Abstract
Two mono-Schiff base Mn(III) complexes were used to catalyze the hydrolysis of p-nitrophenyl picolinate (PNPP) in micellar solutions of n-lauroylsarcosine sodium (LSS) and polyoxyethylene(23) lauryl ether (Brij35). The results show that both catalysts enhance the hydrolysis of PNPP by over two orders of magnitude relative to the spontaneous rate at 25 °C. Moreover, Mn(III)-promoted hydrolysis of PNPP was faster in LSS micellar solution than in both Brij35 micellar solution and pure buffer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydrolase are enzymes that catalyze a variety of hydrolysis reaction [1]. In the study of the mechanism of these enzymes, artificial hydrolases have attracted much attention as model systems [2].
In the past two decades, many studies [3–11] have focused on the synthesis of artificial enzymes with various structures, especially with respect to access to the active site and hydrophobic microenvironment. As expected, a relatively open active site is important for the association between guest molecule and enzyme [12]. We have previously investigated catalytic reactivities of Schiff base complexes with various structures toward the hydrolysis of carboxylic and phosphate esters [13, 14], and found that manganese(III) Schiff base complexes containing large pendants gave smaller catalytic activities due to bigger steric hindrance for approaching substrate molecules. Nevertheless, the hydrophobicity of the enzyme’s active site is important for substrate docking [15]. Hence, metallomicellar systems [16] have been widely studied as enzyme models for esterolysis. Mostly, cationic single-chain surfactants were used as monomers for the formation of micelle aggregates, while zwitterionic and nonionic surfactants have been less studied [17].
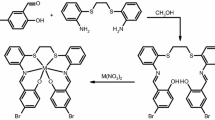
In this paper, we report the reactivity of two manganese(III) Schiff base complexes (Fig. 1) toward the hydrolysis of p-nitrophenyl picolinate (PNPP), a classic model substrate for studies of natural carboxylesterases, in zwitterionic surfactant n-lauroylsarcosine sodium (LSS) or nonionic polyoxyethylene(23) lauryl ether (Brij35) micellar solution, respectively. For comparison, a blank run in non-micellar system was also carried out. The structure–function relations as well as the distinct effects of the two micellar solutions for the two catalysts are discussed.
Experimental section
All reagents, unless otherwise indicated, were of analytical grade and used without further purification. Ultrapure water used in the preparation of all the solutions was obtained from a Water Purification System (Nex Power 1000, Human Corporation, Korea) to achieve a resistivity of at least 16 MΩ cm−1. The resulting pH of reaction solution was 7.00. Ionic strength (I) was kept constant at 0.1 mol L−1 with KCl. The surfactants, LSS and Brij35, were bought from Sigma. Tris (trishydroxymethyl aminomethane) was used as received from Aldrich. PNPP [18] and the Schiff base manganese(III) complexes [19] were synthesized as reported. Stock solution of PNPP (1.0 × 10−2 mol L−1) was prepared in absolute acetonitrile. Owing to low water solubility of the complexes, complex stock solutions were prepared in a mixture of ethanol and water.
Each kinetic run was performed in a 1 cm quartz cuvette, which was placed within the thermally equilibrated cell compartment of a GBC 916 UV–vis spectrophotometer (made in Australia), coupled to a thermostatted water bath with a ±0.1 °C error. The hydrolysis reaction was started by the addition of a stock solution of PNPP of desired concentration by microsyringe. Reference cells consisted of identical solutions without added substrate. In all the hydrolysis systems, the final concentration of catalyst was maintained at 1.0 × 10−5 mol L−1. Under pseudo-first-order conditions, the rates of PNPP hydrolysis were determined by monitoring the increase in absorbance of p-nitrophenolate anion at its characteristic absorbance at 400 nm. The data presented here are the averages of three runs, and the rate constant was reproducible within ±3%.
Results and discussion
Figure 2 shows the variations in pseudo-first-order rate constant (k obs) as a function of PNPP concentration in the three systems studied. As seen in Fig. 2, the hydrolysis rates increase linearly (correlation coefficient r ≈ 0.98) along with the increase in [PNPP] for each catalytic system, which suggests that saturation is not occurring and that the investigated hydrolysis reaction is pseudo-first-order. As compared to the background rate constant k 0 [18], \( {\text{MnL}}^{1}{}_{2} {\text{Cl}} \) exhibited 486-, 186- and 218-fold increase in rate for the hydrolysis of PNPP in LSS, Brij35, and buffer systems, respectively, under conditions of 25 °C, I = 0.1 M (KCl), [Complex] = 1.0 × 10−5 mol L−1. In comparison, \( {\text{MnL}}^{2}{}_{2} {\text{Cl-}} \)catalyzed hydrolysis of PNPP was accelerated by a factor of 626 for LSS, 210 for Brij35, and 252 for buffer under the same conditions. In the entire concentration range of PNPP, \( {\text{MnL}}^{2}{}_{2} {\text{Cl}} \) exhibited 1.1–1.5 times the rate enhancement of \( {\text{MnL}}^{1}{}_{2} {\text{Cl}} \) for the three reaction media.
Dependence of k obs on the concentration of PNPP catalyzed by the two complexes in three micellar solutions, respectively. Conditions: I = 0.1 M (KCl), [MnL2Cl] = 1.0 × 10−5 mol L−1, [LSS] = 5.0 × 10−3 mol L−1, [Brij35] = 1.0 × 10−3 mol L−1. Open rhombus \( {{{\text{MnL}}^{1}{}_{2} {\text{Cl}}} \mathord{\left/ {\vphantom {{{\text{MnL}}^{1}{}_{2} {\text{Cl}}} {\text{LSS}}}} \right. \kern-\nulldelimiterspace} {\text{LSS}}}, \) open circle \( {{{\text{MnL}}^{1}{}_{2} {\text{Cl}}} \mathord{\left/ {\vphantom {{{\text{MnL}}^{1}{}_{2} {\text{Cl}}} {\text{Brij35}}}} \right. \kern-\nulldelimiterspace} {\text{Brij35}}}, \) open triangle \( {{{\text{MnL}}^{1}{}_{2} {\text{Cl}}} \mathord{\left/ {\vphantom {{{\text{MnL}}^{1}{}_{2} {\text{Cl}}} {\text{Buffer}}}} \right. \kern-\nulldelimiterspace} {\text{Buffer}}}, \) solid rhombus \( {{{\text{MnL}}^{2}{}_{2} {\text{Cl}}} \mathord{\left/ {\vphantom {{{\text{MnL}}^{2}{}_{2} {\text{Cl}}} {\text{LSS}}}} \right. \kern-\nulldelimiterspace} {\text{LSS}}}, \) solid circle \( {{{\text{MnL}}^{2}{}_{2} {\text{Cl}}} \mathord{\left/ {\vphantom {{{\text{MnL}}^{2}{}_{2} {\text{Cl}}} {\text{Brij35}}}} \right. \kern-\nulldelimiterspace} {\text{Brij35}}}, \) solid triangle \( {{{\text{MnL}}^{2}{}_{2} {\text{Cl}}} \mathord{\left/ {\vphantom {{{\text{MnL}}^{2}{}_{2} {\text{Cl}}} {\text{Buffer}}}} \right. \kern-\nulldelimiterspace} {\text{Buffer}}} \)
Hydrolysis of PNPP proved to be faster in LSS micellar solution than in nonionic Brij35 micellar solution. For comparison, a blank experiment in the absence of surfactant (only buffer solution) was also preformed. The rate constants of PNPP hydrolysis in various media decreased in the order LSS > Buffer > Brij35, indicating that the inhibition of PNPP hydrolysis occurred in nonionic Brij35 micellar solution.
Proposed mechanism of catalyzed hydrolysis
Normally, metal-catalyzed hydrolysis of carboxylic esters involves a pseudo-intramolecular nucleophilic attack of the metal-bound hydroxide at the carbonyl of the substrate molecule [20]. In this process, the formation of a catalyst–substrate intermediate (left image in Scheme 1) is the key step, in which the substrate PNPP is activated by the central manganese acting as a Lewis acid. Hence, an originally intermolecular reaction is changed to a pseudo-intramolecular reaction [21], a favorable thermodynamic process. The Mn(III)-bound hydroxyl then attacks the carbonyl group of the activated ester to give a hexa-coordinated intermediate, which then releases a p-nitrophenate anion. In this way, a five-membered cyclic transition state (TS) is generated (right image in Scheme 1). Next, the catalytic active species is released, following the substitution of picolinic acid by another water molecule.
Under the conditions used in this work, the Mn(III)-catalyzed hydrolysis of PNPP in all three media is pseudo-first-order. A classic double-reciprocal equation [13] is given as below:
We are able to depict profiles of k −1obs − [PNPP]−1 (refer to supporting information, SI) for MnL2Cl/LSS, MnL2Cl/Brij35, and MnL2Cl/Buffer systems. These plots shown in SI allowed the evaluation of association constants (K s) and first-order rate constants (k) for product formation. The results of these calculations are summarized in Table 1. The data indicate that the association of PNPP to \( {\text{MnL}}^{2}{}_{2} {\text{Cl}} \) is stronger than that of PNPP and \( {\text{MnL}}^{1}{}_{2} {\text{Cl}} \) in all media used in this work. For both catalysts, the largest rate acceleration for PNPP hydrolysis was observed in LSS micellar solution compared to Brij35 micelles and buffer.
To gain insights into structure–activity relationships of natural hydrolases, we have studied catalytic activities of different mono-Schiff base Mn(III) complexes containing benzoaza-15-crown-5 or morpholine pendants toward hydrolysis of model substrates [13, 14]. These earlier obtained results showed that the affinity of substrate for the complexes containing morpholine pendants is stronger than for those containing benzoaza-15-crown-5 pendants.
In the present work, catalytic activities of the two complexes follow the order \( {\text{MnL}}^{1}{}_{2} {\text{Cl}}\; < \; {\text{MnL}}^{2}{}_{2} {\text{Cl}} . \) Considering complex structures, one possible explanation for this trend is that perhaps the variation of 4-substituted groups in aromatic amine moiety has an important effect on the rate of hydrolysis. That is to say, the 4-substituted aza-15-crown-5 inside L1 constructs a relatively crowded active site of \( {\text{MnL}}^{1}{}_{2} {\text{Cl}} \) leading to a weaker association between PNPP and \( {\text{MnL}}^{1}{}_{2} {\text{Cl,}} \) which is unfavorable for the hydrolysis of PNPP. This is supported by the values of the associate constants K s collected in Table 1. These observations obtained are in accordance with our previous reports involving in the effects of steric hindrance on the reactivities of related complexes [13, 14]. Furthermore, the biggest difference (~1.49-fold) in respective linkage strength between PNPP and the two Mn(III) complexes was obtained in LSS micellar solution, which probably results from the different effects of the various media on the local distribution of PNPP and catalyst in micelle aggregates.
Smaller molecule was generally more easily solubilized in micellar solutions [22]. In this work, therefore, the smaller \( {\text{MnL}}^{2}{}_{2} {\text{Cl}} \) should attain a higher local concentration in a small volume of micelle. Then, this enhances the collision frequency of reactants in a small volume of micelle aggregates so as to accelerate the rate of PNPP hydrolytic reaction.
Effects of reaction media on the catalytic hydrolysis
Surfactants in aqueous solutions can affect the kinetic behavior of enzymatic reactions either below or above their critical micelle concentration [23]. Typically, the micelle aggregate exerts its role on the basis of two main effects: hydrophobic interactions concentrate the reactants in the small aggregate volume and charge interactions produce more favorable dissociation equilibria of the nucleophile.
As mentioned above, the rate of PNPP hydrolysis catalyzed by both complexes followed the order: LSS > Buffer > Brij35. This is possibly due to the differences in the local concentration of substrate and catalyst in the three media. In LSS micellar solution, the positive species MnL2(H2O)2 should attain high local concentration via an electrostatic attraction between the positive MnL2(H2O)2 and the negative head group [17] of LSS. This would increase the association between the MnL2(H2O)2 and hydrophobic PNPP in the Stern layer of LSS micelles, resulting in the highest rates of PNPP hydrolysis in LSS micellar solution.
As shown in Table 1, kK s values for both complexes in pure buffer solution are both ca. 1.2-fold higher than those in Brij35 media, implying that this surfactant has a negative influence on the catalysis. The hydrophobic PNPP molecules should be easily embedded in the interior of Brij35 micelle. However, the positive MnL2(H2O)2 molecules would locate in the interfacial region of the neutral Brij35 micelles. This separation of complex and substrate in the micelles may explain the reduced rate in this case.
Conclusion
In summary, the results show that \( {\text{MnL}}^{2}{}_{2}{\text{Cl}} \) has a higher activity than \( {\text{MnL}}^{1}{}_{2} {\text{Cl}} . \) The steric hindrance of the 4-substituted aza-15-crown-5 group could be an important factor for the decreased activity of \( {\text{MnL}}^{1}{}_{2} {\text{Cl}} \) relative to \( {\text{MnL}}^{2}{}_{2} {\text{Cl}} . \) In structural terms, this suggests that exposure of the active site is an important consideration in the design of artificial hydrolases. Among three reaction media studied, LSS micelles promote the hydrolysis of PNPP through favorable electrostatic and hydrophobic interactions.
References
Dugas H (1998) In: Cantor CR (ed) Bioorganic chemistry: a chemical approach to enzyme action. Springer, New York, pp 252–254
Wilcox DE (1996) Binuclear metallohydrolases. Chem Rev 96:2435. doi:10.1021/cr950043b
Weston J (2005) Chem Rev 105:2151. doi:10.1021/cr020057z
Scrimin P, Tecilla P, Tonellato U (1991) J Org Chem 56:161. doi:10.1021/jo00001a033
Zhang YL, Liang HC, Zakharov LN et al (2007) Inorg Chim Acta 360:1691. doi:10.1016/j.ica.2006.09.009
Bertoncin F, Mancin F, Scrimin P et al (1998) Langmuir 14:975. doi:10.1021/la971112f
Mancin F, Tecilla P, Tonellato U (2000) Langmuir 16:227. doi:10.1021/la9909594
Polyzos A, Hughes AB, Christie JR (2007) Langmuir 23:1872. doi:10.1021/la0626454
Fornasier R, Scrimin P, Tecilla P et al (1989) J Am Chem Soc 111:224. doi:10.1021/ja00183a034
Jiang WD, Xu B, Lin Q et al (2007) J Colloid Interf Sci 311:530. doi:10.1016/j.jcis.2007.02.056
Hampl F, Liska F, Mancin F et al (1999) Langmuir 15:405. doi:10.1021/la980861+
Miti N, Smith SJ, Neves A et al (2006) Chem Rev 106:3338. doi:10.1021/cr050318f
Jiang WD, Xu B, Li JZ et al (2006) Prog React Kinet Mec 31:11
Jiang WD, Xu B, Zhong JB et al (2008) J Chem Sci 120:411. doi:10.1007/s12039-008-0065-5
Valérie F, Alain B, Pierre L (1993) J Mol Catal 85:45. doi:10.1016/0304-5102(93)87122-O
Scrimin P, Tecilla P, Tonellato U (1992) J Phys Org Chem 5:619. doi:10.1002/poc.610051002
Jiang BY, Xiang Y, Du J et al (2004) Colloids Surf A 235:145. doi:10.1016/j.colsurfa.2003.10.019
Sigman DS, Gutsche CT (1972) J Am Chem Soc 94:1724. doi:10.1021/ja00760a051
Zeng W, Li JZ, Mao ZH et al (2004) Adv Synth Catal 346:1385. doi:10.1002/adsc.200404094
Weijnen JGJ, Koudijs A, Schellekens GA et al (1992) J Chem Soc Perkin Trans 2:829
Satchell DPN, Satchell RS (1978) Kinetic studies of metal ion catalysis of heterolytic reaction. Annu Rep Prog Chem Sect A Inorg Phys Chem 75: 25–48 (Chap 3)
Choi TS, Shimizu Y, Shirai H, Hamada K (2000) Dyes Pigm 45:145. doi:10.1016/S0143-7208(00)00015-2
Abuin E, Lissi E, Duarte R (2003) Langmuir 19:5374. doi:10.1021/la030050s
Acknowledgments
We would like to thank the financial support given by Scientific Research Foundation for the PhD (Sichuan University of Science & Engineering, No: 07ZR14) and Key Scientific and Technological Project (No: 208118) issued by the Ministry of Education.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, B., Jiang, W., Zhang, J. et al. Effect of manganese(III) Schiff base complexes on the hydrolysis of p-nitrophenyl picolinate. Transition Met Chem 34, 293–296 (2009). https://doi.org/10.1007/s11243-009-9193-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-009-9193-0