Abstract
Formation of cracks as a result of desiccation is a ubiquitous phenomenon in nature which is influenced by various factors including the environmental conditions, the properties of soil and the evaporating fluid. In this work, a comprehensive series of experiments is conducted to investigate the salinity effects on patterns formation during desiccation of bentonite layers. To do so, mixtures of bentonite and NaCl solutions were prepared with salt concentrations ranging from 3 to 15 %. The mixture was placed in a petri dish mounted on a digital balance to record the evaporation dynamics in an environmental chamber in which the ambient temperature and relative humidity were kept constant. An automatic digital camera was used to record cracking dynamics formed during the desiccation of clay layers. Results illustrated that the salt concentration had significant effects on the initiation, propagation, morphology and general dynamics of cracks. It was found that higher salt concentrations resulted in larger crack lengths due to the effects of NaCl on colloidal interactions among particles as well as the drying behavior. Additionally, using scanning electron microscopy, the influence of salt concentration on the patterns of cracks was investigated at different scales down to a few hundred nanometers. The present results provide new insights into the salinity effects on the cracking patterns and dynamics during desiccation of clay layers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Clay minerals occur in all types of sediments and sedimentary rocks and are a common constituent of hydrothermal deposits. Clay minerals normally experience cracking when they undergo desiccation. The desiccation cracks can be particularly extensive in sediments with a large amount of montmorillonite clay, which is a smectite with a high shrink–swell capacity (Luckham and Rossi 1999). Montmorillonite is also the predominant component of bentonite clay, which is often used as a buffer to contain contaminants and prevent leachates from entering the local environment. Presence of cracks in muddy sediments modifies several characteristics of the medium such as pore structure, porosity, and permeability, which in turn influences various flow and transport processes.
Soil cracking is a global issue which influences stability of soil, water evaporation, preferential flow through the macro-porosity as a result of cracking, water management and groundwater contamination. Early work by Kirkham and Selim (1970) and Adams et al. (1969) showed the effects of the presence of cracks on the evaporation from soil. More recently, Kamai et al. (2009) investigated the evaporation process from surface-exposed fractures and illustrated how the drying process is significantly modified as a result of the presence of fractures. The importance of the presence of cracks in clayey soil and its hydrological consequences motivated many scientists to investigate cracking patterns and dynamics under a variety of boundary conditions (e.g., Hallett and Newson 2001, 2005; Tang et al. 2010, 2011; Trabelsi et al. 2012; Sanchez et al. 2013; DeCarlo and Shokri 2014a; Thiery et al. 2015). Hallett and Newson (2005) investigated how the soil clay content and pore water salinity influenced the ductile fracture mechanics of soil. Tang et al. (2010) investigated the influence of temperature on desiccation cracking behavior of clayey soil. Inasawa and Yamaguchi (2012) studied one-dimensional cracking of silica colloidal suspension in a thin glass cell and observed periodic cracks perpendicular to the drying direction and provided a mathematical model, revealing the inverse relation between the drying rate and crack spacing. Shorlin et al. (2000) studied the two-dimensional dynamics of crack patterns forming during desiccation of a thin layer of alumina–water slurry and found that the cracking wavelength (indicating the crack length) increases with layer. Colina and Roux (2000) experimentally investigated the geometry and the kinetics of cracks forming during desiccation of a thin layer of paste consisting of clay, sand and water. They concluded that the crack spacing is mainly controlled by the thickness of the drying sample. Vogel et al. (2005) used Minkowski numbers to quantify the dynamics of the crack network forming during desiccation of two-dimensional samples of varying ratios of sand and bentonite, and found that the crack length densities remain constant, though area densities increase with higher clay content.
Thanks to the recent advances in imaging techniques, a few studies have emerged which help to understand the desiccation cracks in three-dimensional systems (see Goehring 2008; Gebrenegus et al. 2011; DeCarlo and Shokri 2014b). For example, Gebrenegus et al. (2011) studied the crack dynamics of sand–bentonite mixtures by using X-ray computed tomography. They assessed the impact of physicochemical factors on general cracking behavior and adopted a stochastic model to describe the evolution of crack aperture distributions. DeCarlo and Shokri (2014b) investigated the effect of NaCl concentration on the dynamics and morphology of cracks formed during desiccation of three-dimensional bentonite samples using four-dimensional X-ray computed tomography.
Despite considerable research efforts over the past few decades to address the patterns and dynamics of cracks forming during clay desiccation, many questions remain unanswered in this topic due to the complexity and the nonlinear nature of the problem. The effects of several factors such as composition of the clay, physical and chemical properties of the evaporating liquid, and complex colloidal interactions on cracking patterns and dynamics have not yet been fully determined. One of the open questions is the effects of salt concentration on the cracking patterns and dynamics. In natural clayey soils, salt is normally present in the environment which significantly modifies the drying behavior together with the associated cracking patterns and morphology. Motivated by the important hydrological applications of saline soil cracking as well as the evaporation process from salty porous media (Norouzi Rad et al. 2013; Shokri 2014), the aim of the present study is to investigate the salinity effects on the evaporation process from saline clay layers together with the associated dynamics and patterns of cracks forming during desiccation of clay layers. To achieve this aim, a comprehensive series of experiment and analysis is carried out in the present work which is discussed in the following.
2 Materials and Methods
2.1 Experimental Setup
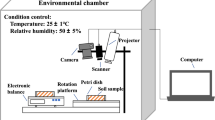
Sodium montmorillonite clay, hereafter, referred to as ‘bentonite’ (Wyo-Ben, MT, USA, Big Horn CH 200) was used in the experiments. Mixtures of bentonite and NaCl solutions were prepared with NaCl concentration varying from 3 to 15 % (by weight) to investigate the drying dynamics. Total of 33 runs of experiments including several repetitions were conducted to measure the crack patterns and morphology in desiccating bentonite layers. Bentonite powder was then dispersed into the salt solutions with a constant solid–liquid mass ratio of 1:1.5. Fifty grams of the mixed slurries were then poured into petri dishes (with an inner diameter of 150 mm). Efforts were made to carefully make the surface of the slurry in petri dishes as smooth and flat as possible. The petri dishes were mounted on digital balances to record the evaporation dynamics as influenced by the salt concentration. The evaporation experiments were conducted in an environmental chamber in which, throughout the whole experiment, the relative humidity and ambient temperature were kept constant at 30 % and \(35\,^{\circ }\hbox {C}\), respectively.
In order to capture the general dynamics and patterns of the cracks, a digital camera (Canon PowerShot SX120 IS) was installed over the top of the desiccating samples. Color images (resolution of approximately 60 microns/pixel) were taken at one-minute intervals from the surface of the clay, which was exposed to air for evaporation. During the experiment, special attention was given to minimize the reflection effects on the images by adjusting the lighting intensity.
To investigate the effects of salt concentration on the morphology, length, scaling characteristics and the general dynamics of cracks forming during bentonite desiccation, several MATLAB codes were developed to segment the images into black and white (representing the crack and matrix, respectively) and subsequently, to analyze the segmented images. The segmentation algorithm is quite similar to the ones described in Shokri et al. (2008) and Shokri and Or (2013), and, therefore, it is not given here.
In order to analyze the underlying patterns, length and density of cracks as influenced by the salt concentration, the segmented images were skeletonized using a standard skeletonization algorithm in MATLAB using the ‘bwmorph’ function (DeCarlo and Shokri 2014a). Figure 1 provides a comparison between the original image recorded by the camera and the corresponding segmented and skeletonized image. In addition, at the end of each experiment, the dried bentonite layer was scanned using the scanning electron microscopy (SEM) to observe any micro-cracks which have not appeared in the images recorded by the digital camera. Combining the images recorded by the digital camera with the SEM method provided an opportunity to understand the salinity effects on cracking patterns at different scales, down to a few hundred nanometers.
2.2 Rheological Characteristics of the Desiccating Slurries
Rheological analysis was performed on the bentonite slurries used in the present study in order to delineate the viscoelastic performance of the slurries, which have direct effects on the strength and cracking behavior of the material. In this part of the experiment, a stress-controlled rheometer (AR-G2 from TA instruments) was used to measure the yield stress of the slurries with a 60-mm-diameter flat base plate and a 1 mm separation. The dynamic yield stress measurements were conducted by measuring a decreasing flow curve from 50 to \(10^{-3}\, \hbox {s}^{-1}\) and determining the asymptote of the low shear rate limit (shown in the inset of Fig. 2a). The static yield stress was obtained through finding the maximum shear stress, measured by applying a constant shear rate of \(10^{-2}\, \hbox {s}^{-1}\) on the slurry, as shown in Fig. 2a.
Static and dynamic yield stresses for bentonite slurries with different NaCl concentrations are shown in Fig. 2b, where a significant decrease in both yield stresses can be seen with increasing the salt concentration. Similar behavior was observed in other studies (e.g., Van Olphen 1963; DeCarlo and Shokri 2014a; Chang and Leong 2014). In the next section, the rheological characteristics of the slurries and their effects on cracking morphology and dynamics in the desiccating bentonite samples will be discussed in more detail.
a Rheological measurement for bentonite slurry mixed with 10 % NaCl solution. Static yield stress is the maximum stress marked with the horizontal line. Inset: After pre-shearing, shear rate decreased from 50 to \(10^{-3}\, \hbox {s}^{-1}\). A plateau was observed and dynamic yield stress was measured as the asymptote value marked with the horizontal line. b Static and dynamic yield stresses for bentonite slurries mixed with solutions of different NaCl concentrations. In both cases, increasing salt concentration results in decreasing yield stress of the slurry
3 Results and Discussions
3.1 Drying Curves and Cracks Formation
The first measurements to be discussed here are associated with the water loss and evaporation rates. Figure 3 shows the typical evaporative water losses and the drying curves, measured during desiccation of bentonite layers with different salt concentrations. Results in Fig. 3a show that the evaporation rate is strongly influenced by the initial salt concentration; higher salt concentration results in lower evaporation rate. This is due to the effect of salt concentration on the saturation vapor pressure above the evaporation surface. Higher NaCl concentration lowers the saturation vapor pressure above the surface. Therefore, increasing the salt concentration in the evaporating solution will reduce the driving force for evaporation, consequently lowering the evaporative water losses as shown in Fig. 3a, b. Figure 3b also shows that for a given elapsed time from the onset of evaporation, the sample with higher salt concentration is wetter. The presence of more water in the evaporating bentonite layer in samples with higher salt concentration is likely to influence the stress development in the desiccating samples, and hence the cracking of the material and its dynamics (Scherer 1990).
Furthermore, the results in Fig. 3 suggest that the evaporation curves from each sample follow a distinct three-stage process, i.e., an initial period of constant evaporation rate, followed by a sharp decreasing transition period that is followed by a decreasing and very slow evaporation regime. All primary cracks (i.e., first-generation cracks), and a majority of secondary cracks that joined the primary ones at \(90^{\circ }\), nucleated and completed their formation, entirely within the first and the transition periods. This is visually illustrated in Fig. 4 for one typical example (i.e., bentonite layer saturated with 7 % salt solution). The above-mentioned three stage of the evaporation process together with the associated cumulative mass loss curve (presented in the inset of Fig. 4g) is presented in Fig. 4g. Figure 4a–e shows that the majority of the cracks formed during the first and transition stage of the evaporation process. Figure 4f shows that even after 18 h, the crack patterns did not change notably compared to the cracks already present in Fig. 4e.
The third stage of the evaporation corresponds to the period, during which the crack polygons start to dry completely by progressively drying one after each other. This is reflected in the color of the recorded images (shown in Fig. 4) by changing from dark gray (indicating wet surface) to light gray or white (indicating dry surface). The recorded images in all cases show that when the primary cracks form, the secondary cracks begin to initiate in erratic positions within each large-enough crack polygons bounded by the primary cracks. The initial growth of the secondary cracks is normal to the position of the boundaries. Their growth terminates when they rejoin the existing primary crack perpendicularly. Although the images are not presented here, the recorded images in our experiments qualitatively show that the salt concentration has a considerable effect on the initiation time and duration of cracking in bentonite samples. We generally observed later cracking initiation and shorter cracking period as NaCl concentration increased. Similar results were observed in DeCarlo and Shokri (2014b).
3.2 Crack Morphology and Patterns
This section will discuss the effects of salt concentration on the patterns and morphology of cracks forming during bentonite desiccation. Figure 5 shows a selection of segmented (non-skeletonized) samples taken at the end of the desiccation experiments. Qualitative inspection of Fig. 5 suggests that increasing the salt concentration leads to fewer cracks at the evaporation surface. Additionally, the extent of cracking in the final crack patterns was quantified as a function of salt concentration. The two primary parameters computed from the segmented images are the crack density \((\hbox {mm}\, ^{-1})\) and crack length (mm). Crack density is defined as \(A_{s}/S\), where \(A_{s}\) is the crack area in the skeletonized image and S is the total sample surface area. Crack length \(L_{c}\) was estimated following the procedure proposed by Colina and Roux (2000). As part of this procedure, first a set of random lines were drawn with horizontal and vertical orientations. Then, the number of intersections between the line and the cracks were counted. From these intersections, the average length of segments (identified as the mean crack length) was computed by dividing the length of the field of view by the number of intersections.
The results obtained from the above procedure are presented in Fig. 6. To ensure the reproducibility of the data and the observed trend, six rounds of experiments were conducted and in each round desiccating samples with different concentrations (ranging from 3 to 10 %) were used. For the case of concentrations higher than 10 %, precipitated salt covered the surface of the desiccating clay layer (see e.g., Fig. 8f) making it difficult to analyze the crack patterns. The data shown in Fig. 6 confirm that increasing the salt concentrations would result in larger crack lengths and fewer cracks at the surface (i.e., lower crack density). One possible explanation for such outcomes could be related to the drying behavior; water evaporation is more when NaCl concentration is less according to Fig. 3. This is due to the higher driving force for water evaporation as a result of higher saturation vapor pressure above the surface of the sample with the lower salt concentration. Negative stress develops in the desiccating clay samples as a results of water evaporation (Scherer 1990). This induces tensile stresses in the bentonite layers which results in cracking and branching at the surface of the clay sample when exceeding the strength of the material. The above justification is consistent with the results observed in Fig. 6 as well as the data reported in DeCarlo and Shokri (2014a).
a Average crack length formed at the desiccating clay surface, and b crack density, defined as the cumulative crack length divided by the total desiccating area at the end of the experiments versus NaCl initial concentration (c). Totally six rounds of experiments were conducted as indicated in the legend
The trend observed in Fig. 6 can be also described by considering the chemistry and colloidal interactions of the clay particles as influenced by the ion concentration (Van Olphen 1963, 1964; Luckham and Rossi 1999; DeCarlo and Shokri 2014b). Several studies have been conducted in the past to delineate the structure of bentonite and its characteristics when dispersed in a liquid (Goehring et al. 2010). The interaction among the negatively charged clay particles is influenced by a phenomenon referred to as the ‘Electric Double Layer’ (EDL) (Gouy 1917; Grahame 1947; Van Olphen 1963, 1964; Luckham and Rossi 1999; DeCarlo and Shokri 2014b). EDL plays an important role in the stability of the colloidal system. Theoretical analyses of the interaction between colloidal particles have been conducted by Derjaguin and Landau (1941) and Verwey and Overbeek (1948), known as the ‘DLVO theory.’ According to this theory, the interaction between clay particles in suspension is influenced by the repulsive electrostatic and the attractive van der Waals forces. The repulsive electrostatic force in the clay suspension is influenced by the properties of the liquid such as the ion concentration and pH of the liquid. When the properties of clay suspension are modified, patterns, morphology and dynamics of cracks formed due to desiccation of clay will be modified.
When salt concentration increases, the thickness of the EDL is compressed (Luckham and Rossi 1999). This considerably reduces the repulsive forces among the particles that influence the configuration of particle association in the colloidal system. At low and high salt concentrations, clay particles form entangled and stacked clay network, respectively, which significantly modifies the mechanical properties of the slurry (Luckham and Rossi 1999; DeCarlo and Shokri 2014b). Entangled clay network includes formation of a three-dimensional ‘voluminous card-house structure,’ and stacked clay network exhibits a highly compact, thicker and possibly larger flakes described as quasi-continuous layers of flexible units (Van Olphen 1963; Van Damme and Ben Ohoud 1990; Luckham and Rossi 1999). In fact, the decrease in the yield stress, as observed during the rheological experiment mentioned earlier in Sect. 2.2, reflects a transition from a three-dimensional card-house structure to a stacked structure with the addition of large amount of salt. Consequently, this transition affects the mechanical behavior of the suspension during desiccation. One could conclude that at lower salt concentrations, bentonite samples exhibited the card-house association. This inevitably induces defects in the bentonite structure, which can weaken the material and promote extensive cracking at desiccating locations.
3.3 Fractal Characteristics
The final crack patterns observed at the clay surface was reminiscent of the fractal patterns (Sahimi 2003). In order to fully establish this, the fractal dimension, \(D_{f}\), of the crack patterns was calculated using the ‘box-counting method.’ As explained by several researchers (e.g., Sahimi 1993, 2003; Shokri and Sahimi 2012), in the box-counting method, the fractal object is covered by boxes of side length, r. The number of such boxes, denoted as N(r), which are required to cover the entire object is counted and then plotted versus r. An example of this method is presented in the inset of Fig. 7a which corresponds to the desiccating bentonite with 4 % salt concentration. \(D_{f}\) is estimated as the (negative) slope of the linear part of the plot on the log-log scale. The box sizes varied from eight pixels \((\sim 0.48\,\hbox {mm})\) to the size of the image. The fractal dimensions of the final crack patterns in all samples are plotted in Fig. 7a. The errors in all cases were \(<\)1 %. The results show that the fractal dimension slightly decreases as the salt concentration increases. Rowe (1962) showed that the fractal dimensions of crack networks are tied to the development of the crack area. This is consistent with the results presented here, as the fraction of the crack area decreases with salt concentration.
Furthermore, Fig. 7b shows a strong linear relation between the computed fractal dimension and the crack density in each sample. The average \(D_{f}\) of all samples was 1.59, with the maximum and minimum values of 1.67 and 1.43, respectively. These values are within the range of the fractal dimensions of crack patterns in clay reported in the literature (Preston et al. 1997; Colina and Roux 2000; Mal et al. 2006).
a Computed fractal dimension (\(D_{f}\)) of the crack networks versus the initial salt concentration (the inset shows the procedure used to compute the fractal dimension in each case). b Fractal dimension of the final crack patterns versus the crack density. Totally six rounds of experiments were conducted as indicated in the legend
3.4 SEM Imaging
At the end of each experiment, detailed visualizations were carried out using the scanning electron microscopy (SEM, ZEISS SUPRA 55VP) to investigate cracks patterns at micro- and nanoscale. The aim was to evaluate the existence and patterns of crack and the precipitated salt at scales far smaller than the resolution of the camera used in the laboratory experiments. At the end of the desiccation experiment, one random polygon (formed through desiccation) was removed from the surface for SEM imaging as shown in Fig. 8a. Typical examples of the crack patterns observed in the desiccating bentonite with the salt concentration of 4 and 15 % are presented in Fig. 8a and f, respectively. In the case of 15% salt concentration, the entire surface was covered by precipitated salt due to evaporation at the surface; hence, no crack was observed macroscopically at the surface. Figure 8b, g confirm the existence of cracks at microscale in bentonite. This is in contrast to, for example, desiccating kaolinite behavior in which only macroscale cracks are formed as a result of desiccation (DeCarlo and Shokri 2014a). Also shown in Fig. 8 is the complex pattern and localization of precipitated salt in bentonite. For example, Fig. 8c–e show the non-uniform precipitation patterns, present at the surface of desiccating bentonite for the case with 4 % NaCl concentration; Fig. 8d shows parts of bentonite being partially covered by salt, while no precipitated salt can be seen in Fig. 8e, in which the exfoliated structure of the bentonite clay is observed. In the case of 15 % salt concentration, the entire surface was covered by salt, but the SEM images still show the presence of cracks at microscale (see Fig. 8g, i), which was not visible in the images recorded by the digital camera. Finally, Fig. 8h and j demonstrates the complex patterns of the precipitated salt at the surface of the desiccating bentonite with 15 % salt concentration.
Scanning electron microscope images of cracked clay surfaces in the case of bentonite mixed with NaCl solution of 4 % a–e and 15 % concentration f–j with the magnification factor ranging from 40 to 3000. b, c, g and i indicate the presence of micro-cracks which were not observed in the images recorded by the camera. d Surface partly covered by the precipitated salt (indicated by the light gray). e shows the exfoliated structure of bentonite clay and h, j illustrate the complex structure of the precipitated salt in the case of 15 % salt concentration
4 Summary and Conclusions
The aim of the present work was to investigate the effects of NaCl concentration on the clay rheology as well as the patterns and morphology of cracking observed during desiccation of thin layers of bentonite dispersed in salt solution. Laboratory experiments were conducted to record the drying behavior and the cracking dynamics with a relatively high spatial and temporal resolution under well-controlled atmospheric conditions. Yield stresses of the desiccating samples with different salt concentrations were separately determined. In addition, scanning electron microscopy (SEM) imaging was used to evaluate the cracking and salt precipitation patterns at smaller scales (down to a few hundred nanometers).
Results illustrated the salinity effects on cracking morphology and patterns forming during desiccation of bentonite. Larger crack lengths and lower crack density (defined as the cumulative length of the crack divided by the evaporating area) were obtained as salt concentration increased in the solution. The observed behavior was attributed to the effects of the presence of salt on the bentonite colloidal interactions that tend to modify the electric double layer and the structure of the particle association in the slurry. Another factor affecting crack morphology and patterns could be attributed to the drying behavior as it was considerably modified due to different salt concentrations. Higher salt concentration resulted in lower saturation vapor pressure immediately above the evaporating surface, thus reducing the driving force for evaporation. Consequently, less evaporation occurred in the desiccating samples with higher salt concentration. The reduction in the evaporation causes less tensile stress (which is developed in bentonite as a result of desiccation) which consequently results in fewer cracking. The findings which have emerged from the present study are useful in various hydrological applications such as water management, evaporation from land surface, analysis related to soil stability and general hydrology of arid and semiarid areas.
References
Adams, J.E., Ritchie, J.T., Burnett, E., Fryrear, D.W.: Evaporation from a simulated soil shrinkage crack. Soil Sci. Soc. Am. J. 33, 609–613 (1969)
Chang, W.Z., Leong, Y.K.: Ageing and collapse of bentonite gels-effects of Li, Na, K and Cs ions. Rheol. Acta. 53, 109–122 (2014)
Colina, H., Roux, S.: Experimental model of cracking induced by drying shrinkage. Eur. Phys. J. E 1, 189–194 (2000)
DeCarlo, K.F., Shokri, N.: Effects of substrate on cracking patterns and dynamics in desiccating clay layers. Water Resour. Res. 50, 3039–3051 (2014a)
DeCarlo, K.F., Shokri, N.: Salinity effects on cracking morphology and dynamics in 3-D desiccating clays. Water Resour. Res. 50, 3052–3072 (2014b)
Derjaguin, B., Landau, L.D.: The theory of stability of highly charged lyophobic sols and coalescence of highly charged particles in electrolyte solutions. Acta Physicochim. U.R.S.S. 14, 633-652 (1941)
Gebrenegus, T., Ghezzehei, T.A., Tuller, M.: Physicochemical controls on initiation and evolution of desiccation cracks in sand-bentonite mixtures: X-ray CT imaging and stochastic modeling. J. Contam. Hydrol. 126(1), 100–112 (2011)
Goehring, L.: On the scaling and ordering of columnar joints. Ph.D. thesis, Department of Physics, University of Toronto (2008)
Goehring, L., Conroy, R., Akhter, A., Clegg, W.J., Routh, A.F.: Evolution of mud-crack patterns during repeated drying cycles. Soft Matter 6, 3562–3567 (2010)
Gouy, G.: Sur la function électrocapillaire. Ann. Phys. (Paris) 9, 129–184 (1917)
Grahame, D.C.: The electrical double layer and the theory of electrocapillarity. Chem. Rev. 41(3), 441–501 (1947)
Hallett, P.D., Newson, T.A.: A simple fracture mechanics approach for assessing ductile crack growth in soil. Soil Sci. Soc. Am. J. 4, 1083–1088 (2001)
Hallett, P.D., Newson, T.A.: Describing soil crack formation using elastic-plastic fracture mechanics. Soil Sci. Soc. Am. J. 56, 31–38 (2005)
Inasawa, S., Yamaguchi, Y.: Self-organized pattern formation of cracks perpendicular to the drying direction of a colloidal suspension. Soft Matter 8, 2416–2422 (2012)
Kamai, T., Weisbrod, N., Dragila, M.I.: Impact of ambient temperature on evaporation from surface-exposed fractures. Water Resour. Res. 45, W02417 (2009)
Kirkham, D., Selim, H.M.: Soil temperature and water content changes during drying as influenced by cracks: a laboratory experiment. Soil Sci. Soc. Am. J. 34, 565–569 (1970)
Luckham, P.F., Rossi, S.: The colloidal and rheological properties of bentonite suspensions. Adv. Colloid Interface Sci. 82, 43–92 (1999)
Mal, D., Sinha, S., Dutta, T., Mitra, S., Tarafdar, S.: Fractal crack patterns in Laponite films and their scaling behavior. Fractals 14, 283–288 (2006)
Norouzi Rad, M., Shokri, N., Sahimi, M.: Pore-Scale dynamics of salt precipitation in drying porous media. Phys. Rev. E 88, 032404 (2013)
Preston, S., Griffiths, B.S., Young, I.M.: An investigation into sources of soil crack heterogeneity using fractal geometry. Eur. J. Soil Sci. 48, 31–37 (1997)
Rowe, P.W.: The stress-dilatancy relation for static equilibrium of an assembly of particles in contact. Proc. R. Soc. London A 269, 500–527 (1962)
Sahimi, M.: Flow phenomena in rocks: From continuum models to fractals, percolation, cellular automata, and simulated annealing. Rev. Mod. Phys. 65(4), 1393–1534 (1993)
Sahimi, M.: Heterogeneous Materials II. Springer, New York (2003)
Sanchez, M., Atique, A., Kim, S., Romero, E., Zielinski, M.: Exploring desiccation cracks in soils using a 2D profile laser device. Acta Geotech. 8, 583596 (2013)
Scherer, G.W.: Theory of drying. J. Am. Ceram. Soc. 73(1), 3–14 (1990)
Shokri, N.: Pore-scale dynamics of salt transport and distribution in drying porous media. Phys. Fluids. 26, 012106 (2014)
Shokri, N., Lehmann, P., Vontobel, P., Or, D.: Drying front and water content dynamics during evaporation from sand delineated by neutron radiography. Water Resour. Res. 44, W06418 (2008)
Shokri, N., Or, D.: Drying patterns of porous media containing wettability contrasts. J. Colloid Interface Sci. 391, 135–141 (2013)
Shokri, N., Sahimi, M.: The structure of drying fronts in three-dimensional porous media. Phys. Rev. E 85, 066312 (2012)
Shorlin, K.A., de Bruyn, J.R., Graham, M., Morris, S.W.: Development and geometry of isotropic and directional shrinkage-crack patterns. Phys. Rev. E 61(6), 6950–6957 (2000)
Tang, C.S., Cui, Y.J., Tang, A.M., Shi, B.: Experimental evidence on the temperature dependence of desiccation cracking behavior of clayey soils. Eng. Geol. 114, 261–266 (2010)
Tang, C.S., Cui, Y.J., Shi, B., Tang, A.M., Liu, C.: Desiccation and cracking behaviour of clay layer from slurry state under wetting-drying cycles. Geoderma 166, 111–118 (2011)
Thiery, J., Rodts, S., Keita, E., Chateau, X., Faure, P., Courtier-Murias, D., Kodger, T.E., Coussot, P.: Water transfer and crack regimes in nanocolloidal gels. Phys. Rev. E 91, 042407 (2015)
Trabelsi, H., Jamei, M., Zenzri, H., Olivella, S.: Crack patterns in clayey soils: experiments and modeling. Int. J. Numer. Anal. Meth. Geomech. 36, 1410–1433 (2012)
Van Damme, H., Ben Ohoud, M.: Disorder and Fracture. Plenum, NewYork, NY (1990)
Van Olphen, H.: Introduction to Clay Colloid Chemistry. Interscience, New York, NY (1963)
Van Olphen, H.: Internal mutual flocculation in clay suspensions. J. Colloid Interface Sci. 19, 313–322 (1964)
Verwey, E.J.W., Overbeek, J.T.G.: Theory of the Stability of Lyophobic Colloids: The Interaction of Sol Particles Having an Electric Double Layer. Elsevier, New York, NY (1948)
Vogel, H.J., Hoffman, H., Roth, K.: Studies of crack dynamics in clay soil I. Experimental methods, results, and morphological quantification. Geoderma 125, 203–211 (2005)
Acknowledgments
We would like to thank Emmanuel Keita for his assistance with rheology measurements. The SEM imaging was conducted at Boston University Photonics Center which is acknowledged. Also, the partial funding from chaire innovative solution for a sustainable habitat between Saint-Gobain and ENPC in France is appreciated.
Author information
Authors and Affiliations
Corresponding author
Additional information
Nima Shokri would like to dedicate this paper to one of the greatest soil physicists of our time, Professor Dani Or, on the occasion of his 60th birthday.
Rights and permissions
About this article
Cite this article
Shokri, N., Zhou, P. & Keshmiri, A. Patterns of Desiccation Cracks in Saline Bentonite Layers. Transp Porous Med 110, 333–344 (2015). https://doi.org/10.1007/s11242-015-0521-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11242-015-0521-x