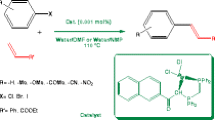
Abstract
A novel pathway for cross-coupling reaction of terminal alkenes with aryl halides (Heck cross-coupling) has been described using a new phosphine-free poly(N-vinyl carbazole) anchored palladium(II) complex as catalyst in aerobic conditions. The catalyst was found to be highly active for the couplings of a variety of substituted and non-substituted aryl halides with terminal alkenes and to smoothly afford the corresponding desired products in good to excellent yields. The catalyst can be reused at least six times without noticeable decrease in catalytic activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Heck reaction [1–3], a Pd-catalyzed C–C coupling between aryl halides and terminal alkenes, is a robust and efficient method for carbon–carbon bond formation and remains a flourishing area of research [4, 5]. The reaction has been applied to many areas, including natural products [6–8] and fine chemicals synthesis [9–11]. Usually, aryl halides are reacted with alkenes in the presence of palladium and a suitable base. Soluble palladium compounds, generally phosphine palladium complexes, are the most efficient catalysts for the Heck reaction [12–18]. However, phosphine ligands are expensive, toxic, unrecoverable and sensitive to air and moisture and therefore environmentally unfavorable. The development of catalyst systems with non-phosphine ligands [19–30] is now of practical interest and thus has been receiving great attention. With the development of green chemistry, the design and preparation of green catalysts have attracted much attention recently, among which heterogeneous catalysts have been widely employed [31–36]. The immobilization of metal catalysts on solid supports has become a valuable tool for the efficient separation of organic products and catalyst recycling. Polymers play a significant role in this area, offering different ways of metal attachment to the polymer matrix via covalent or non-covalent bonding, through hydrogen bridges, as well as through ionic, hydrophobic or fluorous interactions [37–41].
The recent discovery of polymer-based Schiff base ligands and of their effectiveness as catalysts offers an opportunity to develop phosphine-free heterogeneous catalysis. Several authors have recently reported polymer-anchored palladium complexes active for the Heck reaction under phosphine-free conditions. Schubert and coworkers reported stabilized palladium nanoparticles in star-shaped block copolymers [42]. Steel et al. described palladium N-heterocyclic carbine complexes anchored to polymers, and it was claimed that a true heterogeneous recyclable catalyst was functioning [43].
The excellent catalytic activity of poly (N-vinyl carbazole) anchored Pd(II) complex for the hydrogenation reactions of various organic substrates prompted us to extend our investigations into other catalytic reactions. In the present work, we have examined the catalytic activity of poly (N-vinyl carbazole) anchored Pd(II) complex in the Heck cross-coupling reaction between aryl halides and terminal alkenes under phosphine-free reaction conditions. The effects of various reaction parameters for optimization of the reaction conditions were also studied. This polymer-anchored metal complex exhibited high catalytic performance and can be easily recovered and reused without significant loss of catalytic activity.
Experimental
All the reagents were analytical grade and used as such without further purification. Solvents were purified and dried according to standard procedures. Poly (N-vinyl carbazole) (Art.No. 368350–5) was purchased from Aldrich; [Pd(PhCN)2Cl2], Pd(OAc)2 and PdCl2 were procured from Arora Matthey. Other reagents were purchased from Merck.
The palladium content was determined by Varian, USA, AA240 atomic absorption spectrophotometer (AAS). A Perkin–Elmer, USA, 2400C elemental analyzer was used to collect microanalytical data (C, H and N). Surface morphology of functionalized polystyrene ligand and metal complex was analyzed using a scanning electron microscope (ZEISS EVO40, England) equipped with EDX facility. Fourier transform infrared (FTIR) spectra for the catalyst and its precursors were recorded over the wave number range from 400 to 4,000 cm−1 on a Perkin–Elmer, USA, FTIR 783 spectrophotometer using KBr pellets. UV–Vis spectra were taken using a Shimadzu, Japan, UV-2401PC double beam spectrophotometer having an integrating sphere attachment for solid samples. The thermal stability of the immobilized catalyst was determined using a Mettler Toledo, Switzerland, TGA/DTA 851e instrument. The reaction products were analyzed using a Varian, USA, 3400 gas chromatograph equipped with a 30 m CP-SIL8CB capillary column and a flame ionization detector. All reaction products were identified by using an Agilent, USA, GC–MS (QP-5050) equipped with a 30 m HP-5 ms capillary column.
General experimental procedure for Heck reaction
To a suspension of polymer-anchored palladium(II) catalyst in DMF (10 mL), aryl halide (1.0 mmol), alkene (2.0 mmol), K2CO3 (1.5 mmol), 0.5 mol% of catalyst and dodecane as internal standard were added, and the reaction mixture was stirred at 80 °C under air. To study the progress of the reaction, the reaction mixtures were collected at different time intervals and quantified by GC analysis. At the end of the reaction, the catalyst was separated by simple filtration. The filtrate was dried over Na2SO4, filtered, concentrated, and the residue was purified by flash column chromatography on silica gel. The product was analyzed by GC–MS. All the prepared compounds are known and were compared with authentic samples.
Preparation and characterization of poly (3,6-dibenzaldimino-N-vinylcarbazole) Pd(II) complex catalyst
The outline for the preparation of the poly (N-vinylcarbazole) anchored palladium(II) complex, [Pd(C6H4CH=N-Pol)(PhCN)Cl] (Pol=poly-N-vinylcarbazole) is shown in Scheme-1. The functionalized poly(N-vinyl carbazole amine) (3) was prepared according to the method of King and Sweet [44]. The polymer-anchored ligand (4) was prepared by the reaction of poly(N-vinyl carbazole) amine (3 g) with benzaldehyde (10 mL) in dry toluene (30 mL) medium under reflux condition for 72 h in nitrogen atmosphere. For the preparation of poly (N-vinyl carbazole) anchored palladium(II) complex (5), a methanolic solution (25 mL) of Pd(PhCN)Cl (0.7 g) was mixed with poly(3,6-dibenzaldimino N-vinyl carbazole) ligand (2 g), and the reaction mixture was first stirred for 24 h at room temperature and then refluxed for 12 h [45].
Due to insolubility of the [Pd(C6H4CH=N-Pol)(PhCN)Cl] complex in all common organic solvents, its structural investigation was limited only to its physico-chemical properties, SEM-EDX, TGA-DTA, IR and UV–Vis spectral data (Scheme 1). The complete incorporation of the organic substructure in the material was confirmed by elemental analysis (Table 1). The metal content of [Pd(C6H4CH=N-Pol)(PhCN)Cl] complex determined by AAS suggested 12.80 wt% metal loading in the immobilized palladium complex. The SEM images of polymer-anchored ligand and metal complex clearly show the morphological change, which occurred on the surface of the polymer matrix after loading of the metal. EDX data also support the metal attachment on the surface of polymer matrix (Fig. 1).
The catalyst is thermally stable up to 280 °C. The IR spectrum of poly(3,6-dibenzaldimino N-vinyl carbazole) showed characteristic IR peak at 1,630 cm−1 that may be assigned to the C=N stretching vibration of imine, which on complexation shifted toward lower frequency to 1,620 cm−1 suggesting bond formation between Pd and ligand. Other characteristic peaks at 1,590 cm−1 (ν-C=C-stretching, aromatic), 720 cm−1 (orthometallation) [46], 2,290 cm−1 (ν-C≡N of benzonitrile), 455 cm−1 (ν Pd–N) [47] and 355 cm−1 (ν Pd-Cl) [48] also support the formation of the palladium(II) complex. The UV–Vis spectra provided further evidence for the presence of palladium on polymer support. The absorption maxima at 305 nm may be attributed to the π → π* transition in the carbazole and the phenyl moieties, and the absorption at higher range (374, 434 nm) is due to the π → π* and n → π* transitions of the imine system in conjugation with the aromatic nuclei. The Pd(II) carbazole complex shows the bands at 465 and 340 nm which may be assigned to 1A1g → 1A2g and 1A1g → 1B1g transitions, respectively [49].
Heck cross-coupling reactions
To explore the catalytic activity of the present catalyst, we examined the Heck cross-coupling reaction. Generally, Heck reactions are conducted with a tertiary phosphine ligand. The toxicity of phosphines make them unfavorable and environmentally unacceptable. To address these concerns, we performed the Heck reaction under phosphine-free reaction condition. We began our investigation into Heck coupling reaction with the present catalyst using the coupling of bromobenzene with styrene as a model reaction. Since the performance of a successful metal-catalyzed cross-coupling reaction is known to be governed by a number of factors, at first the effects of various metal precursors, bases, solvents, temperatures and catalyst concentrations on the Heck cross-coupling reaction employing [Pd(C6H4CH=N-Pol)(PhCN)Cl] catalyst were surveyed. All the reactions were carried out under air (Scheme 2).
The influences of various metal salts, Pd(OAc)2, PdCl2 and Pd(PhCN)2Cl2 anchored with poly(3,6-dibenzaldimino N-vinylcarbazole) ligand were first studied. The use of Pd(OAc)2 or PdCl2 as a metal precursors resulted in moderate conversion of bromobenzene, whereas Pd(PhCN)2Cl2 exhibited complete conversion at 80 °C (Table 2, entries 1–3). Therefore, Pd(PhCN)2Cl2 was taken as a metal precursor, and further catalytic runs were studied with this catalyst, [Pd(C6H4CH=N-Pol)(PhCN)Cl]. Using metal salts without any ligand gave partial conversion of the desired product (Table 2, entries 4–6). No coupling reaction was observed without catalyst (Table 2, entry 7).
As the palladium compounds are so expensive, the amount used is an important criterion to estimate the value of a catalyst. In order to observe effect of catalyst concentrations on the conversion of bromobenzene and yield of desired product, the reactions catalyzed by [Pd(C6H4CH=N-Pol)(PhCN)Cl] at different concentrations from 0.1 to 1.0 mol% have been studied. The results are summarized in Table 2. From these results, it is seen that reactions at lower catalyst concentration (0.1 mol% of Pd) resulted in poorer conversion (Table 2, entry 8). The catalytic activity increased with an increase in the palladium concentration, and best results were obtained at catalyst concentration of 0.5 mol% Pd (Table 2, entry 3). A further increase in catalyst concentration (from 0.5 to 1.0 mol% Pd) did not significantly improve the conversion (Table 2, entries 10, 11).
Reaction temperature is another important factor for Heck reaction. In general, Heck cross-coupling reaction requires high reaction temperature. It was found that a temperature below 80 °C was not suitable for this cross-coupling as a poor conversion was obtained at 40–70 °C (Table 3, entries 2–5). At room temperature, no cross-coupled product was detected in the reaction medium (Table 3, entry 1). However, good efficiency was reached when the reaction temperature was 80 °C (Table 3, entry 6).
Solvent plays a crucial role for effective cross-coupling reaction. To verify the solvent effect in Heck cross-coupling reactions, a series of reactions were investigated by taking the model reaction in different solvents. The reaction conducted in polar solvent medium like DMF or DMSO was found to be most effective (Table 3, entries 6, 7). The use of THF, MeOH and ACN as solvents led to slower reactions (Table 3, entries 8–10), and no desired cross-coupling products were observed while reactions were carried out in toluene and dichloromethane (Table 3, entries 11, 12). Consequently, DMF was chosen as the medium of choice for this coupling.
To determine the reactivity of the catalyst in various bases, bromobenzene was chosen as the initial coupling partner in combination with styrene as a model reaction for investigation into the optimal base. Various inorganic and organic bases such as Na2CO3, K2CO3, NaOH, NaOAc, KOH, Et3N and NaOtBu were examined. As shown in Table 4, the best performance was observed with K2CO3 (Table 4, entry 2). Other inorganic bases like Na2CO3, NaOH, NaOAc and KOH gave reasonable conversions leading to 35–74% yields (Table 4, entries 1, 3–5). The yield was noticeably low when organic bases such as Et3N and NaOtBu were employed (Table 4, entries 6, 7). No conversion of the reactants was observed in the absence of base (Table 4, entry 8). The catalytic performance of the coupling reaction was also greatly affected by the base–substrate molar ratio employed. The Heck cross-coupling reaction with the present catalyst was carried out under various base–substrate molar ratios and conditions. The results are shown in Table 4 (entries 2, 9–11). The best yield was achieved with 1.5:1 base–substrate ratio (Table 4, entry 2).
On the basis of the above optimized reaction conditions, the coupling reactions between a variety of aryl halides and alkenes were investigated (Scheme 2). The results are summarized in Table 5. Both aryl iodides and aryl bromides could react with aromatic or aliphatic terminal alkenes to give the corresponding coupling products with good to excellent yields. As shown in Table 5, the coupling of activated and deactivated aryl iodides and alkenes proceeded in high yields and more rapidly than aryl bromides. Non-substituted aryl bromide gave moderate yield of coupled product (Table 5, entries 7, 23). Activated aryl bromide like p-bromonitrobenzene and p-bromoacetophenone bearing electron withdrawing group reacted with styrene and methyl acrylate to generate the corresponding product in good yields (Table 5, entries 10, 11, 26, 27). For deactivated aryl bromides, p-bromoanisole and p-bromotoluene bearing electron donating group in their para position, lower yields were obtained under the present reaction conditions (Table 5, entries 8, 9, 24, 25). The coupling of ortho-substituted bromobenzene having nitro group gave the corresponding coupling product with styrene and methyl acrylate in 83 and 76% yield, respectively (Table 5, entries 15, 30). Sterically hindered 1-iodonaphthalene was found to react efficiently under the present reaction conditions (Table 5, entries 14, 29). Heteroaryl bromides, such as 2-bromopyridine, also coupled effectively with styrene providing 78% coupled product (Table 5, entry 13). However, reactivity toward chloroarenes, which was observed by others [50, 51], was not achieved with our catalyst system. Displacement of styrene to methyl acrylate gave lower yields. The entire obtained product was E-stereochemistry, which was identified by GC–MS and 1HNMR.

Comparison of catalytic activity with other reported systems
Table 6 provides a comparison of the results obtained for our present catalytic system with those reported in the literature. From the table, it is seen that present catalyst exhibited higher conversions and yields compared to the other reported systems [52–56]. Reactions conducted at lower temperature and shorter reaction time were required for the Heck cross-coupling reactions using our catalyst.
Heterogeneity test
Heterogeneity and the palladium leaching of the present catalyst were examined by the “Hot filtration test” for the Heck reaction of 4-bromonitrobenzene and styrene. Figure 2 shows the leaching experiment during the reaction over polymer-anchored palladium catalyst. After continuing the reaction for 4 h, the catalyst was removed by filtration using Whatmann filter paper, and the resulting filtrate was subjected to heating for further 4 h reaction. Gas chromatographic analysis of the filtrate solution showed that there was no significant increase in conversion, whereas the catalyst containing portion gave 90% conversion after 4 h reaction (Fig. 2). Palladium leaching was also studied by atomic absorption spectroscopic analysis, indicating that the liquid part of Heck reaction mixture contained less than 0.5 ppm palladium. These results mean that the catalysis by leached palladium is negligible. The efficient catalytic performance is due to the polymer-anchored metal complex not due to leached palladium metal in the solvent.
Reuse of the catalyst
Another notable advantage of the heterogeneous catalyst with this reaction was the reuse of the catalyst. Recycling studies were performed with the present catalyst using the reaction of 4-bromonitrobenzene with styrene. After the first run, the solid catalyst was separated from the reaction medium by simple filtration, washed with dichloromethane and finally dried at 40 °C under reduced pressure. After separation and washing, the heterogeneous catalysts were used for the same reactions under the same reaction conditions as for the initial run without any regeneration procedure. The catalyst was recycled six times to give yields of 95, 93, 93, 91, 91 and 89% consecutively. This reaction conversion shows that the immobilized catalyst can be repeatedly used without any apparent decrease in its catalytic activity and selectivity.
Conclusions
In summary, we have synthesized a new phosphine-free heterogeneous palladium catalyst [Pd(C6H4CH=N-Pol)(PhCN)Cl], which is highly efficient for the Heck cross-coupling reactions of aryl halides with terminal alkenes. The catalyst was found to be air-stable, active and non-polluting under the various reaction conditions screened. The mild reaction conditions, simple experimental procedure, rapid conversion, excellent yields and reusability of the catalyst are notable advantages of the method. High efficiency and economy gain by simple reaction processing and easy recovery and reuse of the catalyst make it an ideal protocol from an industrial point of view.
References
Heck RF, Nolley JP (1972) J Org Chem 37:2320–2322
Plevyak JE, Heck RF (1978) J Org Chem 43:2454–2456
Nicolaou KC, Bulger PG, Sarlah D (2005) Angew Chem Int Ed 44:4442–4489
Cabri AW, Candiani I (1995) Acc Chem Res 28:2–7
Herrman WA, Bromer C, Öfele K, Reisinger C, Riermeier T, Beller M, Fischer H (1995) Angew Chem 107:1989
Burke TR, Liu D-G, Gao Y (2000) J Org Chem 65:6288–6291
Haberli A, Leumann CJ (2001) Org Lett 3:489–492
Link JT (1998) Overman LE metal-catalyzed cross-coupling reactions. In: Diederich F, Stang PJ (eds) Wiley-VCH Verlag, Weinheim, p 231
Bader RR, Baumeister P, Blaser HU (1996) Chimia 50:99
Wu TC (1996) US Patent, 5536870
Eisenstadt A (1998) Palladium in catalysis of organic reactions. In: Herkes FE, Dekker M (eds) New York
Zhao F, Bhanage BM, Shirai M, Arai M (1999) J Mol Catal A Chem 142:383–388
Brase S, De Meijere A (1998) Metal-catalyzed cross-coupling reactions. Wiley-VCH, Weinheim, p 99
Barder TE, Walker SD, Martinelli JR, Buchwald SL (2005) J Am Chem Soc 127:4685–4696
Akba O, Durap F, Aydemir M, Baysal A, Gümgüma B, Özkar S (2009) J Organometal Chem 694:731–736
Surawatanawong P, Fan Y, Hall MB (2008) J Organometal Chem 693:1552–1563
Wang YD, Dutia M, Floyd MB, Prashad AS, Berger D, Lin M (2009) Tetrahedron 65:57–61
Wang PW, Fox MA (1994) J Org Chem 59:5358–5364
Tulloch AAD, Danopoulos AA, Tooze RP, Cafferkey SM, Kleinhenz S, Hursthouse MB (2000) Chem Commun 1247–1248
Shang Y, Wu J, Fan C, Hu J, Lu B (2008) J Organometal Chem 693:2963–2966
Cwik A, Hell Z, Figueras F (2006) Adv Synth Catal 348:523–530
Peris E, Loch JA, Mata J, Crabtree RH (2001) Chem Commun 201–202
Selvakumar K, Zapf A, Beller M (2002) Org Lett 4:3031–3033
Jung IG, Son SU, Park KH, Chung K-C, Lee JW, Chung YK (2003) Organometallics 22:4715–4720
Dıez-Barra E, Guerra J, Hornillos V, Merino S, Tejeda J (2003) Organometallics 22:4610–4612
Consorti CS, Zanini ML, Leal S, Ebeling G, Dupont J (2003) Org Lett 5:983–986
Najera C, Gil-Molto J, Karlstrom S, Falvello LR (2003) Org Lett 5:1451–1454
Masllorens J, Moreno-Manas M, Pla-Quintana A, Roglans A (2003) Org Lett 5:1559–1561
Park SB, Alper H (2003) Org Lett 5:3209–3212
Mazet C, Gade LH (2003) Eur J Inorg Chem 1161–1168
Gladysz JA (2000) Recoverable catalyst, reagents perspective, prospective. Chem Rev 102:3215–3216
Sherrington DC (1998) Chem Commun 2275–2286
Polshettiwar V, Molnar A (2007) Tetrahedron 63:6949–6976
Polshettiwar V, Varma RS (2008) Tetrahedron 64:4637–4643
Song D, Yi WB (2008) J Mol Catal A Chem 280:20–23
Budarin VL, Clark JH, Luque R, Macquarrie DJ, White RJ (2008) Green Chem 10:382–387
Chandrasekhar S, Narsihmulu C, Sultana SS, Reddy NR (2002) Org Lett 4:4399–4401
Gniewek A, Trzeciak AM, Ziółkowski JJ, Kepinski L, Wrzyszcz J, Tylus W (2005) J Catal 229:332–343
Beletskaya IP, Kashin AN, Litvinov AE, Tyurin VS, Valetsky PM, van Koten G (2006) Organometallics 25:154–158
Ribière P, Declerck V, Nédellec Y, Yadav-Bhatnagar N, Martinez J, Lamaty F (2006) Tetrahedron 62:10456–10466
Dahan A, Portnoy M (2003) Org Lett 5(8):1197–1200
Meier MAR, Filali M, Gohy J-F, Schubert US (2006) J Mater Chem 16:3001–3006
Steel PG, Teasdale CWT (2004) Tetrahedron Lett 45:8977–8980
King RB, Sweet EM (1979) J Org Chem 44:385–391
Kharasch MS, Seyler RC, Mayo FR (1983) J Am Chem Soc 60:822–887
Onue H, Moritani I (1972) J Organomet Chem 43:431–436
Durig JR, Layton R, Sink DW, Mitchell BR (1965) Spectrochim Acta 21:1367–1378
Santra PK, Sagar P (2003) J Mol Catal A Chem 197:37–50
Lever ABP (1968) Inorganic electronic spectroscopy, 1st ed. Elsevier, Amsterdam
Molnar A, Papp A (2006) Synlett 3130–3134
Prockl SS, Kleist W, Kohler K (2005) Tetrahedron 61:9855–9859
Polshettiwar V, Hesemann P, Moreau JJE (2007) Tetrahedron Lett 48:5363–5366
Tsai FY, Wu CL, Mou CY, Chao MC, Linc HP, Liua ST (2004) Tetrahedron Lett 45:7503–7506
Cai M, Huanga Y, Zhaob H, Songa C (2004) Reac Func Polymers 59:81–86
Chen T, Gaob J, Shi M (2006) Tetrahedron 62:6289–6294
Nandurkar NS, Bhanage BM (2008) Tetrahedron 64:3655–3660
Acknowledgments
We acknowledge the Department of Science and Technology (DST), Council of Scientific and Industrial Research (CSIR) and the University Grant Commission (UGC), New Delhi, India for funding. One of the authors, K.T., is thankful to the University Grants Commission (Eastern Region), India, for financial support. We also thank the DST and UGC, New Delhi, India for providing instrumental support under the FIST and SAP program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Islam, M., Mondal, P., Roy, A.S. et al. Use of a recyclable poly(N-vinyl carbazole) palladium(II) complex catalyst: Heck cross-coupling reaction under phosphine-free and aerobic conditions. Transition Met Chem 35, 491–499 (2010). https://doi.org/10.1007/s11243-010-9354-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-010-9354-1