS-and-silica showed higher degree of decrease in the acidity and strength of strong acid than that of catalyst bound with APS-and-alumina due to the sodium cations in silica source and the acidic nature of alumina.
Methanol conversions as well as selectivities of the catalysts bound with binary binders are presented in Table 7. All of the prepared catalysts show higher propylene selectivity and lower C1–C4 saturated hydrocarbon selectivity than that of pure HZSM-5. The changes in the product distribution must be due to the decrease in strong acidity and the weakening of strong acid strength of the catalysts bound with binary binders. However, the product distribution of the Z(A10, S10) catalyst was not much different from that of pure HZSM-5, and the propylene selectivity of the Z(A10, S10) catalyst was the 25.6 C mol% which is even lower than others. The Z(P15, A5) and Z(P10, S10) catalysts showed the propylene selectivity of 38.8 and 39.1 C mol%, respectively. The propylene selectivity of the Z(P15, A5) or Z(P10, S10) catalysts is similar to that of the Z(P10) catalyst (Table 3) while the bulk crush strength is much higher. It was proven that the mechanical strength was improved while maintaining the high propylene selectivity in the APS-and-alumina and APS-and-silica binary binder system.
4 Conclusion
The effect of the binders on the acidity, mechanical strength and catalytic performance of HZSM-5 zeolite in MTP process was studied. The HZSM-5 bound with the 10 wt% of APS showed a dramatic increase in the propylene selectivity (41.9 C mol%) due to the decrease in strong acidity as well as due to the weakening of strong acid strength. With larger amount of APS (>20 wt%), the mechanical strength was improved significantly, while methanol was mostly dehydrated into dimethylether without further transformation into hydrocarbons. The binary binder system was proposed to improve the mechanical strength of catalyst which was bound with small amount of APS but showed a high propylene selectivity. The catalyst bound with APS-and-alumina or APS-and-silica showed a comparable propylene selectivity to that of HZSM-5 with the 10 wt% of APS single binder while demonstrated much higher bulk crush strengths. The APS-and-alumina or APS-and-silica was proposed as a good candidate for a binary binder system.
References
Mei C, Wen P, Liu Z, Liu H, Wang Y, Yang W, Xie Z, Hua W, Gao Z (2008) J Catal 258:243
Stöcker M (1999) Micropor Mesopor Mater 29:3
Chang CD, Chu CT-W, Socha RF (1984) J Catal 86:289
Prinz D, Riekert L (1988) Appl Catal 37:139
Zhao TS, Takemoto T, Tsubaki N (2006) Catal Commun 7:647
Marchi AJ, Froment GF (1993) Appl Catal 94:91
Haw JF, Marcus DM (2005) Top Catal 34:41
Bleken F, Bjorgen M, Palumbo L, Bordiga S, Svelle S, Lillerud KP, Olsbye U (2009) Top Catal 52:218
Choudhary VR, Devadas P, Kinage AK, Guisnet M (1997) Appl Catal A. 162:223
Wu X, Alkhawaldeh A, Anthony RG (2002) Stud Surf Sci Catal 143:217
Dorado F, Romero R, Canizares P (2002) Appl Catal A 236:235
Zhang Y, Zhou Y, Qiu A, Wang Y, Xu Y, Wu P (2006) Ind Eng Chem Res 45:2213
Lucas AD, Sanchez P, Funez A, Ramos MJ, Valverde JL (2006) J Mol Catal A 259:259
Sanchez P, Dorado F, Funez A, Jimenez V, Ramos MJ, Valverde JL (2007) J Mol Catal A 273:109
Designation: D 7084-04, ASTM international
Kim SD, Baek SC, Lee YJ, Jun KW, Kim MJ, Yoo IS (2006) Appl Catal A 309:139
Freiding J, Patcas FC, Czarmetzki BK (2007) Appl Catal A 328:210
Khattaf SA, Pavlackova ZM, Ali MA, Cejka J (2009) Top Catal 52:140
Martin A, Berndt H (1994) React Kinet Catal Lett 52:405
Vinek H, Rumplmayr G, Lercher JA (1989) J Catal 115:291
Acknowledgements
This work was supported by the Next-Generation Novel Technology Development project and the Brain Korea 21 project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, KY., Lee, HK. & Ihm, SK. Influence of Catalyst Binders on the Acidity and Catalytic Performance of HZSM-5 Zeolites for Methanol-to-Propylene (MTP) Process: Single and Binary Binder System. Top Catal 53, 247–253 (2010). https://doi.org/10.1007/s11244-009-9412-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-009-9412-0