Abstract
The thermodynamically constrained averaging theory (TCAT) is an evolving approach for formulating macroscale models that are consistent with both microscale physics and thermodynamics. This consistency requires some mathematical complexity, which can be an impediment to understanding and efficient application of this model-building approach for the non-specialist. To aid understanding of the TCAT approach, a simplified model formulation approach is developed and used to show a more compact, but less general, formulation compared to the standard TCAT approach. This new simplified model formulation approach is applied to the case of binary species diffusion in a single-fluid-phase porous medium system, clearly showing a TCAT approach that is applicable to many other systems as well. Recent extensions to the TCAT approach that enable a priori parameter estimation, and approaches to leverage available TCAT modeling building results are also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Porous medium systems have traditionally been modeled at the macroscale in which a point consists of an averaging region over all entities in the system (e.g., phases, interfaces, common curves, common points). Macroscale conservation equations are thus expressed in terms of quantities such as volume fractions, or fluid saturations, as well as effective-medium coefficients (permeability, thermal conductivity, etc.), as a result of formulation scale. While macroscale models are necessary to solve most problems of practical interest because of the length scales involved, microscale approaches have been widely studied over the last two decades as a means to advance fundamental understanding (e.g.,Kischinhevsky and Paes-leme 1997; Ahmadi et al. 2010; Perović et al. 2017).
The formulation of theoretical approaches to formally deduce macroscale models based upon microscale systems that are more fundamentally well-understood has been a long-term pursuit of porous medium theoreticians. It is conceptually appealing to formulate macroscale models that are explicitly connected to their microscale counterparts, including all extensive and intensive variables. This appeal is twofold. First, a firm connection to microscale physics provides explicit definitions for all quantities that are unambiguous. Second, the explicit connection enables microscale analysis and computational approaches to inform macroscale closure relations, thus leveraging the considerable fundamental microscale advances that have occurred in recent years. Several functional and interesting approaches are available in the literature to deduce macroscale models from microscale formulations, among them let us direct the attention in the following paragraphs to the method of volume averaging (MVA) and the thermodynamically constrained averaging theory (TCAT).
The MVA consists of an application of an averaging operator to the differential equations that govern microscale flow or transport. Subsequent application of averaging theorems and spatial decompositions lead to a model that is written in terms of average quantities and spatial deviations. This motivates the formulation, simplification, and formal solution of ancillary boundary-value problems in representative microscale zones in order to deduce the relation between deviation quantities and average properties. Once this relation is derived, it is substituted into the averaged model in order to obtain a macroscale model that is written only in terms of average quantities and effective-medium coefficients. An attractive feature of this method is that it allows one to identify the time and length-scale constraints supporting the macroscale model. In addition, the effective-medium coefficients can be predicted by means of the ancillary problem’s solution mentioned above. The MVA has been extensively used to study the transport of mass with (Ostvar and Wood 2016; Ryan et al. 1980) and without (Eidsath et al. 1983; Plumb and Whitaker 1990) reaction, heat (Nozad et al. 1985; Hager and Whitaker 2002), electrical charge (del Rio and Whitaker 2001), and momentum in both homogeneous (Whitaker 1986) and heterogeneous (Quintard and Whitaker 1988, 1996) porous media as well as in biological systems (Wood and Whitaker 1998; Wood et al. 2002; Golfier et al. 2009; Valdés-Parada et al. 2009) and even in megascale systems (Valdés-Parada et al. 2012). In addition, the MVA has been used to derive jump boundary conditions between heterogeneous media (Ochoa-Tapia and Whitaker 1995), and more recently to determine the dividing surface position where the boundary conditions should be applied (Valdés-Parada et al. 2013). This method has been compared with the method of ensemble averaging (Wood et al. 2003) and also with the homogenization method (Davit et al. 2013) with several points of similarity identified.
The thermodynamically constrained averaging theory (TCAT) has evolved recently as an alternative approach for macroscale model formulation (Gray and Miller 2005, 2014; Miller and Gray 2005), building upon, extending, and modifying earlier work on formal averaging methods. The evolution of TCAT and a comparison with other averaging methods is detailed in a recent review paper (Gray et al. 2013) and need not be repeated here. The TCAT approach has some attractive features, including a firm connection of all extensive and intensive quantities across length scales; the inclusion of a complete set of entities including phases, interfaces, common curves, and common points; thermodynamic constraints derived from microscale thermodynamics; consistency with provable equilibrium constraints; a set of kinematic equations expressing relationships among geometrical quantities derived from averaging theorems and reducing the closure problem needed to formulate closed, solvable macroscale models; and a framework for constructing models of varying sophistication and fidelity. Moreover, even in cases in which solvable microscale models are not available, it is still possible to formulate macroscale models from TCAT in terms of microscale quantities.
In contrast to the MVA, which has to be applied in its entirety in each case, the standard TCAT approach has advanced to generate a broadly applicable entropy inequality, which can support the closure of a hierarchy of models of a given class. The classes of models developed to date include: single-fluid-phase flow (Gray and Miller 2014), megascale models of single-fluid-phase flow (Gray and Miller 2009c), single-fluid-phase flow and species transport (Miller and Gray 2008; Gray and Miller 2009b, 2014), single-fluid-phase flow and heat transport (Gray and Miller 2009a), two-fluid-phase flow (Jackson et al. 2009; Gray and Miller 2011, 2014; Dye et al. 2015; Gray et al. 2015), two-fluid-phase flow and species transport (Rybak et al. 2015), and modeling of the transition between a two-fluid-phase porous medium system and a single-fluid-phase domain (Jackson et al. 2012). These applications are based upon deterministic modeling approaches. The extension of TCAT to stochastic models is yet to be developed. The attractive features of TCAT are accompanied by mathematical complexity and a need for physical intuition in the formulation processes, which has been a barrier to entry for some non-specialists wishing to understand and use the approach. Indeed, the use of physical intuition is a valuable aid that is present in practically all upscaling approaches including TCAT. Decision making based on this intuition is crucial as it can make a difference between a physical and an unphysical upscaled model. Fortunately, TCAT involves steps that allow checking the validity of the approximations made as will be detailed below. Reducing the barriers to entry for TCAT model formulation would be an important contribution to the community. Leveraging a generally broader understanding of the MVA and developing a pedagogical approach of minimal complexity would help promote understanding of the essential features of the TCAT approach.
The overall goal of this work is to reduce the complexity of the standard TCAT model-building approach and to demonstrate how the new approach and available results can be leveraged to derive new models. The specific objectives of this work are: (1) to summarize the steps involved with the TCAT approach; (2) to develop a simplified TCAT approach to reduce the complexity associated with the standard TCAT approach; (3) to illustrate the new model formulation approach for the case of passive diffusion of a dilute species; and (4) to discuss ways in which available approaches and results can be used to build new TCAT models and provide a priori estimation of parameters.
2 Model Formulation
Mechanistic models of porous medium systems are the gold standard for describing mass, momentum, and energy transport in subsurface systems. A rich and diverse set of applications rely upon the solution of such models. However, the formulation of these models is challenging and many open questions remain. Because the length scales of concern in applications are typically much larger than the characteristic length scale of a pore opening in a porous medium system, mechanistic models are typically formulated at a macroscale that represents averaged conditions over some representative volume of the porous medium. Conservation equations are used as the basis for such models, but these equations contain more unknowns than equations. To render the systems solvable additional equations are needed, which take the form of closure relations. Examples of closure relations are equations of state that relate the density of phase as a function of temperature, pressure, and composition of a phase; an approximation for the rate of interfacial mass exchange; and an expression for a reaction rate of a species. Whereas conservation equations are essentially exact, closure relations are typically simple approximations of material behavior that are complicated when viewed at a smaller length scale. For example, the ideal gas law is a simple approximation for the net effect of molecular gas movement and interaction.
Many combinations of conservation equations and closure relations are available in the literature, which lead, combinatorially, to a large set of potential mechanistic models. There are a number of open questions about how mechanistic models of porous medium systems should be formulated for possible different applications. Some of the questions that occur routinely involve, for example, the appropriate form of the conservation equations; the form of the closure relations; how sources of information other than conservation equations alone can be included in the model formulations; how mechanistic models can be formulated to be consistent; and how mechanistic models can take advantage of the knowledge of microscale (or pore scale) physics when it is available, which is increasingly the case nowadays. The TCAT method is a theoretical approach for responding to these model formulation issues. The sections that follow outline the steps involved with this approach, the components involved with the formulation of a model, and how these components interact to yield mechanistic models with a desirable set of properties.
3 Overview of the TCAT Approach
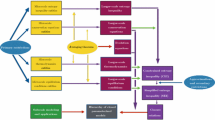
The TCAT model formulation proceeds via a carefully structured process that is shown in Fig. 1, where model formulation proceeds in the direction indicated by the arrows. The end result is a hierarchy of closed parameterized models, which is indicated by the box in the center-bottom of this figure. The entries within the yellow rectangles indicate microscale equations, which can be averaged over a representative elementary volume (REV) to yield the corresponding entries in the maroon rectangles. Evolution equations are derived directly from specific averaging theorems (e.g., Gray and Miller 2014). Larger scale can for example be the macroscale, where a closed, solvable model is sought. The indicated larger-scale equations are used to derive the entropy inequality expressions, which are important components of TCAT and shown in purple. The purpose of these entropy inequalities is to guide the formulation of closure relations, which are combined with conservation and evolution equations to yield the closed and solvable macroscale models sought. It is not necessary to derive each of these components as many of these components already exist and can simply be used. The restrictions and approximation noted in the blue ovals represent decisions the model builder must make, which are discussed in additional detail below. The final upscaled model is identified in gray. This model-building process is detailed in the annotated description that follows:
-
1.
Primary restrictions are imposed, which should specify the entities (phases, interfaces, common curves, and common points) and the phenomena (flow, species transport, heat transport) to be modeled, as well as the microscale thermodynamic theory that is relied upon.
-
2.
A set of macroscale equations, including a balance of entropy, conservation equations, thermodynamic equations, and the macroscale conditions that must apply at equilibrium, is derived from the corresponding microscale equations. This is achieved by application of an averaging operator and the use of a set of transport, divergence, and gradient theorems.
-
3.
The entropy balance equations for all species and all entities are summed resulting in an expression for the rate of entropy production, termed the macroscale entropy inequality (EI), which must be greater than or equal to zero by the second law of thermodynamics.
-
4.
The EI is augmented with a set of conservation and thermodynamic equations corresponding to the entities and processes being modeled, which is done to connect the dissipative entropy producing processes to the rate of entropy production. The form of the equations added assures the EI is not altered, and Lagrange multipliers are used to aid the reduction of the EI to a flux-force form.
-
5.
The EI is manipulated in order to formulate it as close as possible to a flux-force form without introducing approximations. This intermediate version of the augmented EI is denoted as the constrained EI (CEI) and it is characterized by its exact and general nature.
-
6.
In order to produce a strict flux-force form of the CEI, a set of approximations and secondary restrictions are adopted, thus yielding a simplified EI (SEI). In this way, the SEI takes into account particular features of the system of interest. The SEI provides a set of permissibility conditions on closure relations used to complete the macroscale model. Although the closure relations are not unique, they must meet permissiblity constraints.
-
7.
The final ingredient of the macroscale model is a set of evolution equations, which are derived from the averaging theorems and certain approximations. These equations involve macroscopic quantities such as volume fractions, specific interfacial areas, wetted fractions of the solid phase, specific common curve lengths, and curvatures.
-
8.
The macroscale model is formulated by combining the conservation equations, closure relations, and evolution equations.
-
9.
Inverse modeling, involving computational or experimental data, is performed in order to compute the values of the coefficients involved in the model. Microscale information can also be used to evaluate and validate the approximations made and the resultant macroscale model.
The development of scale and thermodynamically consistent models using the TCAT approach requires both mathematical and physical insights, which deserve some additional discussion. These steps are explicitly noted in the approach. In most cases, a TCAT model can be formulated without deriving each of the formulation components that are mentioned above and shown in Fig. 1. For example, the conservation and balance equations and thermodynamic relations have been developed already (Gray and Miller 2014) and can be used without rederiving them; similarly, equilibrium conditions and evolution equations are also available. For the formulation detailed herein, we will use these available model-building components.
While the available TCAT components are useful, the TCAT formulation process requires a variety of restrictions and approximations, which are mentioned in the TCAT components annotated above and graphically depicted in Fig. 1. As a minimum, one must first make primary restrictions to define the system being modeled and the thermodynamics that will be relied upon. Typically, these primary restrictions are the minimal set needed to target a broad class of models. These selections provide the information necessary to develop a CEI. After a CEI is formulated, it is necessary to make approximations to place the CEI in a general flux-force form. These approximations require physical and mathematical insight. This can be a challenging step in the model formulation process. However in cases for which microscale information is available, these approximations can be evaluated and refined as needed, since these results are based on approximations and are always subject to change as improved approximations are developed. The result of this step is an SEI. A general SEI can be developed using an appropriate set of approximations. Because as much generality as is practical is typically sought, the SEI can often be a daunting expression. Therefore, it is usual to examine models that are some subset of the general class of model that is broadly considered. For this effort, secondary restrictions can be applied and used to reduce the SEI to a shorter, more convenient form needed for a specific application. Physical insight is needed to understand how a set of secondary restrictions can be used to reduce an SEI. Once an SEI has been formulated in the most convenient form, this expression must be used to posit a permissible set of closure relations sufficient to produce a closed, solvable model. This step requires care and physical insight as well, and similar to the approximation step, closure relations are also subject to change as they too are approximations. Therefore, even using available formulation components, TCAT involves four specific locations where physical and mathematical insight is needed to produce a closed, solvable model. The TCAT structure makes these steps clear and if a model must be reformulated because it is found to be deficient in some respect, the points where these restrictions or approximations are made are the natural entry points to begin the reformulation. The TCAT method is structured such that these reentry points can be used with the minimal amount of additional work. This is why the CEI and the general SEI are typically viewed as being standard archival results. These equations have already been derived and recorded (Jackson et al. 2012; Gray and Miller 2014; Rybak et al. 2015) for many important classes of models.
4 Simplified TCAT Model Formulation Approach
The standard TCAT model-building approach results in CEIs and SEIs for each major class of model (e.g., Jackson et al. 2012; Gray and Miller 2014; Rybak et al. 2015). These EI expressions are important and of archival value, as we will explore subsequently. However, the general form of the CEI and SEI for porous medium systems is typically multiple page expressions comprised of heavily adorned symbols representing specific sorts of averages of variables related to the dissipative processes that produce entropy and must be approximated to produce closed, solvable models. Both of these expressions are important. The CEI is important because it is an exact expression, subject to the primary restrictions. Since the set of approximations made to reduce the CEI to the SEI are non-unique and subject to change, the CEI is an appropriate place to begin reformulation of a model if needed. Thus a single CEI can lead to multiple SEIs. The SEI is an important expression because it is in a pure flux-force form, which is needed to determine permissibility conditions for closure relations needed to formulate a well-posed model. However, a single SEI only restricts permissible forms of closure relations, which are non-unique. Thus, model reformulation may often start from a given SEI to produce an admissible set of closure relations, making the SEI of archival value.
The TCAT approach has evolved to derive the most general possible forms of the CEI and SEI, such that these general forms can support the broadest possible set of model formulations. The generality of these expressions is reflected in their length and seeming complexity, which can be a barrier to the use of these expressions and adoption of TCAT as a model-building approach. This complexity is also an impediment to the researcher wishing to understand the details of the approach.
To aid the use and hasten the rate of learning the fundamentals of the TCAT framework, we propose a modification to the traditional approach. In summary, we will sacrifice the generality of the standard TCAT approach in order to yield an approach that leads to simple forms of the CEI and SEI. The proposed approach does not affect the steps shown in Fig. 1; it only affects the nature of the primary restrictions made at the onset of the model formulation. Specifically, the model that one wishes to formulate is detailed up front, including all simplifications. The specifics of the model are then posed as a set of primary restrictions. The primary restrictions for a simple model will be much more extensive than would normally be the case; the usual approach is aimed at minimizing the primary restrictions. In contrast, for the pedagogical approach we describe here, we wish to maximize the primary restrictions. The primary restrictions then result in simplifications from the very beginning and can result in a compact CEI and SEI. The final model will be the same as if a general approach was taken and the simple model instance was derived by applying a set of secondary restrictions after the derivation of a general form of the CEI and the SEI, which is the standard approach shown in Fig. 1. The proposed approach can accomplish the objectives of greatly simplifying the TCAT model formulation approach and enabling the approach to be understood clearly without overwhelming detail. To demonstrate this modified approach and fix ideas, we consider a specific example in the following section.
5 Example Application: Single-Phase Passive Diffusion in Homogeneous Porous Media
TCAT has been applied to develop macroscale species transport models for a single-fluid-phase porous medium system that are connected with microscale conservation and balance principles and which are thermodynamically consistent and constrained (Miller and Gray 2008; Gray and Miller 2009b, 2014). The approach taken in these works is consistent with the approach shown in Fig. 1. Gray and Miller (2009b) demonstrated how two different sets of primary restrictions result in two different modeling frameworks. These differences resulted from a specification of whether momentum was modeled for an entity or for a species in an entity.
In both of the existing TCAT models for single-fluid-phase species transport, the general TCAT approach explained above was taken. For this example, we will detail the model that we wish to formulate and cast these details into an expanded set of primary restrictions, compared to the formulations accomplished to date. The steps taken in this modified approach are detailed in the sections that follow. The example case considered is dilute, non-advective and non-reactive species transport in an isothermal, single-fluid-phase, non-deformable porous medium of constant and uniform porosity. The approach that is detailed also corresponds to the model formulation approach shown in Fig. 1.
5.1 Primary Restrictions
The TCAT formulation approach starts with a set of primary restrictions, which detail aspects of the model formulation. As discussed above, a broad set of primary restrictions will be used to simplify the model formulation process. The primary restrictions are:
-
1.
The entities modeled consist of a water phase, denoted with index w, and moving at a constant velocity \({{\mathbf {v}_{w}}}\) that is independent of space and time; and an inert, incompressible, non-deformable solid phase, denoted with index s, that is moving at a constant velocity \({{\mathbf {v}_{s}}}\) that is independent of space and time;
-
2.
The process of concern is limited to non-advective, non-reactive species transport, which implies that \({{\mathbf {v}_{w}}}={{\mathbf {v}_{s}}}\) for all space and time;
-
3.
The system is isothermal;
-
4.
Transfer of mass, momentum, and energy does not occur between the water and solid phases;
-
5.
A clear separation of length scales exists and the macroscale is above the REV limit; and
-
6.
The solid phase distribution is homogeneous, but not necessarily isotropic, in all relevant morphological measures at the macroscale.
This set of primary restrictions will allow all steps in the TCAT process to be detailed completely and concisely to help readers better understand the primary aspects of the theory. Note that, for this case, the set of entities does not include interfaces, common curves, or common points, which will typically be needed to produce high-fidelity models for multiphase systems. Reducing the number of entities modeled and the processes modeled reduces the scope of each subsequent step in the TCAT formulation, while still preserving the basic model-building structure.
5.2 Microscale Equations
A macroscale TCAT model can be formulated directly from available macroscale conservation, balance, potential, and thermodynamic equations. Microscale equations do play two important roles in TCAT models. First, for a complete understanding of a TCAT model formulation, it is useful to understand the formulation of microscale equations and the application of an averaging operator to these microscale equations to yield components of the macroscale model. Second, because all macroscale quantities are explicitly described in terms of microscale quantities, knowledge of these quantities is useful for evaluating components of macroscale TCAT models, including the evaluation of certain approximations relied upon to close macroscale models. Because of these important uses, we will briefly summarize a set of microscale equations required to build a macroscale TCAT model for the single-fluid-phase species transport case being considered.
The relevant microscale equations include a conservation of mass equation for a species in a phase, a conservation of energy equation, a gravitational potential equation, an entropy balance equation, a thermodynamic equation, a fluid potential identity, and a thermodynamic condition classifying the equilibrium state of the system. Given the restrictive conditions placed on the solid phase, no solid phase equations are needed. Similarly, a conservation of momentum equation is not required because the mean relative velocity of the two phases is zero. The primary restrictions also lead to many simplifications of the equations that can be formulated. These simplifications will be mentioned in turn for the microscale equations summarized below.
The relevant microscale conservation of mass equation for a species in the water phase for the limiting case defined above is
where t is time, the index w denotes the water phase, \({{\rho _{w}}}\) is the fluid density, \({{\omega }_{{{i}}{w}}}\) is the mass fraction of species \({i}\) in the water phase, \({{\mathbf {u}}^{\,{}}_{{i}w}}\) is the deviation velocity of species \({i}\) in the water phase, and the material derivative operator is defined as
Indeed, since the velocities of the fluid and solid phases are zero in this example, the material derivative is equal to the partial time derivative; this is generally not the case for TCAT models. Furthermore, because the conservation equations apply in any inertial frame of reference, the standard TCAT approach references all velocities to a common reference velocity. This is done by expressing all equations in material derivative form, which is the approach that is followed here as well for consistency with the standard approach. The terms for advective transport and reaction vanish for the particular case being considered due to the primary restrictions noted above.
The microscale conservation of energy equation for the water phase for the case at hand is
where \({{K}^{\,{}}_{Ew}}\) is a kinetic energy term due to velocity fluctuations, \({{\mathbf {q}_{w}}}\) is a non-advective energy flux vector resulting from velocity fluctuations, and \({{{{h}^{\,{}}_{w}}}}\) is a body source of energy. As a result of the primary restrictions noted above, several terms vanish from the conservation of energy equation, including internal energy, all terms involving the mean velocity, and a summation of the constant body source fluctuation velocity product term.
The microscale gravitational potential equation for the water phase is
where \({\psi _{}}\) is a body force potential per unit mass, which for this example is a result of gravitational acceleration alone, \(\mathcal {J}_s\) is the index set of species.
The microscale entropy balance equation for the water phase is
where \({{\eta _{w}}}\) is the entropy density, \({{\mathbf {\varphi }_{w}}}\) is a non-advective entropy flux vector, \({{b_{w}}}\) is an entropy source density, and \({{{\Lambda }_{w}}}\) is the entropy production rate density. It can be observed that none of the variables in Eq. (5), aside from the independent variables associated with space and time, appear in any of the other conservation equations.
The microscale differential thermodynamic equation for the water phase is
where \({{{\theta }_{w}}}\) is the temperature, and \({{{\mu _{{i}w}}}}\) is the chemical potential. Eq. (6) is a dynamic equation that expresses the relationship among variables close to equilibrium. A term related to the change in internal energy has been dropped as a result of the isothermal primary restriction. Note that this equation includes variables that occur in both the conservation of mass equation and the balance of entropy equation. Thus, this dynamic equation makes a connection between the second law of thermodynamics and the conservation equations that includes the dissipative process for which a closure relation is needed. This parallel will exist at the macroscale as well.
The microscale fluid potential energy identity for this restricted system is
where the material derivative of the species potential vanishes in this case.
Finally, the microscale equilibrium condition for the limiting case described above is
where \({\lambda }_{{\mathcal M}_{i}}\) is a constant. This equation implies that the sum of the chemical and gravitational potentials are constant at equilibrium at the microscale, hence no spatial gradient exists at equilibrium. Details of the formulation of each of the more general microscale equations from which these simplified versions were derived are available in the literature (Gray and Miller 2014).
5.3 Macroscale Equations
The TCAT approach requires that all microscale components be averaged to the macroscale and simplified using an appropriate set of theorems. The details for deriving the macroscale equations have been presented elsewhere (Gray and Miller 2014); thus, we only present the results here. Because of the primary restrictions previously listed, the general macroscale equations can be simplified as was accomplished for the microscale equations. Since the terms that vanish are similar to the microscale case, these simplifications will not be explicitly mentioned. All exchange terms vanish because the water phase is the only active phase, given the restrictions imposed for the solid phase.
The macroscale conservation of mass equation for a species in this restricted system is
where \({{{\epsilon }^{{\overline{\overline{w}}}}}}\) is the volume fraction of the wetting phase, or the porosity for this single-fluid-phase case. Superscripts denote macroscale quantities, and the adornments on the superscripts convey information about how these averages are computed. One must know precisely how an averaged quantity is computed from microscale quantities in order to have the connection between all microscale quantities and all macroscale quantities that we desire. The notation used conveys these details without the need to include averaging operators for each variable. Briefly, unadorned superscripts refer to volume averages, a single overbar refers to quantities that are averaged by weighting with the mass density, and double overbar quantities refer to any other type of average, which is explicitly defined. Further details of the averaging are not needed to understand the TCAT approach, but these details are available to the interested reader (Miller and Gray 2005; Gray and Miller 2014).
The macroscale conservation of energy equation for the w phase in this restricted system becomes

The macroscale gravitational potential equation for this restricted system is
The macroscale balance of entropy equation for this restricted system is
The macroscale differential thermodynamic equation for this restricted system is
where the angle brackets indicate an averaging operator, which can be written in integral form (Gray and Miller 2014). Such an averaging operator was applied to all microscale equations to derive the macroscale forms, but in other cases the operation was evaluated yielding macroscale variables.
A fluid potential energy identity for this restricted system is
Finally, the macroscale equilibrium condition for this restricted system is
where the implications of this condition are analogous to those detailed for the corresponding microscale equation.
The macroscale equations summarized above form a set of equations that can be used to formulate a statement of the second law of thermodynamics that includes the operative conservation, potential, and thermodynamic equations. This entropy inequality will be detailed in the section that follows. The conservation equations are also used to derive the closed model that is sought.
5.4 Constrained Entropy Inequality
The entropy production for a system is typically computed by summing over all entities and species in a system. Because of the restrictions applied to this system, the solid phase will not produce any entropy because it is at a fixed temperature and composition, and is incompressible. Thus, the second law of thermodynamics for the system reduces to
To connect the entropy balance equation to the conservation equations and the thermodynamics of the system, the macroscale equations, which are all arranged so that they are equal to zero, can be multiplied by a Lagrange multiplier and added to Eq. (16) to yield the augmented EI given by
which may be written in expanded form by substituting in the macroscale equations giving
Because we wish to derive an EI that ultimately is in a strict flux-force form, we wish to eliminate the material derivatives to the extent possible through an appropriate use of Lagrange multipliers. Since these Lagrange multipliers are applied to terms that are already zero, any selection of values for these multipliers is, in principle, permissible. Removal of the material derivatives to the extent possible is the guiding principle for all TCAT formulations. For this particular case, the material derivatives of terms involving entropy, species mass, and gravitational potential densities occur in multiple locations within Eq. (18), and the species mass densities are of a common form for all species. Because the kinetic energy, velocity fluctuations, chemical potentials, and temperature only occur in a single term, it will be impossible to eliminate these material derivatives without a trivial selection of Lagrange multipliers, which would preclude eliminating the material derivative of entropy. Thus three types of material derivatives can be removed, meaning that constraints are needed for the Lagrange multipliers to produce a unique solution. We formulate these constraints as
which reduces the free Lagrange multipliers to three—matching the number of material derivatives to be eliminated. These free multipliers can be selected to eliminate the material derivatives associated with entropy, species mass, and gravitational potential densities. This problem can be solved by inspection yielding the solution
Substituting the results given in Eq. (20) into Eq. (18) yields
Dropping the material derivatives that cancel from Eq. (21) leads to
Applying the product rule to the term involving the product of potentials and the divergence of the deviation velocities, writing the partial derivative of the gravitational potential in material derivative form, simplifying, and rearranging the resulting expression yields
Equation (23) is a constrained entropy inequality that is consistent with the set of restrictions made for this particular case. No additional assumptions or approximations have been applied beyond the original set of primary restrictions. Thus, to the extent that the primary restrictions are true for the system of concern, Eq. (23) is an exact expression. However, none of the lines of this equation are in a flux-force form. The fourth line of this equation is close to a flux-force form since Eq. (15) requires that the sum of potentials is constant at equilibrium, however kinetic energy terms also appear in this expression. To preserve what is typically an essentially exact result, the TCAT approach produces a CEI that is of archival value. Steps taken to reduce the CEI to a strict flux-force form require assumptions. Thus one CEI can lead to multiple SEIs depending upon the assumptions made. A path to the reduction of the CEI to a strict flux-force form is detailed in the following section.
5.5 Simplified Entropy Inequality
The CEI given by Eq. (23) is a statement of the second law of thermodynamics. The way in which this equation is formulated results in a connection of the conservation, potential, and thermodynamic equations to the entropy balance equation. Subsequent manipulations yielded a reduced expression that is relatively compact but is not in a strict flux-force form. The goal of formulating an SEI is to produce a strict flux-force entropy inequality, which can in turn be used to constrain the form of the closure relations needed to produce a closed, solvable macroscale model. This step requires approximations. These approximations may not be unique and are subject to evaluation and revision. They are necessary however—as such the SEI is an approximate equation.
For the case at hand, two approximations are needed to produce a flux-force form of the SEI. First, it is common at the microscale to consider the relation between source and flux terms that appear in the energy equation and the balance of entropy. Such terms can be equated for the case in which the system is considered thermodynamically simple. By analogy at the macroscale, we consider a simple system in which the source of entropy is related to the heat source, and other sources resulting from material derivatives of fluctuations, such that the first two lines in Eq. (23), vanish. Also by analogy for a simple microscale system, the entropy flux vector is equivalent to the heat flux vector plus the fluctuation terms that appear in the third line of Eq. (23), such that this line is zero as well. Second, the kinetic energy terms that appear in the fourth line of Eq. (23) are higher order and approximated as being negligible. Together these conditions form a set of SEI approximations, which allow the reduction of the CEI to an SEI given by
Equation (24) is in flux-force form because the sum of potentials is constant at equilibrium, which is a condition given by Eq. (15), and therefore the gradient will vanish at equilibrium. The macroscale fluctuation velocity \({{\mathbf {u}}^{{\overline{\overline{{i}w}}}}}\) also vanishes at equilibrium, thus this term involves the product of two terms that both vanish at equilibrium. This is the classical flux-force form that is sought for all SEI expressions, although an SEI for more complicated, and typical, cases will include many more flux-force pairs.
The above reasoning is typical of other TCAT formulation approaches used to transform a CEI to an SEI. Some approximations are needed, which in this case were a simple system and a higher order term approximation. Typically, additional approximations are needed, which may include approximations related to the correlation between product terms. It will also be usual that given a microscale solution, the approximations made to arrive at the SEI can be evaluated to determine for example if a term concluded to be small is indeed small and the extent of the correlation between terms that were approximated as being uncorrelated. Such evaluations can build confidence in the approximations made and the validity of the resulting model.
Because the SEI is used to derive permissibility conditions for closure relations, it is an important equation with archival value. A single SEI can be used to specify multiple sets of closure relations, hence models, in most cases. In the typically general approach taken to derive an SEI, a much longer and more complicated SEI will result than Eq. (24). Simplifications of a general SEI may be made by adding secondary restrictions, which reduce a general SEI to a form that is applicable for a given model instance that is a subset of the model originally specified by the primary restrictions. For example, the isothermal, non-reactive, no inter-entity exchange of any conserved quantity, and the fixed, inert, and isotropic solid phase restrictions could all have been applied after a general SEI was derived, as would normally be the case for a general TCAT formulation approach.
As an example, in formulating Eq. (24) no assumption has been made about the number of species in the w phase, nor has this equation been simplified to account for the constraints given by
and
Let us state a secondary restriction that there are only two chemical species in the w phase, such that \(\mathcal {J}_s=\{A,B\}\) and that species A is a dilute solute and species B would be the solvent, or water, species. Let us then apply Eqs. 25a and 25b to this binary species system to produce a restricted SEI from Eq. (24) of the form
This equation is equivalent to Eq. (24) but it is constrained to ensure independence of the flux and force. Indeed, one of the flux-force pairs has been removed. This is to be expected because only \(N-1\) diffusive velocity equations can be independent. Thus, this result is a physically constrained case that must hold and is needed to ensure independence of all fluxes and forces in the SEI. Furthermore, since the body force potential is only due to gravity, the porosity is constant, and the system is dilute, the difference in body force potentials is neglected, and the restricted SEI becomes
This restricted SEI can be used to produce a closed model as shown in the section that follows.
5.6 Closed Model
A closed macroscale TCAT model is produced by combining an appropriate set of macroscale conservation equations with closure relations and equations of state that follow from an SEI corresponding to the model one wishes to derive. For the example system being considered, Eq. (27) provides the SEI needed to produce a closed model. The conservation of mass equation for a non-reactive species that does not undergo mass transfer between entities may be written as
Because the mean velocity of the fluid phase is assumed to be zero, this equation may be written as
Also, since the solid phase is immobile, incompressible, and spatially invariant at the macroscale, the above equation can be simplified to
Thus our model reduces to a single equation with five unknowns. Equation (27) provides a constraint on the allowable forms of the closure relation needed for \({{{\mathbf {u}}^{{\overline{\overline{{i}w}}}}}}\). A first-order closure approximation for \({{{\mathbf {u}}^{{\overline{\overline{{i}w}}}}}}\) is
where \({{\hat{\varvec{\mathsf {D}}}}}^{ABw}\) is a second-rank symmetric closure tensor for the binary, isothermal system and \({{x}^{{\overline{\overline{{i}w}}}}}\) is the mole fraction of species \({i}\) in entity w. The form given in Eq. (31) may be unsettling to readers expecting the diffusive velocity to be proportional to the gradient of the chemical potential and not to the relative chemical potential. Before moving on, it is opportune to address this issue in some detail. Let us commence by writing the Gibbs–Duhem equation as:
For the isothermal case considered and holding pressure constant, Eq. (32) reduces for a binary species system to
Since Eq. (33) establishes that the gradient in chemical potentials cannot be independent, we can solve for the gradient of the chemical potential of the species B giving
Since \({{\omega }^{{A\,}{\overline{w}}}}\ll {{\omega }^{{B\,}{\overline{w}}}}\) for a dilute solution, in fact vanishingly small in most dilute species cases typically considered, it follows that \(|\nabla _{\mu }^{Bw}\ll \nabla _\mu ^{Aw}|\) and consequently Eq. (31) would reduce to be proportional only to the gradient of the chemical potential of the solute. The same result that appears in Eq. (31) can also be obtained using other approaches, such as with the use of the Stefan-Maxwell equations (e.g., Whitaker 2009).
From Eq. (31), it is evident that an equation relating the macroscale chemical potential with the macroscale mass fraction is needed. To this end, let us derive such a relationship starting with the definition of a macroscale activity coefficient, \({{\hat{{\gamma }}}}^{{\overline{\overline{{i}w}}}}\), consistent with a reference state
which allows the macroscale chemical potential to be expressed as
where \(\mu ^{{\overline{{i}w}}}_0({{{p_{}^{w}}}},{{{{\theta }}^{{\overline{\overline{w}}}}}})\) is a reference chemical potential for species \({i}\) in the w entity, R is the ideal gas constant, and \(MW_{i}\) is the molecular weight of species \({i}\), which enters the formulation because chemical potentials are here defined on a unit mass basis. From this expression, we obtain
Because we are concerned with a dilute system, it follows that
and
Substituting Eq. (39) into Eq. (31) gives
Or since the system contains only two species, it follows that
and the macroscopic flux takes the form
Equation (42) can be written with algebraic rearrangement as
where \(MW_w\) is the molecular weight of the w entity defined as (Bird et al. 2002)
The gradient of mass fraction may be related to the gradient of the mole fraction giving
which can be used to write Eq. (43) as
and noting
allows Eq. (46) to be written as
Substituting Eq. (48) into Eq. (30) yields
For a dilute, isothermal system if we consider water to be essentially incompressible and define the concentration as
then we can write
Finally, the grouping of terms on the RHS preceding \(\nabla C^{Aw}\) is essentially constant for a dilute system giving
where the effective diffusion tensor is
Thus, the TCAT approach yields the standard diffusion equation under the set of derivative assumptions and approximations clearly stated above. Note that in this example Eq. (52) includes a diffusion tensor. The standard TCAT formulation approach ceases at this point, and it is assumed that model parameters, such as \({{\hat{\varvec{\mathsf {D}}}}}\), are material properties that are determined by comparing experimental, or subscale computational results, to model predictions and solving an inverse problem to determine the parameter values. In certain cases, sufficient knowledge of the microscale system, and morphology and topology of the pore space, may exist and be sufficiently simple such that a priori predictions of parameter values can be made. Such an approach has been used routinely in the MVA, but it has not yet been developed or applied for a TCAT model. This approach is presented in another work in which the result from this analysis is also used.
6 Discussion and Conclusions
The essential messages from this work are: (1) the motivation for using TCAT, (2) a clear pedagogical explanation of the method to allow the curious and interested scholar a toe hold into understanding the method, (3) an understanding of how available TCAT results can be used to construct models with desirable features quickly and efficiently without having to master every detail of the method; and (4) ways in which TCAT might be further improved. We expand on these points in turn in the following discussion. TCAT models have a unique set of desirable features, which are summarized as follows.
-
1.
TCAT models are scale-consistent, which means that every quantity that appears in the final macroscale model is precisely defined in terms microscale quantities, which includes all intensive variables, such as temperature. This scale consistency enables the rapidly evolving field of microscale, or pore scale, simulation to be used to inform, evolve, and validate TCAT models.
-
2.
TCAT models include thermodynamics naturally and closure relations needed to formulate well-posed models are assured of being consistent with the second law of thermodynamics. Model formulation approaches that do not include thermodynamics or posit the thermodynamics directly at the macroscale cannot assure this consistency a priori.
-
3.
TCAT models can contain not only phases, but interfaces, common curves, and common points.These lower dimensional entities can be shown to provide a means to more accurately resolve underlying physical mechanisms than models based upon phases alone. This feature is especially important for multiphase systems in which interfaces and common curves form and evolve in time.
-
4.
TCAT provides a framework to formulate a hierarchy of models of varying sophistication. Models from the hierarchy result from different choices in terms of approximations to derive an SEI, which in most cases can be evaluated through microscale simulation, and various sets of closure relations that satisfy a given SEI. This hierarchy of potential models thus enables a path to match the demands of an application with the details of the mechanisms included in the model. This framework also enables an efficient means to reformulate a scale-consistent model if an existing model is found to be deficient. The efficiency results from the natural entry points in the model formulation process based upon archival conservation equations, CEI, and SEI expressions.
This desirable set of TCAT features may provide a compelling motivation to learn the method, but these features are achieved at the expense of some significant detail in the formulation process. It can require person-years of effort by a specialist to formulate a TCAT model hierarchy for a complex application. This level of detail is a severe impediment to learning all of the details of the method. In this work, we have developed an approach that removes much of the detail and length of a formulated complex hierarchy of models in order to demonstrate the basic elements of the approach in a concise manner. Part of this simplicity owes to the example that was chosen. The result obtained in the end is not new, but this was not the intent. The intent was to demonstrate the elements of the theory clearly and concisely. This simplified approach can be applied not only to the diffusion example considered, but to any other TCAT model one wishes to develop. This would simplify the formulation approach for any application compared to starting from scratch and deriving a complete and general hierarchy of models. We expect that this will enable others to learn the fundamentals of TCAT much more efficiently than by studying the currently available literature alone.
Once an understanding of the potential advantages and fundamental approach to deriving TCAT models has been achieved by an individual, our belief is that it will be easier for such an individual to understand how to leverage the expansive work that is available (e.g., Gray and Miller 2014). The general nature of the available model hierarchies provide a basis for efficient model formulation by using available conservation equations and SEIs. For example, the model developed using the new pedagogical approach can also be derived by starting directly with an available modeling hierarchy. We did not do so, because that would not have illustrated all of the major steps in the method.
TCAT models are scale-consistent both in terms of variables that appear in the conservation and thermodynamic equations. This means that all variables that appear in the final macroscale model are defined in terms of precisely defined averages of microscale variables. This connection across scales provides a means to use the results from microscale experiments and simulations to evaluate and validate macroscale TCAT models. This evaluation and validation is useful because like all model formulation methods TCAT requires certain approximations. The scale consistency does provide a sound means to evaluate these approximations. For cases in which closed solvable microscale models do not exist, macroscale TCAT models can still be formulated and will contain variables that are precisely defined in terms of microscale quantities. The multiscale evaluation and validation of such models would be limited by the microscale information that is available.
For certain cases, the microscale–macroscale connection that is part of TCAT can be exploited to derive a priori estimates of model parameters. This can be accomplished by adapting an approach traditionally used in the MVA. Some subtle points with this approach result because of the way in which averages are computed, but such an extension is an example of how growing a community of scholars working with a method can hasten the rate of advancement. The details of this extension will be reported in a future work, along with new results derived based upon this method.
Despite the attractive features of the TCAT approach, several items remain for future work. For example, while several general SEI expressions have been derived for various classes of problems, much work remains to produce complete, specific, and solvable models that are evaluated and validated. Because TCAT models contain non-traditional quantities such as interfacial areas, curvatures, and geometric orientation tensors, methods are needed to observe and approximate such quantities. The novelty of TCAT formulations also means that macroscale simulators are typically not yet developed. Finally, most natural porous medium systems are heterogeneous in nature and an REV may not exist. Stochastic TCAT models have not yet been developed.
Abbreviations
- b :
-
Entropy source density
- C :
-
Species concentration in an entity
- \(\hat{\varvec{\mathsf {D}}}\) :
-
Effective diffusivity tensor
- \({{\hat{\varvec{\mathsf {D}}}}}^{ABw}\) :
-
Second-rank symmetric closure tensor for a binary system
- \(\varvec{\mathsf {d}}\) :
-
Rate of strain tensor
- \({\mathcal E}_{**}^{{\overline{\overline{w}}}}\) :
-
Particular material derivative form of a macroscale entity total energy conservation equation, Eq. (10)
- \({\mathcal G}_{**}^{{\overline{\overline{w}}}}\) :
-
Particular material derivative form of a macroscale body force potential balance equation, Eq. (11)
- h :
-
Energy source density
- \({\varvec{\mathsf {I}}}\) :
-
Identity tensor
- \(\mathcal {J}_s\) :
-
Index set of species
- i :
-
Species qualifier
- \(K_{E}\) :
-
Kinetic energy term due to velocity fluctuations
- \({\mathcal M}_{**}^{{\overline{\overline{{i}w}}}}\) :
-
Particular material derivative form of a macroscale species mass conservation equation, Eq. (9)
- \(MW_i\) :
-
Molecular weight of species i
- \(MW_w\) :
-
Molecular weight for entity w
- p :
-
fluid pressure
- \(\mathbf {q}\) :
-
Non-advective energy flux vector
- \(\mathbf {q}_{\mathbf {g}0}\) :
-
Non-advective energy flux vector due to the product of fluctuations
- R :
-
Ideal gas constant
- \({\mathcal S}_{**}^{{\overline{\overline{w}}}}\) :
-
Particular material derivative form of a macroscale entropy balance, Eq. (12)
- \({\mathcal T}_*^{{\overline{\overline{w}}}}\) :
-
Particular material derivative form of a macroscale differential thermodynamic equation, Eq. (13)
- \({\mathcal T}_{{\mathcal G}*}^{{\overline{\overline{w}}}}\) :
-
Particular material derivative form of the body source potential equation, Eq. (14)
- t :
-
Time
- \({{\mathbf {u}}^{\,{}}_{}}\) :
-
Species deviation velocity vector
- \(\mathbf {v}\) :
-
Velocity vector
- x :
-
Mole fraction of a species in an entity
- \({{\hat{{\gamma }}}}\) :
-
Macroscale activity coefficient
- \({{{\epsilon }^{{\overline{\overline{{{\alpha }}}}}}}}\) :
-
Specific entity measure of the \({{\alpha }}\) entity (volume fraction, specific interfacial area)
- \(\eta \) :
-
Entropy density
- \({\theta }\) :
-
Temperature
- \({\Lambda }\) :
-
Entropy production rate density
- \({\lambda }_{\mathcal E}^w\) :
-
Lagrange multiplier for energy conservation equation
- \({\lambda }_{{\mathcal G}}^w\) :
-
Lagrange multiplier for potential energy balance equation
- \({\lambda }_{{\mathcal M}}^{{i}w}\) :
-
Lagrange multiplier for mass conservation equation
- \({\lambda }_{{\mathcal M}_{i}}\) :
-
Is a constant related to the sum of potentials
- \({\lambda }_{{\mathcal T}}^w\) :
-
Lagrange multiplier for thermodynamic equation
- \({\lambda }_{{\mathcal T}{\mathcal G}}^w\) :
-
Lagrange multiplier for derivative of potential energy equation
- \(\mu \) :
-
Chemical potential
- \(\mu _0\) :
-
Reference chemical potential
- \(\rho \) :
-
Mass density
- \(\mathbf {\varphi }\) :
-
Non-advective entropy density flux vector
- \(\psi \) :
-
Body force potential per unit mass (e.g., gravitational potential)
- \({{\Omega }_{}}\) :
-
Spatial domain
- \({{\Omega }_{}}_w\) :
-
Domain occupied by the wetting phase in the REV
- \({{\omega }_{{}{}}}\) :
-
Mass fraction of a species in an entity
- A :
-
Species qualifier (subscript, superscript)
- B :
-
Species qualifier (subscript, superscript)
- \({\mathcal E}\) :
-
Energy equation qualifier (subscript)
- \({\mathcal G}\) :
-
Potential equation qualifier (subscript)
- \({i}\) :
-
General index denoting a species (subscript, superscript)
- \({\mathcal M}\) :
-
Mass equation qualifier (subscript)
- s :
-
Index that indicates a solid phase (subscript, superscript)
- \({\mathcal T}\) :
-
Thermodynamic equation qualifier (subscript)
- \({\mathcal T}{\mathcal G}\) :
-
Fluid potential energy identity qualifier (subscript)
- w :
-
Entity index corresponding to the wetting phase (subscript, superscript)
- \(\overline{\ }\) :
-
Above a superscript refers to a density weighted macroscale average
- \(\overline{\overline{\ }}\) :
-
Above a superscript refers to a uniquely defined macroscale average
- \({{{\left\langle f \right\rangle _{{{\Omega }_{{{\alpha }}}},{{\Omega }_{}},w}}}}\) :
-
Averaging operator, \(\int _{{\Omega }_{{{\alpha }}}}wf\, \mathrm {d}\mathfrak {r}/\int _{{{\Omega }_{}}} w\, \mathrm {d}\mathfrak {r}\)
- \(\mathrm {D}^{}_{{}}/\mathrm {D}t\) :
-
Material derivative
- \(\text{ CEI }\) :
-
Constrained entropy inequality
- \(\text{ EI }\) :
-
Entropy inequality
- \(\text{ MVA }\) :
-
Method of volume averaging
- \(\text{ REV }\) :
-
Representative elementary volume
- \(\text{ SEI }\) :
-
Simplified entropy inequality
- \(\text{ TCAT }\) :
-
Thermodynamically constrained averaging theory
References
Ahmadi, A., Abbasian Arani, A.A., Lasseux, D.: Numerical simulation of two-phase inertial flow in heterogeneous porous media. Transp. Porous Media 84(1), 177–200 (2010). doi:10.1007/s11242-009-9491-1
Bird, R.B., Stewart, W.E., Lightfoot, E.N.: Transport Phenomena, 2nd edn. Wiley, New York (2002)
Davit, Y., Bell, C., Byrne, H., Chapman, L., Kimpton, L., Lang, G., Leonard, K., Oliver, J., Pearson, N., Shipley, R., Waters, S., Whiteley, J., Wood, B., Quintard, M.: Homogenization via formal multiscale asymptotics and volume averaging: How do the two techniques compare? Adv. Water Resour. 62, 178–206 (2013)
del Rio, J.A., Whitaker, S.: Electrohydrodynamics in porous media. Transp. Porous Media 44(2), 385–405 (2001). doi:10.1023/A:1010762226382
Dye, A.L., McClure, J.E., Gray, W.G., Miller, C.T.: Multiscale modeling of porous medium systems. In: Vafai, K. (ed.) Handbook of Porous Media, 3rd edn, pp. 3–45. Taylor and Francis, London (2015)
Eidsath, A., Carbonell, R., Whitaker, S., Herrmann, L.: Dispersion in pulsed systems III: comparison between theory and experiments for packed beds. Chem. Eng. Sci. 38, 1803–1816 (1983)
Golfier, F., Wood, B.D., Orgogozo, L., Quintard, M., Bués, M.: Biofilms in porous media: development of macroscopic transport equations via volume averaging with closure for local mass equilibrium conditions. Adv. Water Resour. 463–485(32), 3 (2009)
Gray, W.G., Dye, A.L., McClure, J.E., Pyrak-Nolte, L.J., Miller, C.T.: On the dynamics and kinematics of two-fluid-phase flow in porous media. Water Resour. Res. 51(7), 5365–5381 (2015). doi:10.1002/2015wr016921
Gray, W.G., Miller, C.T.: Thermodynamically constrained averaging theory approach for modeling flow and transport phenomena in porous medium systems: 1. Motivation and overview. Adv. Water Resour. 28(2), 161–180 (2005)
Gray, W.G., Miller, C.T.: Thermodynamically constrained averaging theory approach for heat transport in single-fluid-phase porous media systems. J. Heat Transf. 131(10), 101002 (2009a). doi:10.1115/1.3160539
Gray, W.G., Miller, C.T.: Thermodynamically constrained averaging theory approach for modeling flow and transport phenomena in porous medium systems: 5. Single-fluid-phase transport. Adv. Water Resour. 32(5), 681–711 (2009b)
Gray, W.G., Miller, C.T.: Thermodynamically constrained averaging theory approach for modeling flow and transport phenomena in porous medium systems: 7. Single-phase megascale flow models. Adv. Water Resour. 32(8), 1121–1142 (2009c). doi:10.1016/j.advwatres.2009.05.010
Gray, W.G., Miller, C.T.: TCAT analysis of capillary pressure in non-equilibrium, two-fluid-phase, porous medium systems. Adv. Water Resour. 34(6), 770–778 (2011)
Gray, W.G., Miller, C.T.: Introduction to the Thermodynamically Constrained Averaging Theory for Porous Medium Systems. Springer, Cham (2014). doi:10.1007/978-3-319-04010-3
Gray, W.G., Miller, C.T., Schrefler, B.A.: Averaging theory for description of environmental problems: What have we learned? Adv. Water Resour. 51, 123–138 (2013). doi:10.1016/j.advwatres.2011.12.005
Hager, J., Whitaker, S.: The thermodynamic significance of the local volume averaged temperature. Transp. Porous Media 46, 19–35 (2002)
Jackson, A.S., Miller, C.T., Gray, W.G.: Thermodynamically constrained averaging theory approach for modeling flow and transport phenomena in porous medium systems: 6. Two-fluid-phase flow. Adv. Water Resour. 32(6), 779–795 (2009)
Jackson, A.S., Rybak, I., Helmig, R., Gray, W.G., Miller, C.T.: Thermodynamically constrained averaging theory approach for modeling flow and transport phenomena in porous medium systems: 9. Transition region models. Adv. Water Resour. 42, 71–90 (2012). doi:10.1016/j.advwatres.2012.01.006
Kischinhevsky, M., Paes-leme, P.J.: Modelling and numerical simulations of contaminant transport in naturally fractured porous media. Transp. Porous Media 26(1), 25–49 (1997). doi:10.1023/A:1006506328004
Miller, C.T., Gray, W.G.: Thermodynamically constrained averaging theory approach for modeling flow and transport phenomena in porous medium systems: 2. Foundation. Adv. Water Resour. 28(2), 181–202 (2005)
Miller, C.T., Gray, W.G.: Thermodynamically constrained averaging theory approach for modeling flow and transport phenomena in porous medium systems: 4. Species transport fundamentals. Adv. Water Resour. 31(3), 577–597 (2008)
Nozad, I., Carbonell, R.G., Whitaker, S.: Heat conduction in multiphase systems I: theory and experiment for two-phase systems. Chem. Eng. Sci. 40, 843–855 (1985)
Ochoa-Tapia, A.J., Whitaker, S.: Momentum transfer at the boundary between a porous medium and a homogeneous fluid. I: theoretical development. Int. J. Heat Mass Transf. 38(14), 2635–2646 (1995)
Ostvar, S., Wood, B.D.: A non-scale-invariant form for coarse-grained diffusion-reaction equations. J. Chem. Phys. 145(11), 114105 (2016). doi:10.1063/1.4962421
Perović, N., Frisch, J., Salama, A., Sun, S., Rank, E., Mundani, R.P.: Multi-scale high-performance fluid flow: simulations through porous media. Adv. Eng. Softw. 103, 85–98 (2017). doi:10.1016/j.advengsoft.2016.07.016
Plumb, O.A., Whitaker, S.: Diffusion, adsorption and dispersion in porous media: small-scale averaging and local volume averaging. In: Cushman, J.H. (ed.) Dynamics of Fluids in Hierarchical Porous Meida, pp. 97–148. Academic Press, New York (1990)
Quintard, M., Whitaker, S.: Two-phase flow in heterogeneous porous media: the method of large-scale averaging. Transp. Porous Media 3(4), 357–413 (1988)
Quintard, M., Whitaker, S.: Transport in chemically and mechanically heterogeneous porous media. I: theoretical development of region-averaged equations for slightly compressible single-phase flow. Adv. Water Resour. 19(1), 29–47 (1996)
Ryan, D., Carbonell, R., Whitaker, S.: Effective diffusivities for catalyst pellets under reactive conditions. Chem. Eng. Sci. 35, 10–16 (1980)
Rybak, I.V., Gray, W.G., Miller, C.T.: Modeling two-fluid-phase flow and species transport in porous media. J. Hydrol. 521, 565–581 (2015). doi:10.1016/j.jhydrol.2014.11.051
Valdés-Parada, F., Aguilar-Madera, C., Ochoa-Tapia, J., Goyeau, B.: Velocity and stress jump conditions between a porous medium and a fluid. Adv. Water Resour. 62, 327–339 (2013)
Valdés-Parada, F., Alvarez-Ramirez, J., Varela-Ham, J.: Upscaling pollutant dispersion in the mexico city metropolitan area. Physica A 391, 606–615 (2012)
Valdés-Parada, F., Porter, M., Narayanaswamy, K., Ford, R., Wood, B.: Upscaling microbial chemotaxis in porous media. Adv. Water Resour. 32, 1413–1428 (2009)
Whitaker, S.: Flow in porous media I: a theoretical derivation of Darcy’s law. Transp. Porous Media 1, 3–25 (1986)
Whitaker, S.: Derivation and application of the Stefan–Maxwell equations. Rev. Mex. Ing. Quím. 8(3), 213–243 (2009)
Wood, B.D., Cherblanc, F., Quintard, M., Whitaker, S.: Volume averaging for determining the effective dispersion tensor: closure using periodic unit cells and comparison with ensemble averaging. Water Resour. Res. 39(8), 1210 (2003). doi:10.1029/2002WR001723
Wood, B.D., Quintard, M., Whitaker, S.: Calculation of effective diffusivities for biofilms and tissues. Biotechnol. Bioeng. 77(5), 495–516 (2002)
Wood, B.D., Whitaker, S.: Diffusion and reaction in biofilms. Chem. Eng. Sci. 53(3), 397–425 (1998)
Acknowledgements
The work of CTM was supported by Army Research Office grant W911NF-14-1-02877 and National Science Foundation grant 1619767. The contributions of BDW were supported by National Science Foundation, grant EAR-1521441. The efforts of W.G. Gray to review and comment on this work are appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miller, C.T., Valdés-Parada, F.J. & Wood, B.D. A Pedagogical Approach to the Thermodynamically Constrained Averaging Theory. Transp Porous Med 119, 585–609 (2017). https://doi.org/10.1007/s11242-017-0900-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11242-017-0900-6