Abstract
Formation of carbon nanofibers (CNFs) and carbon nanotubes (CNTs) through the decomposition of ethylene at 973 K was achieved using various metal catalysts covered with silica layers. CNFs of various diameters were formed by ethylene decomposition over a Co metal catalyst supported on the outer surface of the silica. In contrast, silica-coated Co catalysts formed CNTs with uniform diameters by ethylene decomposition. Silica-coated Ni/SiO2 and Pt/carbon black also formed CNTs with uniform diameters, while CNFs and CNTs with various diameters were formed over Ni/SiO2 and Pt/carbon black without a silica coating. These results indicate that silica layers that envelop metal particles prevent sintering of the metal particles during ethylene decomposition. This results in the preferential formation of CNTs with a uniform diameter.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Carbon nanotubes (CNTs) and carbon nanofibers (CNFs) have attracted a great deal of interest because they have remarkable and unique magnetic, electronic, chemical and mechanical properties. Catalytic decomposition of hydrocarbons (CCVD, catalytic chemical vapor deposition) is a promising method for the production of CNTs and CNFs. Supported transition-metal catalysts such as Ni and Co are frequently utilized for the production of CNTs and CNFs through the decomposition of hydrocarbons [1, 2]. The properties of these nano-scaled carbons are strongly related to their diameters. Therefore, CNTs and CNFs with uniform diameters are required for the use of these carbon materials in applications such as catalysis and sensing. In hydrocarbon decomposition over supported metal catalysts, the metal particles decompose hydrocarbon molecules and deposit carbon atoms on the metal surface. The carbon atoms then diffuse throughout the surface and within the bodies of the metal particles, resulting in the growth of CNTs and CNFs [3, 4]. The diameters of CNTs and CNFs formed from the metal catalysts are similar to that of the metal particles [5]. Therefore, the metal particles in the catalysts must be uniform in size to form CNTs and CNFs with uniform diameters [6]. However, metal nanoparticles in conventional supported metal catalysts are easily aggregated during hydrocarbon decomposition, since the reactions are generally carried out at temperatures higher than 900 K [7]. Supported metal catalysts with good tolerance for sintering at high temperatures should thus be investigated for the preferential formation of CNTs and CNFs with uniform diameters.
We have prepared metal nanoparticle catalysts covered with silica layers using a microemulsion [8–10]. This method of preparation allows metal particles, such as Ni, Pt and Rh of a few nanometers in diameter to be uniformly covered with silica layers. In addition, we recently reported that metal nanoparticles supported on carriers were covered with silica layers of a few nanometers thickness by hydrolysis of silicon alkoxides such as tetraethoxysilane (TEOS) [11]. We demonstrated that the metal particles in these silica-coated catalysts showed good tolerance for sintering, even at high temperatures, because the metal nanoparticles were covered with silica layers. These silica-coated metal catalysts are thus expected to be effective for the preferential formation of CNFs and CNTs with uniform diameters through hydrocarbon decomposition.
In the present study, silica-coated metal nanoparticles were used as catalysts for ethylene decomposition to form CNTs and CNFs. We thus demonstrate that silica-coated metal nanoparticles form CNFs and CNTs with a uniform diameter.
2 Experimental
2.1 Preparation of Silica-Coated Co Catalysts Using a Microemulsion
Silica-coated Co catalysts (coat-Co) were prepared by using a water-in-oil microemulsion. The microemulsion system was prepared by adding aqueous Co(NO3)2 into a surfactant solution in cyclohexane. Polyoxyethylene (n = 5) nonylphenyl ether (NP5) was used as the surfactant. Nanoparticles containing Co cations were synthesized by addition of aqueous NH3 into the microemulsion system. Hydrolysis and polycondensation of tetraethoxysilane (TEOS) were done at 323 K by addition of TEOS and aqueous NH3 to the microemulsion system. The precipitates obtained were washed several times with isopropanol after filtration and then calcined at 773 K for 2 h under an air stream. A silica-supported Co catalyst (Co/SiO2) was prepared by a conventional impregnation method for comparison. The silica supports (BET surface area = 42 m2 g−1) were prepared in a similar manner to that of coat-Co. These Co catalysts were reduced with hydrogen at 773 K for 1 h prior to ethylene decomposition.
2.2 Coverage of Supported Metal Catalysts with Silica
A silica-supported Ni catalyst (Ni/SiO2) and carbon black-supported Pt catalyst (Pt/CB) were covered with silica layers. The surface area of silica and CB was estimated to be 42 and 195 m2 g−1, respectively. Ni(3.4 wt%)/SiO2 and Pt(10 wt%)/CB were prepared by a conventional impregnation method. Coverage of Ni/SiO2 with silica was done using hydrolysis of TEOS. Hydrolysis and polycondensation of TEOS were done at 323 K by addition of TEOS and aqueous NH3 to an ethanol solution containing Ni/SiO2. The obtained precipitates were calcined at 623 K under an air stream, followed by reduction with hydrogen at 773 K. Silica-coated Ni/SiO2 is denoted as coat-Ni/SiO2 hereafter.
The coverage of Pt/CB with silica layers was done using successive hydrolysis of 3-aminopropyl-triethoxysilane (APTES) and TEOS [11]. Pt/CB was dispersed into a mixed solution of ethanol and water after which triethylamine and APTES were added at 333 K. Hydrolysis of TEOS was then carried out by addition of TEOS to this solution. The sample was then dried at 333 K in air, followed by treatment with hydrogen at 623 K for 3 h. The silica-coated Pt/CB (coat-Pt/CB) was reduced with hydrogen at 673 K prior to ethylene decomposition. The reduction temperature for Pt/CB with hydrogen was lower than that for coat-Ni/SiO2 and coat-Co, since the reduction of Pt/CB at high temperature resulted in the hydrogenation of CB supports.
2.3 Formation of CNFs and CNTs Through Ethylene Decomposition
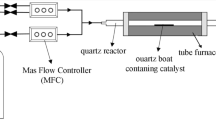
Decomposition of ethylene over the metal catalysts was done using a conventional gas flow system with a fixed catalyst bed at atmospheric pressure. Before ethylene decomposition, the catalysts were treated with hydrogen at the required temperatures. The temperature was increased to 973 K under an Ar stream after the remaining hydrogen in the reactor was purged with Ar. The formation of CNFs and CNTs was accomplished by contact of the reactant gas (pure ethylene or a mixed gas of ethylene and hydrogen) with the catalysts.
TEM images of the catalysts before and after ethylene decomposition were obtained with a JEOL JEM-3000F. The catalyst samples were dispersed ultrasonically in 2-propanol. An aliquot of the solution was dropped onto a grid for the measurement of the TEM images.
3 Results and Discussion
3.1 Catalytic Performance of Silica-Coated Co Catalysts
Figure 1 shows the TEM images of fresh Co/SiO2 and coat-Co catalysts. These catalysts were reduced with hydrogen at 773 K prior to obtaining the TEM images. In the TEM image of fresh Co/SiO2, spherical silica particles with diameters of 20–30 nm were observed and darker spots were observed as well. The darker spots in this TEM image are assigned to Co metal particles, as described below. Co metal particles in fresh Co/SiO2 seemed to be present on the outer surfaces of the silica particles but the shape of the Co metal particles could not be clearly seen in this TEM image. Spherical particles with a diameter of ca. 20 nm are also present in the TEM image of fresh coat-Co and no darker spots were observed. The loading of Co was estimated by X-ray fluorescence (XRF) spectra to be 5.0 wt% for Co/SiO2 and 5.4 wt% for coat-Co. The Co species in fresh coat-Co would thus have to be highly dispersed in the silica.
Figure 2 shows TEM images of Co/SiO2 and coat-Co after ethylene decomposition at 973 K. The carbon yield in ethylene decomposition for 1 h of time on stream was estimated to be 29 mol-C/mol-Co for Co/SiO2 and 18 mol-C/mol-Co for coat-Co. The carbon yield was less for coat-Co due to the coverage of Co catalysts with silica. Some Co species that are covered with silica can not grow CNTs through ethylene decomposition. In the TEM images of Co/SiO2 after ethylene decomposition, fibrous carbons and aggregated metal particles were observed [12]. The diameters of these fibrous carbons ranged from 20 to 50 nm. The fibrous carbons seemed to have a hollow structure but the hollow structure was not complete as shown in Fig. 2b. In addition, Co metal particles were observed at the tip or within the body of the fibrous carbons. The Co metal particles decomposed ethylene to grow the fibrous carbons. The coat-Co decomposed ethylene to form multi-walled CNTs as observed in TEM images (c) and (d). The diameters of the CNTs formed on coat-Co were smaller and more uniform than the diameters of fibrous carbons formed on Co/SiO2. The graphite layers of the CNTs on coat-Co were parallel to the axes of the tubes, whereas the graphite layers of the fibrous carbons on Co/SiO2 were highly disordered. These results indicate that coat-Co can form CNTs with a uniform diameter through ethylene decomposition [6, 13, 14]. The darker spots, which implied the presence of Co metal particles, are seen in the TEM image (c) of coat-Co after ethylene decomposition, while the aggregated Co species are not seen in the TEM image of fresh coat-Co. These results suggest that the structure of the Co species in coat-Co was changed during ethylene decomposition. The structure of the Co species in fresh and used coat-Co catalysts was examined by Co K-edge XANES spectra. The results are shown in Fig. 3. The Co K-edge XANES spectrum of fresh Co/SiO2 was very similar to that recorded for Co foil, suggesting that the Co species in fresh Co/SiO2 were present as Co metal. The XANES spectrum of Co/SiO2 did not change appreciably after the growth of fibrous carbons by ethylene decomposition at 973 K. Therefore, the Co metal in Co/SiO2 catalyzed ethylene decomposition to form fibrous carbons. In contrast, the XANES spectrum of fresh coat-Co was similar to the XANES spectrum of Co3O4, rather than that of Co foil, i.e., peaks which are characteristic of Co3O4 were observed at about 7710 and 7725 eV in the XANES spectrum of fresh coat-Co catalyst. This result strongly suggests that most Co species in fresh coat-Co exist in the oxidized state. Contact between ethylene and fresh coat-Co at 973 K changed the features of the XANES spectra. A shoulder peak at 7710 eV which is characteristic of Co foil appeared and the intensity of the peak at 7725 eV, due to Co oxides, decreased after contact between ethylene and coat-Co. The oxidized Co species in fresh coat-Co were thus reduced with hydrogen and/or carbon atoms deposited on Co species to Co metal particles during ethylene decomposition at 973 K. These metal particles worked as catalytically active sites for the growth of CNTs with uniform diameters. The Co metal particles in coat-Co do not seriously aggregate during ethylene decomposition as they are covered with silica. The coat-Co can therefore form CNTs with small and uniform diameters by ethylene decomposition.
The coat-Co catalysts produced single or double walled CNTs through methane decomposition at 1073 K. The TEM images of coat-Co after methane decomposition are shown in Fig. 4. The shape of the fibrous carbons formed by methane decomposition over coat-Co was significantly different from that of CNTs formed by ethylene decomposition, as shown in Fig. 2. The high resolution TEM image in Fig. 4b strongly suggests that bundles of single or double-walled CNTs are formed during methane decomposition over coat-Co [15–17]. From the results described above, we concluded that the coat-Co catalysts prepared using microemulsion were effective for the formation of CNTs with uniform diameters by hydrocarbon decomposition.
3.2 Catalytic Performance of Ni/SiO2 and Pt/CB Covered with Silica
Ni/SiO2 and Pt/CB were uniformly covered with silica layers to suppress the sintering of metal particles in these catalysts during ethylene decomposition. Figure 5 shows TEM images of fresh and used Ni/SiO2 catalysts which were not covered with silica. Many darker spots were observed in the TEM image of fresh Ni/SiO2. The XRD of fresh Ni/SiO2 showed the presence of crystallized Ni metal in the catalyst (the result is not shown). The darker spots in the TEM image of fresh Ni/SiO2 are thus assigned to Ni metal particles. The diameter of Ni metal particles in fresh Ni/SiO2 ranged from 3 to 10 nm. CNFs were observed in the TEM image for Ni/SiO2 after ethylene decomposition, and Ni metal particles were present at the tips of the CNFs. The Ni metal particles at the tips of the CNFs decomposed ethylene to form the CNFs. The CNFs were observed to have a wide distribution (5–50 nm) of diameters in the TEM image. The diameter distribution of Ni metal particles in fresh Ni/SiO2 is narrower than the diameter distribution of CNFs formed on the catalysts. Therefore, Ni metal particles in Ni/SiO2 severely aggregate during ethylene decomposition and the larger Ni metal particles result in the formation of CNFs with various diameters.
Figure 6 shows TEM images of the coat-Ni/SiO2 catalyst before and after ethylene decomposition. Spherical silica particles with diameters from 80 to 100 nm were observed in the TEM image (a) for fresh coat-Ni/SiO2, whereas the diameters of silica particles in Ni/SiO2 before the silica-coating ranged from 20 to 30 nm as shown in Fig. 5a. Ni metal particles could not be found in the TEM image of the fresh coat-Ni/SiO2 catalyst. Many Ni metal particles were, however, supported on the silica particles in Ni/SiO2 before coverage with silica, as shown in Fig. 5a. These results strongly suggest that the Ni/SiO2 catalyst can be uniformly covered with silica using the hydrolysis of TEOS. This coat-Ni/SiO2 was used as a catalyst for ethylene decomposition. As shown in TEM image (b) of Fig. 6, coat-Ni/SiO2 also formed CNFs by ethylene decomposition despite the Ni metal particles being covered with thick silica layers. The diameters of the CNFs formed on coat-Ni/SiO2 were more uniform and smaller than the diameters of the CNFs formed on Ni/SiO2 without the silica coating. We concluded that the coat-Ni/SiO2 can form CNFs with uniform diameters because silica, which envelops the Ni metal particles, prevents aggregation of Ni metal particles during ethylene decomposition. The carbon yield in ethylene decomposition for 30 min of time on stream for the coat-Ni/SiO2 was significantly less (3 mol-C/mol-Ni) than that for the Ni/SiO2 (500 mol-C/mol-Ni). The TEM images in Fig. 6 show that the coat-Ni/SiO2 is covered with thick silica layers. Many Ni metal particles in coat-Ni/SiO2 may thus not grow CNFs during ethylene decomposition. We have previously reported that Ni metal particles with diameters from 40 to 100 nm show higher activity for hydrocarbon decomposition than Ni metal particles with diameters <40 nm [18]. These are reasons why the carbon yield for Ni/SiO2 decreases by the coverage with silica.
Pt/CB was also used as a catalyst for the growth of nano-scaled carbons through ethylene decomposition. CNTs and CNFs are usually modified with another phase to produce a highly functionalized composite. For example, CNT-supported Pt metal is used as an active electrocatalyst for the oxygen reduction reaction at the cathode in proton-exchange membrane fuel cells (PEMFCs) [19]. The CNTs and CNFs which are formed by hydrocarbon decomposition over the metal catalysts intrinsically contain metal particles, as shown previously. The CNTs and CNFs are thus used as highly functionalized composites without further modification [20]. Precious metals such as Pt show excellent chemical performance in catalytic and electrochemical reactions. The formation of nano-scaled carbons by hydrocarbon decomposition over precious metal catalysts is of interest for the preparation of CNT-precious metal composites. Only a few reports have described the formation of CNFs and CNTs by hydrocarbon decomposition over precious metal catalysts [21, 22].
Figure 7 shows the TEM images of fresh Pt/CB and fresh coat-Pt/CB catalysts. Pt metal particles with diameters from 1 to 5 nm were observed on the surface of CB in the TEM images of fresh Pt/CB. This Pt/CB catalyst was covered with silica layers using successive hydrolysis of APTES and TEOS. In the TEM images for fresh coat-Pt/CB catalysts, Pt metal particles with diameters from 1 to 5 nm were observed. The outer surface of coat-Pt/CB seems to be covered with thin silica layers. The content of Pt, CB and SiO2 in coat-Pt/CB was estimated by XRF spectra and thermogravimetric analysis under an air stream to be 9.1, 68.4 and 22.5 wt%, respectively. These results strongly suggest that Pt/CB can be covered with silica layers by the successive hydrolysis of APTES and TEOS.
Figure 8 shows TEM images of Pt/CB and coat-Pt/CB after ethylene decomposition. As shown in Fig. 8a, CNTs were formed by ethylene decomposition over Pt/CB. In addition, Pt metal particles were frequently observed at the tip of CNTs and their diameters were similar to that of the CNTs. Pt metal particles thus have catalytic activity in ethylene decomposition and form CNTs. The diameters of CNTs formed on Pt/CB ranged widely from 10 to 30 nm, while the diameter of Pt metal particles in fresh Pt/CB ranged from 1 to 5 nm as shown in Fig. 7a. These results indicate that Pt metal particles in Pt/CB are seriously aggregated during ethylene decomposition at 973 K, resulting in the formation of CNTs with various diameters. In contrast to Pt/CB, coat-Pt/CB formed CNTs with uniform diameters by ethylene decomposition as shown in Fig. 8b. The diameters of CNTs formed on coat-Pt/CB were more uniform and smaller than the diameters of CNTs formed on Pt/CB without silica coating. Pt metal particles could not be found at the tips or within the bodies of CNTs formed by coat-Pt/CB catalysts. The CNTs likely grow via a base-growth mechanism during ethylene decomposition over coat-Pt/CB, i.e., Pt metal particles are always present on the CB supports during ethylene decomposition. The carbon yield in ethylene decomposition for 90 min of time on stream was improved from 32 mol-C/mol-Pt to 97 mol-C/mol-Pt by covering Pt/CB with silica layers. It is likely that small Pt metal particles have higher activity for promoting the growth of CNTs in ethylene decomposition compared to larger ones. In fact, Pt metal particles with diameters larger than 30 nm in Pt/CB after ethylene decomposition had surfaces covered with thick carbon layers (the results are not shown). The carbon yield in ethylene decomposition over Pt/CB would therefore be improved by coverage with silica layers.
4 Conclusion
This study investigated the catalytic activity of silica-coated metal catalysts for the growth of CNFs and CNTs through ethylene decomposition. The coverage of metal catalysts with silica layers resulted in the preferential formation of CNFs and CNTs with uniform diameters. The inhibition of metal particle aggregation during ethylene decomposition at 973 K by silica layer coverage brought about the formation of CNTs and CNFs with uniform diameters.
References
Moisala A, Nasibulin AG, Kauppinen EI (2003) J Phys: Condens Matter 15:S3011
Dupuis A (2005) Prog Mater Sci 50:929
Baker RTK (1989) Carbon 27:315
Hofmann S, Sharma R, Ducati C, Du G, Mattevi C, Cepek C, Cantoro M, Pisana S, Parvez A, Cervantes-Sodi F, Ferrari AC, Dunin-Borkowski R, Lizzit S, Petaccia L, Goldoni A, Robertson J (2007) Nano Lett 7:602
Takenaka S, Ogihara H, Otsuka K (2002) J Catal 208:54
Han S, Yu T, Park J, Koo B, Joo J, Hyeon T, Hong S, Im J (2004) J Phys Chem B 108:8091
Otsuka K, Ogihara H, Takenaka S (2003) Carbon 41:223
Tago T, Hatsuta T, Miyajima K, Kishida M, Tashiro S, Wakabayashi K (2002) J Am Ceram Soc 85:2188
Takenaka S, Hori K, Matsune H, Kishida M (2005) Chem Lett 34:1594
Takenaka S, Umebayashi H, Tanabe E, Matsune H, Kishida M (2007) J Catal 245:395
Takenaka S, Arike T, Matsune H, Tanabe E, Kishida M (2008) Carbon 46:365
Takenaka S, Ishida M, Serizawa M, Tanabe E, Otsuka K (2004) J Phys Chem B 108:11464
Ciuparu D, Chen Y, Lim S, Haller GL, Pfefferle L (2004) J Phys Chem B 108:503
Hernadi K, Fonseca A, Nagy JB, Siska A, Kiricsi I (2000) Appl Catal A 199:245
Terrones M, Grobert N, Olivares J, Zhang JP, Terrones H, Kordatos K, Hsu WK, Hare JP, Townsend PD, Prassides K, Cheetham AK, Kroto HW, Walton DRM (1997) Nature 388:52
Cheng HM, Li F, Sun X, Brown SDM, Pimenta MA, Marucci A, Dresselhaus G, Dresselhaus MS (1998) Chem Phys Lett 289:602
Colomer JF, Stephan C, Lefrant S, Tendeloo GV, Willems I, Kónya Z, Fonseca A, Ch Laurent, Nagy JB (2000) Chem Phys Lett 317:83
Takenaka S, Kobayashi S, Ogihara H, Otsuka K (2003) J Catal 217:79
Lee K, Zhang J, Wang H, Wilkinson DP (2006) J Appl Electrochem 36:507
Yang J, Liu D, Kariuki NN, Chen LX (2008) Chem Commun 329
Takagi D, Homma Y, Hibino H, Suzuki S, Kobayashi Y (2006) Nano Lett 6:2642
Ritschel M, Leonhardt A, Elefant D, Oswald S, Büchner B (2007) J Phys Chem C 111:8414
Acknowledgements
This research was supported by a Grant-in-Aid of Scientific Research (B) (No. 19360361) under the Ministry of Education, Culture, Sports, Science and Technology in Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iguchi, T., Takenaka, S., Nakagawa, K. et al. Production of Carbon Nanotube by Ethylene Decomposition over Silica-Coated Metal Catalysts. Top Catal 52, 563–570 (2009). https://doi.org/10.1007/s11244-009-9191-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-009-9191-7