Abstract
Three silver-based complexes, namely [Ag2(bpa)2](Brtp)·6H2O (1), [Ag3(bpa)3](Hdcdcpb)·9H2O (2), and [Ag2(bpa)2(oa)]·2H2O (3), were synthesized via the reactions of AgNO3, 1,2-bis(4-pyridyl)ethane (bpa), and 2-bromoterephthalic acid (H2Brtp), 2,3-dicarboxyl-(2′,3′-dicarboxylazophenyl)benzene (H4dcdcpb) or 4,4′-oxybisbenzoic acid (H2oa) in aqueous alcohol/ammonia solution at room temperature. Complexes 1 and 2 consist of 1D infinite cationic [Ag(bpa)] n+ n chains, interspersed with non-coordinated and completely deprotonated Brtp2− or partly deprotonated Hdcdcpb3− anions acting as discrete counter-ions to balance the charge. The lattice water molecules are situated among the framework of the crystal structure and show rich hydrogen-bonding interactions, while the presence of Ag…N and Ag…Ag contacts strengthens the frameworks. Complex 3 consists of 2D neutral [Ag2(bpa)2(oa)] in which the completely deprotonated oa2− units both act as bidentate ligands and as counter-ions. All three complexes exhibit luminescent properties, which can be assigned to ligand-to-metal charge transfer and Ag…Ag interactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metal–organic frameworks (MOFs, also called coordination polymers) are a relatively new class of porous materials with high diversity, which have attracted much attention in recent decades due to not only their diverse structures [1–3], but also their many potential applications, such as gas storage [4, 5], catalysis [6, 7], conductive materials [8, 9], drug delivery systems [10, 11], sensors [12, 13], magnetic materials [14, 15], gas separation materials [16], and photocatalysis [1, 17–20].
MOFs constructed from metal atoms as templates linked together by multifunctional organic ligands as linkers, can display a variety of infinite supramolecular networks [2, 7, 8, 19, 21, 22]. The construction of such MOFs is highly influenced by such factors as the coordination preferences of the metal, the structural characteristics of polydentate organic ligands, the metal–ligand ratio and the choice of counter-ions [2, 7, 8, 21, 23–26]. It is noteworthy that counter-ions, especially anions, often play important roles in determining the structures of sliver(I) complexes [2, 7, 8, 21, 22]. Compared to inorganic anions, organic carboxylate anions are more numerous and versatile [2, 7, 8, 21, 22, 24]. Therefore, in recent years, our group has focused on syntheses of silver complexes containing different organic carboxylate anions in order to explore how the self-assembly process can be influenced by these organic anions [1, 2, 7, 8, 21, 22, 24, 27–29]. The key to targeted construction of a desired framework is usually the selection of organic carboxylate anions as ligands or/and counter-ions. In some cases, a subtle alteration of organic ligands can lead to a new architecture. Furthermore, with the aid of supramolecular interactions such as hydrogen-bonding, π–π staking interactions, Ag…Ag and Ag…N contacts, various high-dimensional silver(I) coordination polymers can be built up from low-dimensional Ag(I) complexes [2, 7, 8, 20–22, 24].
In this paper, we present three silver complexes, namely [Ag2(bpa)2](Brtp)·6H2O (1), [Ag3(bpa)3](Hdcdcpb)·9H2O (2) and [Ag2(bpa)2(oa)]·2H2O (3), constructed from 1,2-bis(4-pyridyl)ethane (bpa), 2-bromoterephthalic acid (Br-H2tp), 2,3-dicarboxyl-(2′,3′-dicarboxylazophenyl)benzene (H4dcdcpb), and 4,4′-oxybisbenzoic acid (H2oa) (as shown in Scheme 1), in order to investigate the influence of different organic anions on the crystal structures and properties of the resulting silver complexes.
Experimental
All chemicals were commercially available reagent grade and used without further purification. C, H, N elemental analyses were obtained with an Elementar Vario EL-III instrument. FTIR spectra in the region (400–4000 cm−1) were recorded on a PerkinElmer Spectrum 100 Fourier transform infrared spectrophotometer with KBr pellets. Powder X-ray diffraction (PXRD) patterns were recorded using a Dandonghaoyuan DX-2700B diffractometer employing Cu Kα radiation. Luminescence spectra were recorded on a Hitachi F-7000 fluorescence spectrophotometer equipped with a Xenon flash lamp at room temperature.
Synthesis of complex 1
An ammonia solution (125 mL, 0.5 mol/L) of AgNO3 (0.21 g, 1.25 mmol) and 2-bromoterephthalic acid (0.31 g, 1.25 mmol) was added dropwise to an EtOH solution (125 mL) of bpa (0.23 g, 1.25 mmol). The clear mixture was stirred for 15 min and then allowed to evaporate slowly at room temperature. Block-like light white crystals of [Ag2(bpa)2](Brtp)·6H2O (1) were obtained after 4 weeks. Anal. calcd. for C32H39Ag2BrN4O10 (%): C, 41.1; H, 4.2; N, 6.0. Found: C, 41.2; H, 4.3; N, 6.0. IR (KBr)/cm−1: 3333(m), 3030(m), 2925(w), 1933(w), 1606(s), 1557(s), 1495(w), 1423(m), 1360(s), 1219(w), 1177(s), 1100(m), 1076(w), 1012(m), 991(w), 917(w), 828(s), 810(m), 778(s), 718(m), 659(w), 546(m), 488(m), 409(w).
Synthesis of complex 2
Synthesis of block-like red crystals of Ag3(bpa)3](Hdcdcpb)·9H2O (2) followed the same procedure as for 1, except that 2-bromoterephthalic acid was replaced by 2,3-dicarboxyl-(2′,3′-dicarboxylazophenyl)benzene. Anal. calcd. for C52H61Ag3N8O17 (%): C, 44.8; H, 4.4; N, 8.0. Found: C, 44.7; H, 4.4; N, 8.0. IR (KBr)/cm−1: 3369(m), 3070(m), 1931(w), 1660(w), 1606(s), 1580(s), 1560(s), 1500(w), 1460(m), 1441(m), 1420(m), 1410(w), 1380(s), 1221(w), 1154(s), 1080(m), 1062(w), 1011(m), 989(w), 854(m), 827(s), 797(w), 776(m), 659(w), 574(s), 546(m), 400(w).
Synthesis of complex 3
Synthesis of block-like white crystals of [Ag2(bpa)2(oa)]·2H2O (3) followed the same procedure as for 1, except that 2-bromoterephthalic acid were replaced with 4,4′-oxybisbenzoic acid. Anal. calcd. for C38H36Ag2N4O7 (%): C, 52.0; H, 4.1; N, 6.4. Found: C, 52.1; H, 4.1; N, 6.4. IR (KBr)/cm−1: 3417(m), 3030(m), 2926(m), 1599(s), 1553(s), 1500(m), 1400(m), 1311(m), 1290(m), 1266(m), 1251(m), 1218(w), 1161(s), 1114(m), 1010(m), 991(w), 866(m), 828(s), 809(m), 777(m), 659(w), 547(m), 495(m).
X-ray crystallography
X-ray single-crystal data collection for all three complexes was performed with a Bruker Smart 1000 CCD area detector diffractometer with graphite-monochromatized MoKα radiation (λ = 0.71073 Å) using φ–ω mode at 298(2) K. The SMART software [30] was used for data collection and the SAINT software [30] for data extraction. Empirical absorption corrections were performed with the SADABS program [31]. The structures were solved by direct methods (SHELXS-97) [32] and refined by full-matrix least-squares techniques on F 2 with anisotropic thermal parameters for all of the non-hydrogen atoms (SHELXL-97) [32]. All hydrogen atoms were located by Fourier difference synthesis and geometrical analysis. These hydrogen atoms were allowed to ride on their respective parent atoms. All structural calculations were carried out using the SHELX-97 program package [32]. Crystallographic data and structural refinements for the complexes are summarized in Table 1. Selected bond lengths and angles are listed in Table 2.
Results and discussion
IR spectra
In the IR spectra of all three complexes, a strong and broad absorption at 3333, 3369, and 3417 cm−1 for 1, 2, and 3, respectively, is assigned to the O–H stretching vibration, showing the presence of lattice water molecules. Sharp bands at 1557 and 1423 m−1 (for 1), 1580 and 1420 cm−1 (for 2), and 1599 and 1410 cm−1 (for 3) are attributed to the asymmetric and symmetric vibrations of the respective carboxylate groups.
Crystallographic analysis of complex 1
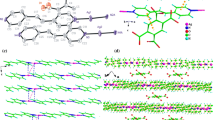
The crystal structure of complex 1 reveals infinite cationic chains of [Ag2(bpa)2] 2n+ n cations, Brtp2− anions, and lattice water molecules, as illustrated in Fig. 1a. In the cationic chains of [Ag2(bpa)2] 2n+ n , the Ag(1) and Ag(2) atoms, in linear geometry, are coordinated by the two nitrogen atoms from two different bpa ligands, as also seen for previously reported AgI complexes [1, 2, 7, 8, 21, 22] (Fig. 1a; Table 2). The oxygen atoms of the lattice water molecules interact with the Ag centers through weak Ag…O interactions [Ag(1)…O(5) = 2.734(4) Å and Ag(2)…O(6) = 2.859(4) Å, respectively], in which the Ag…O distances are shorter than their van der Waals contact distance of 3.24 Å [33].
a Asymmetric unit of [Ag2(bpa)2](Br-tp)·6H2O (1) and coordination environments around the AgI atoms. Water molecules and corresponding H atoms are omitted for clarity. b Packing view of the sandwich-like framework built from anionic and cationic sheets along the a-axis for complex 1. c The anionic layer constructed from Br-tp2− and water molecules in complex 1. d The cationic layer formed from Ag-bpa chains in complex 1 via Ag…N interactions
In complex 1, bpa acts as a typical 4,4′-bipyridine-like bidentate ligand, linking two Ag centers via the nitrogen atoms from two pyridyl rings to form infinite 1-D [Ag2(bpa)2] 2n+ n chains. The dihedral angles between the two pyridyl rings of the two different bpa ligands, are 171.3°. The adjacent cationic [Ag2(bpa)2] 2n+ n chains are packed into 2-D cationic sheets in an ABBA pattern via Ag…N interactions [Ag…N contacts ranging from 3.502(46) to 3.840(45) Å], as shown in Fig. 1d, similar to a previously reported AgI complex [7]. The completely deprotonated Brtp2− anion acts as counter-ion to compensate the charge of [Ag2(bpa)2] 2n+ n , which is joined into anionic sheets with the aid of lattice water molecules via intermolecular hydrogen-bonding interactions, as depicted in Table 3 and Fig. 1c. The neighboring cationic and anionic sheets are further joined into a 3D sandwich-like framework along the a-axis, as shown in Fig. 1b. The lattice water molecules of complex 1 are situated within the 3D framework and stabilized by hydrogen-bonding interactions.
Crystallographic analysis of complex 2
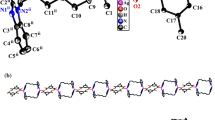
As illustrated in Fig. 2a, the crystal structure of complex 2 consists of three chains of [Ag(1)(bpa)] n+ n , [Ag(2)(bpa)] n+ n ,and [Ag(3)(bpa)] n+ n cations, partly deprotonated Hdcdcpb3− moieties, and lattice water molecules. The coordination environment of AgI is similar to that observed in complex 1, involving a linear coordination geometry with two nitrogen atoms from different bpa ligands. The Ag atoms interact with the oxygen atoms of the water molecules through weak Ag…O interactions, and the partly deprotonated Hdcdcpb3− anions act as counter-ions to balance the cationic [Ag3(bpa)3] 3n+ n chains.
a Asymmetric unit of [Ag3(bpa)3](Hdcdcpb)·9H2O (2) and coordination environments around the AgI atoms. Lattice water molecules and corresponding H atoms are omitted for clarity. b Packing view of the sandwich-like framework built from anionic and cationic sheets along the b-axis for complex 2. c The cationic layer formed from Ag-bpa chains in complex 2. d The anionic layer constructed from Hdcdcpb3− and water molecules in complex 2
The adjacent cationic [Ag(1)(bpa)] n+ n , [Ag(2)(bpa)] n+ n and [Ag(3)(bpa)] n+ n chains are connected by Ag…Ag and Ag…N interactions [Ag(1)…Ag(2) contacts 3.4846(10) Å, Ag(1)…Ag(3) contacts 3.5903(10) Å, Ag(1)…N contacts 3.5346(65) and 3.521(66) Å, Ag(2)…N contacts 3.3988(65) Å, and Ag(3)…N contacts 3.5045(65) Å] into 2D cationic sheets (A sheets), as shown in Fig. 2c. The partly deprotonated Hdcdcpb3− anions are linked into anionic sheets (B sheets) with the aid of lattice water molecules via intermolecular hydrogen-bonding interactions, as depicted in Table 3 and Fig. 2d. The neighboring cationic and anionic sheets are further joined into a 3D sandwich-like framework (as shown in Fig. 2b) with the ABAB pattern by hydrogen-bonding and electrostatic interactions, which is similar to previously reported AgI complexes [22, 24].
Crystallographic analysis of complex 3
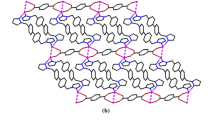
The crystal structure of complex 3 reveals that [Ag2(bpa)2(oa)]·2H2O is made up of infinite neutral 2D sheets of [Ag2(bpa)2(oa)] n moieties and lattice water molecules, as illustrated in Fig. 3a. In the 2D sheet of [Ag2(bpa)2(oa)] n , each Ag atom has a T-shaped coordination geometry provided by two nitrogen atoms from different bpa ligands and an oxygen atom from a COO−, as illustrated in Table 2.
a Asymmetric unit of [Ag2(bpa)2(oba)]·2H2O (3) and coordination environments around the AgI atoms. Lattice water molecules and corresponding H atoms are omitted for clarity. b 2-D network built up of 1-D cationic silver-bpa chains linked by oa2− ligands. c Packing view of the 3-D framework built from rich hydrogen-bonding interactions along a-axis for 3
The coordination mode of bpa is similar to those of bpy-like ligands in similar complexes [2, 7, 8, 20–22], such that two Ag atoms are linked via the nitrogen atoms from two pyridyl rings to form 1-D [Ag(bpa)] n+ n chains. The fully deprotonated oa2− ligand, in which the dihedral angle between the two benzene rings is 123.2°, connects two Ag atoms via O(1) and O(3) in bis-monodentate mode, to form 1-D anionic chains. The neighboring cationic [Ag(bpa)] n+ n chains are further linked into 2-D sheets, as illustrated in Fig. 3b and Table 2. The 2-D sheets are connected into a 3-D framework by rich hydrogen-bonding interactions provided by lattice water molecules, as illustrated in Fig. 3c.
A series of complexes of silver(I) with sandwich-like frameworks has been reported previously [1, 2, 7, 8, 21, 22]. These complexes consist of 2D cationic sheets constructed from parallel 1-D infinite bpy/bpe/bpp-silver cationic chains via ligand-unsupported Ag…Ag and Ag…N interactions, interspersed with anionic sheets constructed from organic anions and water molecules via rich hydrogen-bonding interactions. The coordination environment of the AgI is generally either linear [1, 2, 7, 8, 21, 22, 24], T-shaped [2, 7, 8, 21, 22], trigonal or tetrahedral [8, 22]. Generally, the counter-ions can be present in coordinated, uncoordinated, or mixed modes, such that coordinated anions normally increase the dimensionality of the crystal structures, while uncoordinated anions may help to extend the crystal structures via hydrogen-bonding, π–π stacking, and/or ligand-unsupported Ag…Ag and Ag…N interactions [2].
Fluorescence properties of the complexes
The solid-state emission spectra of these complexes have been investigated at room temperature (Fig. 4). Intense bands in the emission spectra were observed at 354 nm (λ ex = 290 nm) for 1, 335 nm (λ ex = 308 nm) for 2 and 375 nm (λ ex = 310 nm) for 3. According to the literature, these emission bands can be assigned to ligand-to-metal charge transfer and Ag…Ag interactions. Some silver-based complexes with Ag…Ag interactions show emission with similar energies [21, 34].
In order to check the phase purities of the complexes which were used to study their fluorescence properties, powder X-ray diffraction (PXRD) patterns have been checked at ambient temperature, as listed in Fig. 5. For all three complexes, the measured PXRD patterns agree well with those calculated from the X-ray single-crystal diffraction data, confirming high-phase purities. The slight differences in intensities may be attributed to the preferred orientations of the crystalline powder samples [19, 35].
Conclusions
Three silver(I) complexes have been synthesized and characterized by single-crystal diffraction. Complexes 1 and 2 contain novel and fascinating sandwich-like frameworks built up of cationic [Ag(bpa)] n+ n layers and anionic layers with the aid of supramolecular interactions, via ligand-unsupported Ag…Ag and Ag…N interactions and hydrogen-bonding interactions, in which the deprotonated Brtp2− and Hdcdcpb3− moieties do not participate in coordination with silver, only playing the role of charge compensation. Complex 3 is built up of 2D [Ag2(bpa)2(oa)] sheets, in which the exo-multidentate oa2− ligands not only act as O-donors to link the silver centers, but also as counter-ions to compensate the cationic charge of the crystal structure. All three complexes are luminescent.
Supplementary materials
CCDC 1412329, 1412330, and 1412331 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
References
Wang C-C, Jing H-P, Wang P (2014) Three silver-based complexes constructed from organic carboxylic acid and 4, 4′-bipyridine-like ligands: syntheses, structures and photocatalytic properties. J Mol Struct 1074:92–99
Wang C-C, Wang P, Guo G-L (2012) 3D sandwich-like frameworks constructed from silver chains: synthesis and crystal structures of six silver (I) coordination complexes. Transit Met Chem 37:345–359
Sun L-B, Li J-R, Lu W, Gu Z-Y, Luo Z, Zhou H-C (2012) Confinement of metal–organic polyhedra in silica nanopores. J Am Chem Soc 134:15923–15928
Rosi NL, Eckert J, Eddaoudi M, Vodak DT, Kim J, O’Keeffe M, Yaghi OM (2003) Hydrogen storage in microporous metal–organic frameworks. Science 300:1127–1129
Llabrés i Xamena FX, Abad A, Corma A, Garcia H (2007) MOFs as catalysts: activity, reusability and shape-selectivity of a Pd-containing MOF. J Catal 250:294–298
O. Ohmori, M. Fujita (2004) Heterogeneous catalysis of a coordination network: cyanosilylation of imines catalyzed by a Cd (II)-(4, 4′-bipyridine) square grid complex. Chem Commun 2004:1586–1587
Wang C-C, Wang P, Guo G-S (2010) Synthesis and crystal structures of four mixed-ligand silver (I) complexes with sandwich-like structure. Transit Met Chem 35:721–729
Wang C-C, Wang P, Feng LL (2012) Influence of organic carboxylic acids on self-assembly of silver (I) complexes containing 1, 2-bis (4-pyridyl) ethane ligands. Transit Met Chem 37:225–234
Lin P, Henderson RA, Harrington RW, Clegg W, Wu C-D, Wu X-T (2004) New 1-and 2-dimensional polymeric structures of cyanopyridine complexes of AgI and CuI. Inorg Chem 43:181–188
Kitagawa S, Uemura K (2005) Dynamic porous properties of coordination polymers inspired by hydrogen bonds. Chem Soc Rev 34:109–119
Horcajada P, Chalati T, Serre C, Gillet B, Sebrie C, Baati T, Eubank JF, Heurtaux D, Clayette P, Kreuz C (2010) Porous metal-organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat Mater 9:172–178
Wong KL, Law GL, Yang YY, Wong WT (2006) A highly porous luminescent terbium–organic framework for reversible anion sensing. Adv Mater 18:1051–1054
Achmann S, Hagen G, Kita J, Malkowsky IM, Kiener C, Moos R (2009) Metal–organic frameworks for sensing applications in the gas phase. Sensors 9:1574–1589
Habib HA, Sanchiz J, Janiak C (2008) Mixed-ligand coordination polymers from 1, 2-bis (1, 2, 4-triazol-4-yl) ethane and benzene-1, 3, 5-tricarboxylate: trinuclear nickel or zinc secondary building units for three-dimensional networks with crystal-to-crystal transformation upon dehydration. Dalton Trans 2008:1734–1744
Janiak C, Vieth JK (2010) MOFs, MILs and more: concepts, properties and applications for porous coordination networks (PCNs). New J Chem 34:2366–2388
Kandiah M, Nilsen MH, Usseglio S, Jakobsen S, Olsbye U, Tilset M, Larabi C, Quadrelli EA, Bonino F, Lillerud KP (2010) Synthesis and stability of tagged UiO-66 Zr-MOFs. Chem Mater 22:6632–6640
Wang C-C, Li J-R, Lv X-L, Zhang Y-Q, Guo G (2014) Photocatalytic organic pollutants degradation in metal–organic frameworks. Energy Environ Sci 7:2831–2867
Wang C-C, Zhang Y-Q, Li J, Wang P (1083) Photocatalytic CO 2 reduction in metal–organic frameworks: a mini review. J Mol Struct 2015:127–136
Wang C-C, Zhang Y-Q, Zhu T, Zhang X-Y, Wang P, Gao S-J (2015) Four coordination compounds constructed from 1,10-phenanthroline and semi-flexible and flexible carboxylic acids: hydrothermal synthesis, optical properties and photocatalytic performance. Polyhedron 90:58–68
Wang C-C, Jing H-P, Zhang Y-Q, Wang P, Gao S-J (2015) Three coordination compounds of cobalt with organic carboxylic acids and 1,10-phenanthroline as ligands: syntheses, structures and photocatalytic properties. Transit Met Chem 40:573–584
Wang C-C, Li H-Y, Guo G-L, Wang P (2013) Synthesis, characterization, and luminescent properties of a series of silver (I) complexes with organic carboxylic acid and 1, 3-bis (4-pyridyl) propane ligands. Transit Met Chem 38:275–282
Wang C-C, Guo G-L, Wang P (2013) Synthesis, structure, and luminescent properties of three silver (I) complexes with organic carboxylic acid and 4, 4′-bipyridine-like ligands. Transit Met Chem 38:455–462
Tong M-L, Wu Y-M, Ru J, Chen X-M, Chang H-C, Kitagawa S (2002) Pseudo-polyrotaxane and β-sheet layer-based three-dimensional coordination polymers constructed with silver salts and flexible pyridyl-type ligands. Inorg Chem 41:4846–4848
Wang C, Wang P (2013) Synthesis and crystal structure of three mixed-ligand silver (I) complexes constructed from 1, 2-Di (4-pyridyl) ethylene and different organic carboxylate anions. Russ J Coord Chem 39:194–200
Chen C-L, Kang B-S, Su C-Y (2006) Recent advances in supramolecular design and assembly of silver (I) coordination polymers. Aust J Chem 59:3–18
Zhang J-P, Kitagawa S (2008) Supramolecular isomerism, framework flexibility, unsaturated metal center, and porous property of Ag (I)/Cu (I) 3, 3′, 5, 5′-tetrametyl-4, 4′-bipyrazolate. J Am Chem Soc 130:907–917
Wang C-C, Wang P (2011) Syntheses and crystal structures of two silver (I) complexes with organic carboxylic acid and bidentate N-donor ligands. Chin J Struct Chem 30:811–818
Wang C-C, Song Y-X, Wang P (2011) Syntheses, crystal structure and optical property of two bis-ligand silver (I) complexes containing diphenic acid and bidentate N-donor ligands. Chin J Inorg Chem 27:361–366
Wang C-C, Wang P, Guo G-L (2012) Influence of different organic carboxylate anions on the crystal structure of silver (I) coordination polymers. Chin J Struct Chem 31:1008–1020
Bruker (2003) SAINT, Version 6.28. Bruker AXS Inc., Madison
Bruker (2000) SADABS, V2.03. Bruker AXS Inc., Madison
Sheldrick GM (1997) SHELX-97. Göttingen University, Germany
Yeh C-W, Chen T-R, Chen J-D, Wang J-C (2009) Roles of anion and solvent in the self-assembly of silver(I) complexes containing 2, 3-diphenylquinoxaline. Cryst Growth Des 9:2595–2603
Omary MA, Patterson HH (1998) Temperature-dependent photoluminescence properties of Tl [Ag (CN)2]: formation of luminescent metal–metal-bonded inorganic exciplexes in the solid state. Inorg Chem 37:1060–1066
Hao J-M, Yu B-Y, Van Hecke K, Cui G-H (2015) A series of d 10 metal coordination polymers based on a flexible bis (2-methylbenzimidazole) ligand and different carboxylates: synthesis, structures, photoluminescence and catalytic properties. CrystEngComm 17:2279–2293
Acknowledgments
We thank the financial support from National Natural Science Foundation of China, the Beijing Natural Science Foundation and Scientific Research Key Program of Beijing Municipal Commission of Education (KZ201410016018, KM201510016017), the Training Program Foundation for the Beijing Municipal Excellent Talents (2013D005017000004), the Importation and Development of High-Caliber Talents Project of Beijing Municipal Institutions (CIT&CD201404076), the Scientific Research Common Program of Beijing Municipal Commission of Education (KM201510016017), and Open Research Fund Program of Key Laboratory of Urban Stormwater System and Water Environment (Ministry of Education).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, J., Wang, CC., Wang, P. et al. Three silver complexes constructed from organic carboxylic acid and 1,2-bis(4-pyridyl)ethane ligands: syntheses, crystal structures, and luminescent properties. Transition Met Chem 40, 821–829 (2015). https://doi.org/10.1007/s11243-015-9978-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-015-9978-2