Abstract
Three new mixed ligand complexes [Mn(4-pytone)2(bipy)2]bipy (1), [Mn(pot)2(en)2] (2) and [Mn(4-mot)2(en)2] (3) (4-pytone = 5-(4-pyridyl)-1,3,4-oxadiazole-2-thione, pot = 5-phenyl-1,3,4-oxadiazole-2-thione, 4-mot = 5-(4-methoxy-phenyl)-1,3,4-oxadiazole-2-thione) have been prepared containing bipy/en as coligands. The starting material potassium N-(aryl-carbonyl)-hydrazinecarbodithioates (RCONHNHCSSK) underwent cyclization during complexation in the presence of bipy or en to give the corresponding 5-aryl-1,3,4-oxadiazole-2-thiones. The complexes have been characterized by physicochemical techniques and single crystal X-ray structure determination. In all cases, the manganese has a six coordinate octahedral arrangement coordinated by 4N atoms of two bipy/en and two covalently bonded N atoms of the oxadiazole-2-thione anions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Manganese is an essential trace element, forming the active site of a number of metalloproteins. In metalloproteins, manganese can exist in any of five oxidation states or in mixed valence states. The most important natural role of manganese is in the oxidation of water in green plant photosynthesis, where its presence in photosystem II is essential [1, 2]. Our current interest in the metal complexes of 1,3,4-oxadiazoles arises from their numerous biological applications such as anti-tuberculostatic, anti-inflamatory, analgesic, antipyretic, and anticonvulsant agents [3, 4]. Earlier, we reported on the Cu(II) and Ni(II) mixed ligand complexes of 5-(4-pyridyl)-1,3,4-oxadiazole-2-thione/thiol and 5-(3-pyridyl)-1,3,4-oxadiazole-2-thione/thiol [5, 6]. Although some work has been reported on the binary complexes of 5-phenyl-1,3,4-oxadiazole-2-thione [7, 8], 5-(4-pyridyl)-1,3,4-oxadiazole-2-thione [9–13], and 5-(2-pyridyl)-1,3,4-oxadiazole-2-thione [14], scanty information is available on the mixed ligand complexes of these ligands. 1,3,4-Oxadiazole-2-thiones are biologically active compounds and information about their 3-dimensional structures may be of interest for rational drug design. Since 1,3,4-oxadiazole-2-thiones can exist in both thione and thiol forms in solution [15, 16], it is of interest to investigate the bonding modes in their complexes. Aromatic–aromatic (π–π) interactions are important non-covalent intermolecular forces similar to hydrogen bonding in contributing toward self assembly or molecular recognition processes. Due to the presence of aromatic rings in the ligands under investigation, such π–π stacking is envisaged in the present system [17–19]. In view of this, we have prepared and characterized the Mn(II) complexes of 5-(4-pyridyl)-1,3,4-oxadiazole-2-thione, 5-phenyl-1,3,4-oxadiazole-2-thione, and 5-(4-methoxy-phenyl)-1,3,4-oxadiazole-2-thione in the presence of ethylenediamine or bipyridyl coligands.
Experimental
Commercial reagents were used without further purification, and all experiments were carried out in open atmosphere. Isonicotinic acid hydrazide (Sigma Aldrich), CS2 (S D Fine Chemicals, India), and KOH (Qualigens) were used as received. All the solvents were purchased from Merck and used after purification. Carbon, hydrogen, and nitrogen contents were estimated on a Carlo Erba 1108 model microanalyzer. Magnetic susceptibility measurements were undertaken at room temperature on a Cahn Faraday balance using Hg[Co(NCS)4] as the calibrant. Electronic spectra were recorded on a Shimadzu 1700 UV–Vis spectrophotometer as Nujol mulls [20]. IR spectra were recorded in the 4000–400 cm−1 region as KBr pellets on a Varian Excalibur 3100-FT IR spectrophotometer. 1H and 13C NMR spectra were recorded in d6-DMSO on a JEOL AL 300 FT NMR spectrometer using TMS as internal reference.
Synthesis of K+(H2L)−
Potassium N-(pyridine-4-carbonyl) hydrazine carbodithioate and potassium N′-(4-methoxybenzoyl)]-hydrazine carbodithioate [K+(H2L)−] were prepared by adding CS2 (1.5 mL, 25 mmol) dropwise to a suspension of isonicotinic acid hydrazide (2.7 g, 20 mmol or 4-methoxy benzoic acid hydrazide (3.32 g, 20 mmol) in methanol (30 mL) in the presence of potassium hydroxide (1.2 g, 20 mmol). The reaction mixtures were stirred continuously for 30 min, and the solids that separated were filtered off, washed with EtOH, and dried. Yield: 90%. Mp 305 °C. Anal. Found: C, 33.4; H, 2.4; N, 16.8; S, 25.5%. Calc. for C7H6N3OS2K (251.36): C, 33.5; H, 2.4; N, 16.7; S, 25.5%. 1H NMR (300 MHz, DMSO-d6): δ = 10.60 (s, 2H, NH), 8.65,7.85 (m,4H, pyridine ring). 13C NMR (DMSO-d6, TMS): δ 201.05(>C=S), 163.90(>C=O), 131.77(C3), 150.56(C6), 140.27 (C5), 121.01(C4), 118.63(C7). IR (KBr, cm−1): ν(NH) 3,289 m, 3,182 m; ν(C=O) 1,676 s; ν(N–N) 1,062 s; ν(C=S) 993 s; pyridine ring 667.
Yield: 85%. Mp 245 °C. Anal. Found: C, 38.6; H, 3.1; N, 10.0; S, 22.9%. Calc. for C9H9O2N2S2K (280.40): C, 38.6; H, 3.2; N, 10.1; S, 22.6%. 1H NMR (300 MHz, DMSO-d6): δ = 3.80 (s, 3H, OCH3), 7.0–7.7(d, 4H, C6H5), 9.65(m, 2H, NH). 13C NMR (DMSO-d6, TMS): δ 178.90 (C=S), 160.38 (C=O), 117.87 (C1), 113.67 (C2), 126.64 (C3), 128.95 (C4), 55.28 (OCH3). IR (KBr, cm−1): ν(NH) 3,246 m, 3,169 m; ν(C=O)1,637 s; ν(N–N) 1,000 m; ν(C=S) 886.
Synthesis of 5-phenyl-1,3,4-oxadiazole-2-thione (Hpot)
The 5-phenyl-1,3,4-oxadiazole-2-thione (Hpot) was synthesized by the method described earlier [21].
Synthesis of [Mn(bipy)2(4-pytone)2]⋅bipy (1)
A solution of N-(pyridine-4-carbonyl)-hydrazine carbodithioate [(K+(H2L)−] (0.50 g, 2 mmol) in water (10 mL) was added to an aqueous solution (10 mL) of Mn(OAc)2⋅4H2O (0.25 g, 1 mmol). This mixture was stirred for 1 h at room temperature. The resulting precipitate was filtered off, washed thoroughly with EtOH, and air dried. This was suspended in MeOH to which 2-2′ bipyridyl (0.80 g, 4 mmol) was added and stirred for 2 h at room temperature (Scheme 1). The resulting clear yellow solution was filtered and kept for crystallization. Yellow crystals of 1 suitable for X-ray analysis were obtained by the slow evaporation of the above-mentioned methanolic solution over a period of 12 days.
Yield: 55%. Mp 220 °C. Anal. Found: C, 60.0; H, 3.6; N, 19.1; S, 7.3; Mn, 6.2%. Calc. for C44H32MnN12O2S2: C, 59.9; H, 3.7; N, 19.1; S, 7.3; Mn, 6.3. IR (KBr, cm−1): ν(C=N) 1,612; ν(C–O–C) 1,280w; ν(N–N) 1,060; ν(C=S) 945 m; ν(Mn–N) 489 w, μB = 5.85 BM.
Synthesis of [Mn(pot)2(en)2] (2) and [Mn(4-mot)2(en)2] (3)
The reactions of Mn(OAc)2·4H2O with Hpot and potassium N′-(4-methoxy-benzoyl)-dithiocarbazate in a 1:2 M ratio in methanol gave yellow precipitates which were filtered off, washed with ethanol–water mixture (50:50), and dried. A methanol (5 mL) solution of ethylenediamine (0.30 mL, 4 mmol) was added to a methanol suspension (20 mL) of each of the above-mentioned compounds (1 mmol), and after shaking for a few minutes, the resulting clear yellow solution was filtered and kept for crystallization. Yellow crystals of 2 and 3 suitable for X-ray analyses were obtained by the slow evaporation of the above-mentioned solutions over a period of 7–10 days. Yield: 52%. Mp 225 °C. Anal. Found: C, 45.3; H, 4.9; N, 21.1; S, 12.1; Mn, 10.4%. Calc. for C20H26MnN8O2S2 (2): C, 45.3; H, 4.9; N, 21.2; S, 12.1; Mn, 10.3. IR data (KBr, cm−1); ν(NH) 3,256 m (en), ν(C=N) 1,598; ν (C–O–C) 1,280w; ν(N–N)1,095; ν(C=S) 1,130 m; ν(Mn–N) 530 w, μB = 5.90 BM.
Yield: 55%. Mp 195 °C. Anal. Found: C, 44.8; H, 5.1; N, 19.0; S, 10.9; Mn, 9.3%. Calc. for C22H30MnN8O4S2 (3): C, 44.8; H, 5.1; N, 19.0; S, 10.9; Mn, 9.3. m.p.: 195 °C. IR data (KBr, cm−1); ν(NH) 3,234 m (en), ν(C=N) 1,608; ν(C–O–C) 1,260w; ν(N–N)1,072; ν(C=S) 977 m; ν(Mn–N) 471 w, μB = 5.85 BM.
Crystal structure determination and refinement
Crystals suitable for X-ray analyses of the complexes 1, 2, and 3 were grown at room temperature. Data for the structure of 1 were obtained at 173(2) K, on a Nonius Kappa CCD diffractometer using the COLLECT program [22]. Cell refinement and data reductions used the programs DENZO and SCALEPACK [23] and SIR 97 [24] was used to solve the structure of complex 1. The data for complexes 2 and 3 were recorded at 296(2) and 295(2) on an Oxford Diffraction Gemini diffractometer [25] equipped with CrysAlis Pro. A graphite monochromated Mo Kα (λ = 0.71073 Å) radiation source was used for all the complexes. The structures were solved by direct methods and refined (SHELX-08) against all data by full matrix least-square on F 2 using anisotropic displacement parameters for all non-hydrogen atoms. All hydrogen atoms were included in the refinement at geometrically ideal positions and refined with a riding model [26]. The MERCURY package was used for molecular graphics [27]. Molecular structure diagrams were generated by the use of the ORTEP-3 for windows program [28].
Results and discussion
As a part of our studies on metal complexes of 1,3,4-oxadiazole-2-thione [3, 6], we report herein the preparation, spectroscopic and X-ray studies of three new mixed ligand complexes of Mn(II) with 5-aryl-1,3,4-oxadiazole-2-thiones. Compounds 1, 2, and 3 were obtained by shaking [Mn(H2L)2] or [Mn(pot)2] with a methanol solution of ethylenediamine or bipy taken in 1:4 M ratio. Scheme 1 depicts the synthesis of these complexes. The complexes are stable toward air and moisture for several days. The compounds 1, 2, and 3 are insoluble in ethanol and chloroform but are soluble in MeOH and DMF, and melt at 220, 225, and 195 °C respectively.
The complexes were fully characterized by magnetic susceptibility measurements, IR, UV–Vis, and X-ray spectroscopies. The analytical data for the complexes (see experimental section) corroborated well with their respective formulations.
The IR spectrum of complex 1 shows no band due to ν(NH) indicating loss of both hydrazinic protons upon complexation. The appearance of two new bands for ν(Mn–N) at 515 and 587 cm−1 suggests the bonding of Mn(II) with bpy and one hydrazinic nitrogen after loss of proton(s). In complex 1, ν(C=S) shows a very small negative shift when compared to 4-pytone showing that the exocyclic sulfur is not participating in bonding. The IR spectra of complexes 2 and 3 show bands in the region of 3,256–3,234 cm−1 due to the N–H stretching vibrations of en which are shifted to lower frequencies than those encountered in free en [29]. A negative shift in ν(NH) and appearance of two new bands near 530 cm−1 due to ν(Mn–N) suggest the formation of a chelate with en and bonding of the oxadiazole nitrogen with Mn(II). Both complexes 2 and 3 show a very small negative shift in ν(C=S), showing that the exocyclic sulfur is not participating in bonding; rather, this small shift can be attributed to the involvement of sulfur in hydrogen bonding with the NH2 hydrogens of ethylenediamine. The IR data are thus consistent with the presence of an 1,3,4-oxadiazole moiety in compounds 1, 2, and 3 [30].
The magnetic moment values of 5.85–5.90 B.M. for the three complexes suggest the presence of high-spin Mn(II) with five unpaired electrons. The electronic spectrum of 1 shows a band at 648 nm that may be assigned to the 6A1g → 4T1g transition in an octahedral geometry. The presence of a band around 540–514 mm assigned to the 6A1g → 4T2g transition suggests a high-spin octahedral geometry for complexes 2 and 3 [31].
Description of structures 1, 2 and 3
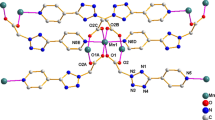
The molecular structures of 1, 2, and 3 were determined by single crystal X-ray diffraction. The details of data collection, structure solution, and refinement are listed in Table 1. ORTEP diagrams of complexes 1, 2, and 3 with atom numbering schemes are shown in Figs. 1, 3, and 4, respectively. Selected bond lengths and angles are given in Tables 2, 3, and 4. The single crystal X-ray diffraction studies indicate that the ligands adopt the thione form in complexes 1, 2, and 3 (Figs. 1, 3, and 4).
The X-ray structure of complex 1 shows that the coordination environment of manganese is fulfilled by two (4-pytone)− anions and two bidentate N, N′-bipy. The two bipy ligands bond to manganese via Mn1–N1, Mn1–N2, Mn1–N3, and Mn–N4 at the distances of 2.255(4), 2.320(4), 2.289(3), and 2.262(4) Å, respectively (Table 2). The resulting complex has a distorted octahedral geometry. Weak intermolecular C–H···N interactions between the oxadiazole nitrogen and CH hydrogen atoms of coordinated bpy stabilize the structure of compound 1 (Fig. 2). The nitrogens of uncoordinated bipy present in the structure are associated through intermolecular hydrogen bonding with the CH hydrogen of the 4-pytone ligand. The geometry and bonding parameters within the bpy molecule agree with those of other bpy complexes [32, 33]. In addition, complex 1 is stabilized by weak π···π interactions occurring between pyridine (Cgpy) and 1,3,4-oxadiazole (Cgoxa) rings from a nearby molecule with a distance of 3.701 Å (Fig. 2).
The molecular structure of 2 shows that in the centrosymmetric unit of [Mn(en)2(pot)2] the metal ion is six coordinate, bonding through four nitrogens of en and two oxadiazole nitrogens. The complex consists of two ethylenediamine ligands that chelate manganese in the equatorial positions and two 1,3,4-oxadiazole-2-thione ligands in the apical positions, in a trans manner. The Mn–N distances are in the range of 2.094–2.1170 Å (Table 3) which is normal for Mn–N amine coordination. The bite angle for the MnC2N4 five membered rings is 82.42°(9), indicating a minor distortion from octahedral geometry in the molecule. The manganese in D4h symmetry is bonded to four nitrogen atoms of two en ligands that offer interesting hydrogen bonding potential. The elements of the structure are linked together in the crystal packing via intramolecular N–H···N interactions between the oxadiazole nitrogen atoms and NH2 hydrogen atoms of the en. The N–H···S intramolecular interactions occur between the thione sulfur of oxadiazole and NH2 hydrogen atoms of en. The arrangement of the monomeric [Mn(en)2(pot)2] unit in a three dimensional architecture along the a axis provides a supramolecular network (see supplementary material F-1). The hydrogen bonding parameters are listed in Table 5. The geometry and bonding parameters within the en molecule agree with those of other en complexes [34, 35].
The single crystal X-ray diffraction studies of complex 3 indicate that the ligand (4-mot−) adopts the thione form with two independent complexes in the asymmetric unit; in each unit, the Mn atom is on a center of inversion. The two 4-mot− ligands are covalently bonded to manganese in a distorted octahedral geometry (Fig. 4) having axial bond angles of 90.60(10) and 89.56(8)° but equatorial bond angles of 82.73(11) and 82.99(11), respectively, in units containing Mn(1) and Mn(2). The 4-mot anions occupy the trans positions, bonded through oxadiazole nitrogens at the distances of 2.108 Å Mn(1) and 2.158 Å Mn(2), respectively. The four equatorial sites in complex 3 are occupied by the two bidentate N,N′-ethylenediamine coligands. The bond length for Mn(1)–N(1A) (Noxa) is shorter than the corresponding bond length in Mn(2), showing stronger bonds in Mn(1) when compared to Mn(2). The two Mn–N(en) distances in both the units are different. The Mn(1)–N(12A) bond length is longer than Mn(2)–Mn–N(12B), while Mn(1)–N(11A) is shorter than Mn(2)–N(11B). Due to stronger Mn(1)–N(1A) (Noxa) bonding, the C(1A)–S(1A) bond is longer in unit 1 when compared to unit 2. The binding of manganese with en involves the formation of two five membered chelate rings with bite angles of 82.73(11)° and 82.99(11)° in Mn(1) and Mn (2), respectively, again showing a slight distortion from ideal octahedral geometry. The two 4-methoxy-phenyl rings growing in apposite directions in the Mn(2) unit are almost parallel (∠BCA = 90.76 Å) having centroid to centroid (Cg···Cg) separation of 3.619 Å but displaced with respect to each other by 0.175 Å with a displacement angle of 20.94°, which is well within the reported values for π···π interactions (Fig. 5) [36]. Weak intermolecular N–H···N interactions between the oxadiazole nitrogen and NH2 hydrogen atoms of en ligands and N–H···S interactions (Table 6) between thione sulfur of oxadiazole and CH2 and NH2 hydrogens of en stabilize the crystal structure of compound 3 (supplementary material F-2).
Conclusion
This paper reports on the syntheses and crystal structures of three new manganese complexes of 5-(4-pyridyl)-1,3,4-oxadiazole-2-thione, 5-phenyl-1,3,4-oxadiazole-2-thione, and 5-(4-methoxy-phenyl)-1,3,4-oxadiazole-2-thione containing ethylenediamine or bipy as the coligand. In [Mn(4-pytone)2(bipy)2]bipy (1), [Mn(pot)2(en)2] (2), and [Mn(4-mot)2(en)2] (3), the metal has a six coordinate octahedral arrangement involving 4N atoms of two bipy or en ligands and two covalently bonded N atoms of oxadiazole-2-thione anions. The crystal structures of the complexes are stabilized by intermolecular and intramolecular hydrogen bonding. In addition, complex 3 is stabilized by π–π interactions between two 4-methoxy-phenyl rings from adjacent layers.
Supplementary material
CCDC 748647, 708243, and 748648 contain the supplementary crystallographic data for [Mn(4-pytone)2(bpy)2]bpy (1), [Mn(pot)2(en)2] (2), and [Mn(4-mot)2(en)2] (3), respectively. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data center, 12 Union Road, Cambridge CB2 IEZ, UK; fax: (+44)1223-336-033; or e-mail: deposit@ccdc.cam.ac.uk.
References
Wieghardt K (1989) Angew Chem Int Ed 28:1153. doi:10.1002/anie.198911531
Greenwood NN, Earnshaw A (1997) Chemistry of the Elements, 2nd edn. Reed educational and Professional, Oxford
Jakubkiene V, Burbuliene MM, Mekuskiene G, Udrenaite E, Gaidelis P, Vainilavicius P (2003) Il Farmaco 58:323. doi:10.1016/S0014-827X(02)00022-8
Tripathi P, Pal A, Jancik V, Pandey AK, Singh J, Singh NK (2007) Polyhedron 26:2597. doi:10.1016/J.poly.2006.12.046
Singh NK, Bharty MK, Dulare R, Butcher RJ (2009) Polyhedron 28:2443. doi:10.1016/J.poly.2009.04.030
Singh M, Butcher RJ, Singh NK (2008) Polyhedron 27:3151. doi:10.1016/J.poly.2008.08.007
Amin OH, Al-Hayaly LJ, Al-Jibori SA, Al-Allaf TAK (2004) Polyhedron 23:2013. doi:10.1016/J.poly.2004.05.006
Zhang ZH, Tian YL, Guo YM (2007) Inorg Chim Acta 360:2783. doi:10.1016/J.ica.2006.11.020
Zhang ZH, Li CP, Tang GM, Tian YL, Guo YM (2008) Inorg Chem Commun 11:326. doi:10.1016/J.poly.2006.09.008
Wang YT, Tang GM (2007) Inorg Chem Commun 10:53. doi:10.1016/Jinoche.2006.09.010
Du M, Zhang ZH, Zhao XJ, Xu Q (2006) Inorg Chem 45:5785. doi:10.1021/ic060129v
Wang YT, Tang GM (2007) Inorg Chem Commun 10:53. doi:10.1016/Jinoche.2006.09.010
Xu HX, Ma JP, Huang RQ, Dong YB (2005) Acta Cryst E61:m2462. doi:10.1107/S1600536805034987
Wang YT, Tang GM, Qiang ZW (2007) Polyhedron 26:4542. doi:10.1016/J.poly.2007.06.026
Obi K, Kojima A, Fukuda H, Hirai K (1995) Bioorg Med Chem Lett 5:2777. doi:10.1016/0960-894X(95)00485-C
Mishra L, Said MK, Itokawa H, Takeya K (1995) Bioorg Med Chem 3:1241. doi:10.1016/0968-0896(95)00095-X
Amabilino DB, Stoddart JF (1995) Chem Rev 95:2725. doi:10.1021/cr00040a005
Claessens CG, Stoddart JF (1997) J Phys Org Chem 10:254. doi:10.1002/(SICI)1099-1395(199705)10:5<254:AID-POC875>3.0.CO;2-3
Hirsch KA, Wilson SR, Moore JS (1997) Chem Eur J 3:765. doi:10.1002/Chem.19970030517
Lee RH, Griswold G, Kleinberg H (1964) Inorg Chem 3:1278. doi:10.1021/ic50019a019
Singh NK, Butcher RJ, Tripathi P, Srivastava AK, Bharty MK (2007) Acta Cryst E63:o782. doi:10.1107/S1600536806052238
Nonius (1998) COLLECT Nonius BV, Delft. The Netherlands
Otwinowski Z, Minor W (1997) In: Carter CW, Sweet RM (eds) Methods in enzymology, macromolecular crystallography, part A, vol 276. Academic Press, London, p 307
Altomare A, Burla MC, Camalli M, Cascarano GL, Giacovazzo C, Guagliardi A, Moliterni AGG, Polidori G, Spagna R (1999) J Appl Cryst 32:115. doi:10.1107/S0021889898007717
Oxford Diffraction (2007) CrysAlis RED and CrysAlis CCD Versions 1.171.31.8. Oxford Diffraction Ltd Abingdon, Oxafordshire, England
Sheldrick GM (2008) Acta Cryst A64:112. doi:10.1107/S0108767307043930
Bruno IJ, Cole JC, Edgington PR, Kessler M, Macrae CF, McCabe P, Pearson J, Taylor R (2002) Acta Crystallogr Sect B58:389. doi:10.1107/S0108768102003324
Farrugia LJJ (1997) Appl Crystallogr 30:565. doi:10.1107/S0021889897003117
Molina P, Tarraga A, Espinosa A (1988) Synthesis 9:690. doi:10.1055/s-1988-27672
Patricia GS, Javier GT, Miguel AM, Francisco JA, Teofilo R (2002) Inorg Chem 41:1345. doi:10.1021/ic015625s
Lever ABP (1984) Inorganic electronic spectroscopy, 2nd edn. Elsevier, Amsterdam
Feng X, Shi XG, Ruan F (2009) Z. Kristallogr. NCS 224:193 ISSN:1433-7266
Chen XM, Huang XY, Xu YJ, Zhu YJ (1995) J Chemical Crystallography 25:605. doi:10.1007/BF01667032
Usuki N, Yamada M, Ohba M, Okawa H (2001) J Solid State Chem 159:328. doi:10.1006/JSSC.2001.9165
Bensch W, Nather C, Schur M (1997) Chem Commun 18:1773. doi:10.1039/a702844j
Janiak C (2000) J Chem Soc Dalton Trans 3885. doi:10.1039/b003010o
Acknowledgments
Authors thank CSIR, New Delhi for financial support by grant No. 01 (2152)07/EMR-II.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Singh, N.K., Bharty, M.K., Kushawaha, S.K. et al. Manganese(II) complexes of 5-(4-pyridyl), 5-phenyl and 5-(4-methoxy-phenyl)-1,3,4-oxadiazole-2-thione containing 2, 2′-bipyridyl/ethylenediamine: synthesis, spectral, and X-ray characterization. Transition Met Chem 35, 337–344 (2010). https://doi.org/10.1007/s11243-010-9332-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-010-9332-7