Abstract
Radish (Raphanus sativus) is a rich source of glucosinolates (GSLs) and their hydrolytic products such as isothiocyanates (ITCs). GSLs and ITCs enhance plant defense responses to biotic and abiotic stresses and are health promoting effect in human. The branched-chain aminotransferase 4 (BCAT4) gene encode an enzyme catalyzing the deamination of methionine in the first step in the chain elongation of aliphatic GSL biosynthesis. Previously, plant transformation in radish has been successfully performed using several methods such as floral dipping, vacuum infiltration and sonic infiltration, protoplast transformation and microspore culture. However, the recalcitrant of regeneration in radish affects the transformation efficiency remain relatively low. Therefore, there is still a need to improve the transformation methods for radish. In this study, we used a simple method for the efficient transformation of radish using Agrobacterium tumefaciens strain GV3101 and tested it with the radish BCAT4 (RsBCAT4) transgene. The PCR, RT-qPCR, Southern blot, GFP fluorescence, and HPLC analyses were used to confirm the transgene integration. Positive correlations between the expression of RsBCAT4 and downstream genes (i.e., CYP79F1, CYP83A1, and GRS1) were also observed in selected T2 transgenic lines. RsBCAT4 transgenic lines exhibited significantly increased levels of aliphatic GSLs compared to the levels in wild type plants, particularly glucoraphasatin. This needle perforation technique is simple in plant transformation method significantly enhancing transformation efficiency in radish, which could be utilized for molecular breeding of radish to improve its traits.
Key message
An efficient protocol for stable transformation of apical meristems of radish (Raphanus sativa L.) seedlings using a needle perforation and Agrobacterium tumefaciens incubation method. We developed a needle perforation and Agrobacterium tumefaciens incubation method for in planta transformation
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radish (Raphanus sativus) is an annual or biennial root vegetable crop that is a member of the Brassicaceae family. It is an economically important crop grown worldwide, especially in Asia. The fleshy taproot of radish is the main edible part of the plant with high nutritional value and reported health benefits (Yu et al. 2016; Sasaki et al. 2020). In general, radishes contain vitamins (B1, B2, B3, B5, B6, B9, and C), minerals, and bioactive compounds. Many studies have reported that radish is a rich source of antioxidants and other vital metabolites such as glucosinolates (GSLs). GSLs are major secondary metabolites produced in Brassicaceae family plants, and their hydrolytic products, in particular isothiocyanates (ITCs), possess beneficial properties such as the prevention of cancer development in humans (Cartea and Velasco 2008; Lim et al. 2020). ITCs are breakdown products from the enzymatic hydrolysis of GSLs by myrosinase in radish (Fahey et al. 2001; Fimognari et al. 2012). GLSs play a positive role in enhancing plant defense systems against a diverse range of biotic and abiotic stresses (Burow and Halkier 2017; Ting et al. 2020). Interestingly, it was recently reported that glucoraphasatin (GRH) is the most abundant aliphatic GSL (60–90%) among the GSLs detected in radish (Yi et al. 2016; Kakizaki et al. 2017).
The biosynthesis of aliphatic GSLs can be divided into three phases: (1) chain elongation of amino acids, (2) core structure formation, and (3) side chain modification (Sønderby et al. 2010; Chhajed et al. 2020). First, the precursor amino acid, methionine (Met) enters the chain elongation phase, where it is deaminated to 4-methylthio-2-oxobutyrate (MTOB) by the enzyme branched-chain aminotransferase 4 (BCAT4) (Schuster et al. 2006). Since BCAT4 is the first enzyme involved in the biosynthesis of aliphatic GSLs, it will be of interest to enhance the nutrient content of radish plants.
Genetic transformation methods have progressed and provide new approaches to study gene functions and identify genes capable of improving agronomic characteristics (Curtis 2011; Matveeva and Lutova 2014; Liu et al. 2018). Among these methods, genetic transformation using Agrobacterium tumefaciens is considered efficient and convenient since it has the following characteristics: (1) A. tumefaciens has the ability to transfer genes from unrelated species, (2) these gram negative bacteria can transfer relatively large DNA segments, (3) the technique requires less time than other procedures, and (4) the technique can be targeted towards a particular trait (Hwang et al. 2017; Lacroix and Citovsky 2019; Li et al. 2020). The ability of A. tumefaciens to transfer transgenes into plant cells, where the transgenes are stably integrated into the host chromosome(s) and expressed, has made these bacterial strains extremely useful in plant genetic engineering.
To develop efficient A. tumefaciens-mediated transformation protocols for radish, several different factors, such as type of explant and plant age, were previously tested to enhance regeneration efficiency. It is known that the effort of producing shoot regenerated from radish has been facing challenges due to the recalcitrance of the cultured radish explants. Several studies reported the ethylene produced by cultured radish explants inhibited shoot regeneration. However, the combination of an ethylene synthesis inhibitor [L-a-2-aminoethoxy vinyl glycine (AVG)] and silver nitrate in a regeneration medium containing 2 mg/l of 6-benzyl adenine (BA) and 1 mg/l of a-naphthaleneacetic acid (NAA) significantly improved the regeneration of hypocotyl explants of Chinese radish cv. Red Coat (Pua and Lee 1995; Curtis et al. 2004). Later on, the application of silver nitrate in shoot regeneration system of seedling radish cv. Jinjudaepyung showed 60% significant improvement compared to the control treatment (Curtis et al. 2004). However, other explants such as hypocotyl and cotyledon are failed for shoot regeneration in the screening using antibiotics (Curtis 2011). Furthermore, it is known that the frequency of shoot regeneration from embryogenic calli and microspores is too low for practical usage in Agrobacterium-mediated transformation (Jeong et al. 1995; Takahata et al. 1996). More recently, the study about radish transformation using cotyledon with pCAMBIA 1301 and pPTN290 shows frequency of transformation was 0.26 and 0.18% respectively (Mi et al. 2008). These studies further emphasized that the combined challenges by recalcitrance cell and low tissue regeneration have led to approach to methods using in planta transformation.
In planta transformation is a protocol used for in vitro regeneration, integrating transfer DNA (T-DNA) into the host genome of cells in or around the apical meristems that are subsequently allowed to grow and produce seeds. In planta transformation methods have been reported to be easy and inexpensive, and they substantially enhance the efficiency of Agrobacterium-mediated transformation. In Brassicaceae family plants, the first in planta method was a floral dipping method, which was successfully demonstrated in Arabidopsis thaliana. Following this, a protocol for Agrobacterium-mediated transformation of radish cultivars was also developed, resulting in genetic transformation frequencies of 1.1–1.4% (Clough and Bent 1998; Curtis 2011). Another in planta method for radish transformation was also developed using ultrasonic and vacuum infiltration (Park et al. 2005). That study indicated that the combination of 2.5–7.5 min sonication and 2.5–7.5 min vacuum treatments could improve the transformation efficiency to 2–22%. However, these procedures are still challenging because the putative transgenic radish plants produce only 50–100 seeds per individual. The objective of the present study was to develop a simple and efficient protocol for Agrobacterium-mediated radish transformation. Our method was tested on radish seedlings using two plant expression cassettes in the T-DNA, the first harboring the RsBCAT4 transgene under the CaMV35S promoter and the second, the bar selectable marker transgene under the CaMV35S promoter. We successfully obtained transgenic plants overexpressing RsBCAT4 and showing increased aliphatic GSL profiles compared to those of non-transgenic radish plants. Therefore, we believe that this protocol will be useful for the development of radish transgenic plants, further contributing to molecular breeding to optimize the quality traits of radish plants.
Materials and methods
Plant materials
Raphanus sativus L. cv. Jinjudaepyung, was chosen as material because it is a homozygous and commercial cultivar cultivated in South Korea was used as genetic material. Radish seeds were sterilized by immersing in 70% ethanol for 3 min and washed twice with sterile distilled water. Then, the seeds were soaked in 2% sodium hypochlorite (v/v) with low agitation mixing (30 rpm) on a shaker for 10 min, followed by briefly rinsing three times with sterile deionized water. The surface-sterilized seeds were transferred to sterilized filter paper for air drying. Then, the dried radish seeds were germinated on Murashige and Skoog medium (Murashige and Skoog 1962) (Duchefa Biochemie, Netherlands) solidified with 4 g/l phytoagar (Duchefa Biochemie) and incubated at 27 °C in the dark. The germinated seeds were then transferred and further incubated for 3 days at 4 °C with illumination. We had performed a preliminary experiment on seedling growth in low (4 °C) or high (28 °C) temperature growth condition. The result showed that seedling was elongated and the hypocotyl become thin and fragile in higher warm temperature. Meanwhile, seedlings grown in low temperature had thick and stumpy hypocotyls which looked suitable for multiple punching (Supplementary Fig. S1).
Vector construction
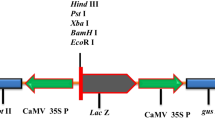
Total RNA was extracted from the radish leaves using TRIzol reagent (Invitrogen, USA). First strand cDNAs were synthesized in 20 µl from 2 µg of total RNA using the AccuPower® RT PreMix (Bioneer, South Korea) with oligo-dT as a primer. Information of full-length sequences of RsBCAT4 was obtained from database of radish (http://radish-genome.org/). Primers were designed specifically using Primer3 online software (https://bioinfo.ut.ee/primer3-0.4.0/). Using the gene specific primer pair (RsBCAT4-fullF and RsBCAT4-fullR), RsBCAT4 transgene was amplified from the cDNA radish sample. The PCR product was purified with Gel Purification kit (Qiagen, Germany) and was A-tailed with normal Taq polymerase for 25 min at 70 °C. The ligation product was then transformed into E.coli strain DH5α using heat shock method. The colony appeared after overnight incubation at 37 °C was confirmed by colony PCR using PCR-premix (Bioneer, Korea). The colonies showing correct insert were picked up and grown in liquid LB media containing appropriate antibiotic for overnight and the plasmid were isolated using Accuprep Nano plus kit (Bioneer, Korea). The RsBCAT4 integration in pGEM-T Easy vector was confirmed by DNA sequencing. For the sub-cloning, A 1086-bp PCR product of RsBCAT4 transgene was amplified from pGEM-T Easy using Pfu enzyme polymerase (Bioneer product) with primers of RsBCAT4_Eco_1F and RsBCAT4_Bam_1086R (Supp. Table S1). The PCR program were followed by: 95 °C pre-denaturation for 5 min followed by 35 cycles at 95 °C for 20 s, annealing 57 °C for 20 s, and extension at 72 °C for 1 min. Furthermore, the PCR product was purified by DNA purification kit (SolGent, Korea), then subsequently subjected to enzyme cutting with EcoRI and BamHI restriction enzymes. Restriction enzyme-treated and purified PCR products were ligated into pEGAD which contains the green fluorescent protein (GFP) gene and the BASTA resistance (bar) gene as a selectable marker (Fig. 1). The pEGAD-RsBCAT4 vector was transformed into Agrobacterium strain GV3101 by freeze and thaw method (Weigel and Glazebrook 2006).
Genetic transformation procedure in radish (Raphanus sativus L.) A The pEGAD-RsBCAT4 construct containing RsBCAT4 and the EGFP (enhanced GFP) coding sequence used for expression in radish plants. This vector contains the CAMV35S promoter (35S), Nopaline synthase (nos) gene, and BASTA® resistance (BARR) gene. B Schematic representation of Agrobacterium-mediated transformation in radish. a Seeds were sterilized and germinated in ½ MS medium then the germinated seeds were then transferred and further incubated for 3 days at 4 °C with illumination. b Upper part of hypocotyl, near apical meristem of radish seedling was perforated with a 25G syringe needle. c Needle-perforated radish seedlings were immersed in the suspension of A. tumefaciens containing the transgene vector for 3 days on MS media with 125 mg/l acetosyringone in darkness. d Infected seedlings were transferred on MS liquid media with 125 mg/l acetosyringone for 2 days at 25 °C with illumination. e Transfer of co-cultivated seedlings in a pot tray for acclimatization. f All transgenic plants were grown in the greenhouse until flowering. Self-pollination by hand was perform in each transgenic line. g Seeds of T1 generation were harvested. h Putative T1 transgenic plants were screened by spraying with 0.03% BASTA® solution
Plant transformation
Seven-day-old seedlings, which have a green and thick stem (2–3 cm length), were chosen for transformation by a needle perforation and A. tumefaciens incubation method. The plant materials were perforated 5–10 times using a small needle (0.1–0.3 mm diameter) in the upper region of the hypocotyl, near the apical meristem (Fig. 2) and then transferred to a prepared flask containing Agrobacterium in liquid MS media. The mixtures were cultured on a shaker (max. 150 rpm) for 30 min in the dark. The liquid MS medium was then removed, and the seedlings were air-dried before being transferred to co-cultivation medium (MS medium containing 125 mg/l acetosyringone). The seedlings were co-cultivated for 3 days at 25 °C in the dark followed by 2 days at 25 °C with illumination. After co-cultivation, the infected plants were eventually transferred into soil for acclimatization (Fig. 2). The acclimatization condition were critical factor for plants to adjust under a change in the new environment. To ensure the survival rate of putative transgenic plants, the infected seedlings were removed from the culture vessels without damaging the roots. To prevent microbial infection, excess agar around the roots were washed three times in sterilized distilled water containing 250 mg/l cefotaxime. Healthy, well-developed rooted seedlings were successfully transplanted into a plastic pot tray containing a mixture of soil and then incubated in 16 h/8 h (light/dark) photoperiod growth chamber at 24 °C. To keep the plants moist, the transparent plastic covers were placed over them for 5 days. The plants were then moved to glass house and irrigated every other day with regular tap water.
Screening and bud pollination of T1 putative transgenic radish plants. A Putative radish T1 transgenic plants that survived BASTA® screenings. After BASTA® treatments, the survived plants were transplanted into individual pots. B Non-transgenic plants in soil. C T1 transgenic plants. D T1 plants successfully bolted and flowered. E Bud-pollination of each individual plant. Red arrows indicate buds and flowers used for bud pollination by hand. F Picture showing successfully pollinated buds of radish T1 transgenic plants
Screening for transgenic plants by spraying with phosphinothricin
BASTA® (Bayer Crop Science, Germany) containing 18% phosphinothricin (PPT) was used for assessment of the putative T1 and T2 transformants. As a preliminary test, to determine the lethal doses of BASTA, 32 plants radish non-transformants at the 4-leaf stage were sprayed with different concentrations of BASTA (0.0, 0.01, 0.03, 0.05, and 0.1% (v/v) (Supplementary Fig. S1). The spraying was repeated one more time after 2 weeks. The surviving plants were recorded after 2 weeks and the numbers finalized 4 weeks after the second spraying at 6 weeks after the first spray application. The minimal concentration (0.03%) causing death of all the non-transgenic plants was used for screening for the putative transgenic plants harboring the pEGAD-RsBCAT4 transgene.
PCR analysis
PCR analysis was performed to determine the presence of the pEGAD-RsBCAT4 transgene in the putative transformants. Genomic DNA from 24 putative T1 transformants was isolated using the urea method. Approximately 1 μg of genomic DNA was used as the DNA template for each PCR amplification. The PCR conditions were 95 °C for 5 min as initial denaturation, followed by 30 cycles at 95 °C for 20 s, 57 °C for 20 s, and 72 °C for 1 min, with a final extension of 72 °C for 5 min. The PCR products were subjected to 1% agarose gel electrophoresis and visualized with a UV transilluminator after EtBr staining. The primers used in this study are described in Supplementary Table 1.
RT-qPCR analysis
Total RNA was isolated from leaf tissue of the T2 lines using TRIzol reagent (Invitrogen, USA). Any residual DNA was removed with DNase I (Sigma-Aldrich, USA), and then the RNA was used as a template for cDNA synthesis using the AccuPower® RT Premix (Bioneer). Specifically, oligo-dT (0.5 μg) was added to 1 μg of RNA in a total volume of 20 μl and was incubated for 5 min at 70 °C. The RNA mixture was subsequently transferred into an RT-Premix tube and incubated for 60 min at 42 °C for reverse transcriptase activity and 5 min at 94 °C for enzyme deactivation. The expression of GSL pathway genes was analyzed using the AccuPower 2 × Greenstar qPCR Master Mix (Bioneer) with three technical replicates. According to the instructions, the reaction mixture was prepared by mixing 10 μl of 2 × Master mix, 1 μl of forward primer (10 pmol), 1 μl of reverse primer (10 pmol), 0.5 μl of 50 × ROX dye, 1 μl of template (cDNA), and 6.5 μl diethyl pyrocarbonate (DEPC)-treated water. The RT-qPCRs were performed in an ABI PRISM 7500 real-time PCR system (Life Technologies, USA) with the following program: 95 °C initial denaturation for 10 min, 40 cycles at 95 °C for 30 s, annealing at 60 °C for 30 s, and extension at 72 °C for 1 min. The RT-qPCR was repeated using three independent biological replicates. The relative expression of each gene was obtained by normalization to a reference gene (ACTIN). The data were analyzed using ABI PRISM 7500 expression suite software (Life Technologies). The primers for RT-qPCR were designed using primer3 online software (http://bioinfo.ut.ee/primer3-0.4.0/) and listed in the Supplementary Table S1.
Southern blot analysis
Genomic DNA was extracted from radish (wild type (WT) and eight BASTA-resistant T1 radish transformants). Equal amounts (10 µg) of genomic DNA were digested with EcoRI-HF (New England Biolabs, USA) and fractionated on a 0.8% agarose gel. The gel was washed twice with denaturation buffer (75 ml of 1.5 M NaCl, 12.5 ml of 0.5 NaOH, and purified H2O up to 250 ml) and twice with neutralization buffer (75 ml of 1.5 M NaCl, 62.5 mL of 0.5 Tris, 0.5 ml of 0.001 M EDTA, and purified H2O up to 250 ml) for 20 min per wash. The fractionated DNA bands were transferred onto Amersham Hybond N+ membranes (Amersham, USA). Prehybridization and hybridization were carried out at 65 °C in the presence of buffered salt solution, Denhardt's solution, and bovine serum albumin (BSA). The blotted membrane-bound DNA bands were hybridized with the 1.96 kb bar gene fragment, 32P-labeled using the DECAprime™ II DNA Labeling System (Thermo Fisher Scientific, USA). The membranes were washed with the following solutions: 50 ml of 2 × SSC, 0.1% (w/v) SDS at 65 °C for 25 min and 0.1 × SSC and 0.1% (w/v) SDS at 65 °C for 25 min. The membranes were then exposed to a phosphor screen for 4 days at − 80 °C using an intensifier screen.
GFP transient expression
To detect GFP (Green Fluorescent Protein) expression in the putative transgenic plants, T1 radish transformants were germinated in half strength MS salt medium. Fluorescence images of 7 days after germination (DAG) seedlings were acquired with a Nikon SMZ-18 stereo microscope. GFP-L with a 460–500 nm excitation filter (transmitting only green light) was used.
Extraction and analysis of GSLs
Whole fresh plant tissues from individual transgenic plants were lyophilized using a vacuum freeze dryer (Ilshin Biobase, Korea) and ground into a fine powder for GSL analysis. Desulfo-glucosinolates (DS-GSLs) were extracted and analyzed as previously described (Nugroho et al. 2019). Briefly, the lyophilized samples were incubated with 70% methanol at 70 °C for 10 min to inactivate myrosinase activity. The methanol extract was then transferred into a polypropylene column (Thermo Fisher Scientific). Sulfatase (11.25 units, Sigma-Aldrich) was added, and the mixture was incubated for 12 h at 37 °C; 0.5 mg/ml of sinigrin (Sigma-Aldrich) was used as an internal standard. Individual DS-GSLs were analyzed with ultra-high-performance liquid chromatography (3000 UHPLC System, Thermo Scientific). Specifically, the DS-GSLs were separated on a C18 reverse phase column (Zorbax XDB-C18, 4.6 × 250 mm, 2.5 μm particle size, Agilent, USA) with a water and acetonitrile gradient system. The samples (20 μl) were injected, and the flow rate was maintained at 1.0 ml min−1. Peaks were identified using standard compounds (Phytoplan, Germany) (Supplementary Table S2), and sinigrin was used for relative quantification (Brown et al. 2003). The contents were analyzed independently as three replicates and presented as μmol kg−1 dry weight (DW).
Results
Transformation of radish seedlings with the radish BCAT4 gene
Using our needle perforation protocol to transform apical meristems of seedlings, young radish seedlings were prepared and used for transformation with the pEGAD-RsBCAT4 plasmid (Fig. 1). We performed 4 independent transformations with the pEGAD-RsBCAT4 vector (Table 1). In detail, non-infected radish cv Jinjudaepyong were used and collected separately in each transformations process for the control plants.
After transformation using our new method, a total of 128 T0 transgenic plants were obtained in the greenhouse. After harvesting the T1 seeds of individual T0 plants, 80 T1 lines survived the 0.03% BASTA treatment (Table 1). To confirm T-DNA integration into the genome of these putative transformants, the presence of the RsBCAT4 transgene was first verified by PCR (Fig. 3). PCR using two primer pairs, bar-F + bar-R and GFP-F + BCAT4-R, was performed to amplify DNA fragments of 400 bp and 1352 bp, respectively (Fig. 3A). Sixty-one T1 transgenic radish plants were deemed positive in the PCR analysis (Table 1 and Fig. 3B). No detectable PCR products were amplified with these primer pairs from the genomic DNA of WT plants.
PCR detection of GFP-BCAT4 and bar coding sequence in radish T1 transgenic lines. A The pEGAD-RsBCAT4 construct showing location of primers used for PCR analysis. Each primer is indicated with red color arrows. B Results of PCR analysis using primer pairs for GFR-F+RsBCAT4R and bar-F+bar-R. Expected PCR fragments of 1352 bp (GFP-F+BCAT4-R) and 400 bp (bar-F+bar-R) were amplified from 61 putative T1 transgenic lines. M, marker; WT, Wild type; P, Plasmid DNA; and the 61 T1 plants
Southern blot analysis of RsBCAT4 T1 transgenic plants
To quantify transcript levels of RsBCAT4 transgenic lines, twenty-four T1 lines were primarily selected and used for RT-qPCR analysis. All tested 24 T1 transgenic lines showed significantly higher expression of RsBCAT4 compared to the level of WT plants (Supplementary Fig. S3A). We further selected eight T2 transgenic lines that exhibited substantially increased RsBCAT4 mRNA transcripts (Fig. 4). To verify incorporation of the RsBCAT4 transgene into the radish genome, Southern blot analysis was performed (Fig. 5A). Genomic DNA from one WT and the eight T1 transgenic plants (#26, #31, #42, #46, #48, #57, #59, and #61) was digested and hybridized with the 32P-labeled BAR probe. The copy number of transgenes in the radish genome, reflected by the number of hybridized bands, varied from one to three. Six transformants (excluding #57 and #46) showed clear, hybridized bands, indicating that the RsBCAT4 transgene was integrated into the radish genome. The faint band in the line #57 and #46 might be caused by incomplete digestion or membrane transfer due to the relatively low quality of genomic DNAs. In contrast, no hybridization signal was detected for genomic DNA of non-transgenic (WT) plants.
Expression of RsBCAT4 in T2 transgenic plants. A RsBCAT4 functions to convert methionine (Met) to 4-methylthio-2-oxobutyrate (MTOB), the first step in aliphatic glucosinolate biosynthesis. B Relative transcript levels of RsBCAT4 were calculated by the normalization to the level of the reference gene, RsACTIN, in eight T2 transgenic lines. Data are shown as means of triplicate samples with standard errors. Student t-test was applied to calculate statistical significance (*P < 0.05, **P < 0.01, and ***P < 0.001)
GFP visualization of RsBCAT4 T1 transgenic plants
GFP analyses of the eight T1 transgenic plants (#26, #31, #42, #46, #48, #57, #59, and #61) were performed to verify transgene expression in the radish transformants. While green fluorescence was detected in all T1 transgenic seedlings, none was observed in the WT plants (Fig. 5B). In addition, PCR analysis of transgenic lines showed the presence of 225 bp GFP transgene in T1 plants (Supplementary Fig. S4B). These data indicate that transgene integration and expression successfully occurred in these radish plants.
Effect of overexpressed RsBCAT4 on GSL profile in radish
GSLs are stress-responsive defense compounds uniquely found in Brassicaceae family crops, including radish. RsBCAT4 is the enzyme responsible for conversion of Met to MTOB, the first step in the biosynthesis of aliphatic GSLs. To explore the effect of overexpression of the RsBCAT4 transgene, we first quantified the transcript level of RsBCAT4 transcripts from twenty-four T1 lines (Supplementary Fig. S4). All T1 transgenic lines exhibited significantly overexpressed level of RsBCAT4 compared to the wild type. Next, we measured the amounts of aliphatic GSLs of twenty-four T1 lines using HPLC (Supplementary Fig. S5). Among twenty-four lines, sixteen lines (67%) exhibited higher level of aliphatic GSL compounds albeit level of individual lines were different. Four (#25, #44, #47, and #57) and other four (#14, #17, #20, and #22) T1 lines showed similar or even lower level of aliphatic GSL compounds. This kind of phenomenon has also been reported in several other studies (Zhang et al. 2015; Kumar et al. 2017). It indicates that metabolic engineering by transgene expression might be somehow beyond our expectation because there are many factors are involved in the complicated metabolic networks in plants. Hence, it is likely that although we have the transgenic plants with high expression of gene interest, it doesn’t always guarantee the increase of metabolite product, thus requiring step to confirm metabolic change. For further analysis in next T2 generation, we selected eight T2 lines showing higher aliphatic GSL compounds. 4-week leaves of eight T2 transgenic plants were subjected to UHPLC analysis to measure GSL content. We compared the total aliphatic GSL content in one WT and eight T2 transgenic plants. UHPLC data showed that overexpression of RsBCA4 substantially increased the amount of aliphatic GSL compounds compared to that in WT radish plants (Figs. 6A and 7). It was previously reported that GRH represents a large portion of the aliphatic GSLs produced in radish (Nugroho et al. 2019) We also observed that GRH was the most abundant GSL compound in all tested radish plants (Figs. 6B and 7). Along with the elevation in GRH, the levels of other aliphatic GSLs significantly increased in most of the tested pEGAD-RsBCAT4 transgenic lines (Fig. 7). Next, we measured the level of three genes downstream of BCAT4 (i.e., RsCYP79F1, RsCYP83A1, and RsGRS1) in the aliphatic GSL pathway. The expression profiles of these three genes in the eight RsBCAT4-overexpressed transgenic lines displayed a similar pattern to that of RsBCAT4 (Fig. 8A), suggesting that overexpression of RsBCAT4 positively affected expression of downstream genes in GSL biosynthesis. Correlation coefficient analysis of the expression profiles of RsBCAT4 and its three downstream genes also indicated significant positive correlations in the transgenic lines (Fig. 8B). Taken together, these data support that overexpression of RsBCAT4 in radish can affect expression of biosynthetic genes in the aliphatic GSL pathway, resulting in increased aliphatic GSL compounds, particularly GSH.
Aliphatic GSL content in pEGAD-RsBCAT4 transgenic lines of radish. A Amount of total aliphatic GSLs (nM/g dry weight) in wild type and eight T2 transgenic plants. B Amount of glucoraphasatin (GRH) in wild type and eight T2 transgenic plants. Vertical red line indicates the level of WT. One-way ANOVA analysis with Duncan post-hoc test was applied to calculate the statistical difference. For the wild type, three biological replications were used in this analysis (n = 3)
Pie charts showing profiles of aliphatic GSL content. Profiles of individual aliphatic GSL compounds from wild type and eight T2 transgenic lines (#26-2, #31-2, #42-2, #46-2, #48-3, #57-5, #59-2, and #61-4) were measured using UHPLC analysis. The total amount of aliphatic GSLs detected in each line is shown below each pie chart. GRH glucoraphasatin, GER glucoerucin, GRE glucoraphanin, PGT progoitrin, GSL glucosinolates. For the wild type, three biological replications were used in this analysis (n = 3)
RT-qPCR quantification of downstream genes in aliphatic GSL pathway. A Expression of three genes (RsCYP79F1, RsCYP83A1, and RsGRS1) downstream of RsBCAT4 in the aliphatic GSL biosynthetic pathway in eight T2 plants. Relative transcript levels of the three downstream genes normalized to level of reference gene, RsACTIN, were calculated in wild type plants (WT) and eight T2 transgenic lines. Data are shown as means of triplicate samples with standard errors. Asterisks indicate a significant difference between lines by Duncan's multiple range test (*P < 0.05, **P < 0.01, and ***P < 0.001). B Correlations of expression between RsBCAT4 and each downstream gene (RsCYP9F1, RsCYP83A1, and RsGRS1). The R-value is the correlation coefficient between the expression of RsBCAT4 and RsCYP83A1, RsCYP79F1 and RsGRS1 in wild type and eight pEGAD-RsBCAT4 transgenic lines, which were 0.96, 0.93, and 0.95, respectively. Therefore, overexpression of RsBCAT4 positively affects expression of each downstream gene in the aliphatic GSL pathway. X axis represents the relative expression of RsBCAT4, while Y axis represents the relative expression of the three downstream genes (RsCYP83A1, RsCYP79F1, and RsGRS1). Correlation coefficients (R-values) were analyzed using Sigma plot 12.0. GSL, glucosinolate
Discussion
Agrobacterium-mediated transformation has been widely used to transfer genes of interest (GOIs) to plant genomes. However, in many crop plants, it is still quite difficult to obtain genetic transformants. Pre-existing protocols should be experimentally fine-tuned to determine the optimal protocol to generate a particular transgenic crop. For successful Agrobacterium-mediated transformation of radish, various issues need to be addressed, which include the type of explant, DNA delivery method into the genome, Agrobacterium inoculation and co-cultivation method, screening method for the transformed plants, and regeneration method of the transgenic plants (Gelvin 2003; Hwang et al. 2017; Liu et al. 2018; Lacroix and Citovsky 2019; Li et al. 2020). Tissue culture is a common method used to generate calluses from explants, in which transgenes are introduced by Agrobacterium into the host plant genome. However, in the case of radish tissue culture, the cultured cells are recalcitrant to regenerating shoots (Jeong et al. 1995; Takahata et al. 1996). Thus, hypocotyls and cotyledons have been mostly used as explants because they have the potential to regenerate shoots. Among these tissues, cotyledon explants require a longer time for organogenesis than hypocotyl explants (Jeong et al. 1995; Bae et al. 2012; Xu et al. 2020).
In planta methods of gene transfer via Agrobacterium have been introduced, which avoid tissue culture steps and produce transgenic plants directly from infected seedlings in a shorter period of time than tissue culture-based transformations (Brown et al. 2003; Yasmeen et al. 2009; Rivera et al. 2012; Shah et al. 2015; De Jonge et al. 2016). The first transgenic radish was reported in early 2001 by Curtis and Nam using in planta transformation, in which primary bolted plants were used as explants. They demonstrated the successful progeny inheritance of antisense GIGANTEA (GI) T-DNA by Southern blot analysis (Curtis and Nam 2001). Another method for radish transformation utilized both ultrasonic- and vacuum infiltration-assisted transfer to seeds (Park et al. 2005). In addition, Agrobacterium-mediated transformation parameters such as bacterial strain, time of inoculation, and type of promoter sequence need to be optimized for successful transformation (Beaujean et al. 1998; Supartana et al. 2005; Gelvin 2017). For the inoculation and co-cultivation of Agrobacterium cells to deliver transgenes into radish seedlings, 125 mg/l of acetosyringone and a long co-cultivation period (3 days in darkness followed by 2 days under light) were shown to be effective compared to the conditions needed for other Brassicaceae family plants (Liu et al. 2018).
Considering the young age of seedlings, young seedlings are fit materials that are easy to be manipulated in plant transformation due to the cell wall is not so rigid. In addition, the physiological and biochemical status of seedlings is also very active which can help the plant to recover fast after the transformation process. That is most likely why the majority of researchers use much older seedlings, such as 5- or even 10-day-old seedlings in other in planta transformation using seedlings (Barillari et al. 2007; Ciska et al. 2008; Baenas et al. 2015; Kitajima et al. 2020). Nevertheless, transformation of seedlings has a limitation. For example, it is usually performed in seedlings having normally a green and thin stem (2–3 cm length). Based on reports using Brassica oleracea plants, low temperatures during germination affect shoot meristem growth and development in the seedlings (Tobeh and Jamaati-E-Somarin 2012; De Jonge et al. 2016; Fu et al. 2017). Thus, to overcome this limitation, we subjected germinating seedlings to a low temperature and light to stimulate strong and stumpy growth. In preliminary test, seedling treated with low temperature (4 °C) showed a shorter and more stumpy hypocotyl than seedling grown at high temperature (28 °C) (Supplementary Fig. S5). In addition, we used a needle to generate tiny physical wounding to increase the delivery of bacteria to plant tissues. To avoid mechanical damage to the apical meristem, the side of the upper hypocotyl was perforated. Under the husk, the epicotyl or plumule (embryonic shoot above the cotyledonary node) gives rise to the shoot apex and, later, germ cells (Rao et al. 2008; Foster 2016). In detail, seedlings were perforated 5–10 times using a small needle (0.1–0.3 mm diameter) to minimize the negative effect of wounding on seedlings' growth and development. Needle perforation is a simple and quick way to create wounds on the seedlings and bacteria delivery, however, if the perforation were done excessively the seedlings may be subject to physical stress and abnormal development caused by the wounds (Trieu et al. 2000; Rivera et al. 2012).
Upon maturation, germ cells (egg and pollen) are produced by apical meristems. An individual germ cell contains a single haploid copy of the genomic DNA. Two germ cells, an egg and a sperm, produce a single fertilized cell, from which the entire body of cells of each transformant in the next generation is derived. Thus, transformants of the T1 and subsequent generations should be true transformants and not chimeras. On the basis of the above considerations, in most of our experiments, we examined the transformation and integration of the transgene in the genome of transformants using plants of the T1 and T2 generation, but not the T0 generation. To the best of our knowledge, the successful transformation of radish using seedlings and the perforation method has not been reported previously, although the possibility that the basal parts of young seedlings are a potential explant source for regeneration and transformation has been noted. In that radish transformation study, the standard method only resulted in transformation efficiencies of 1.4% and 8% for floral dipping and seed Agrobacterium co-culture, respectively (Park et al. 2005; Curtis 2011). Using our method, we were able to achieve a much higher transformation rate (Table 1). In the last decade, there has been strong interest in GSL biosynthesis of Brassicaceae family plants, including radish. The BCAT4 gene encodes an enzyme catalyzing the initial step of chain elongation of amino acids by converting Met to MTOB. Thus, BCAT4 might play a critical role in the biosynthesis of GSLs in radish. In this study, the pEGAD vector harboring the RsBCAT4 transgene driven by a Cauliflower Mosaic Virus 35S (CAMV35s) promoter was used for radish transformation. CAMV35s is a constitutive promoter that is used extensively to drive the expression of genes in the majority of tissues throughout plant development. Regarding transgene integration, it is generally considered that integration occurs at random positions, leading to unwanted side effects and unpredictable gene expression patterns (Gelvin 2017). We observed significant overexpression of RsBCAT4 in most of the transgenic plants, although there was a broad range of RsBCAT4 expression among the different lines (Fig. 4B). Thus, we confirmed that CAMV35S can be used to successfully express the RsBCAT4 transgene in radish plants.
It is also worthy to note that even RsBCAT4 is highly overexpressed in all tested transgenic lines, corresponding metabolites (aliphatic GSLs) are not accordingly increased in tested T2 lines (Fig. 6). This phenomenon was also observed in T1 line as we mentioned above response (Supplementary Fig. S3). Regarding this observation, we reasoned that many factors are involved in the complicated metabolic network and metabolic flux in certain steps can somehow reduce rate of metabolic process. Thus, overexpression of one particular metabolic genes does not always guarantee the increase of target metabolite products. Thus, follow-up selection is essentially required to select the transgenic lines exerting increased production of metabolite of interest.
To date, there are the limited cultivars including Chinese (Pua et al. 1996), Japanese (Hegazi and Matsubara 1992; Park et al. 2005), and Korean ecotypes (Curtis et al. 2004, Cho et al. 2008) used for the genetic transformation in radish. Jinjudaepyung cultivar is a homozygous inbred radish line and are commercially used for the cultivation in South Korea. In addition, this cultivar is mainly used for genetic transformation in previous studies (Curtis et al. 2004; Cho et al. 2008; Curtis 2011). Previously, Jinudaepyung cultivar was shown to have 1.4% transformation efficiency using floral dipping method (Curtis and Nam 2001), 0.28% efficiency using hypocotyl explant regeneration system (Mi et al. 2008). Later, in plant transformation using germinating radish seeds and sonication-vacuum infiltration method significantly increased the transformation efficiency to 8% using cultivar named Kosena (Park et al. 2005). In the present study, we succeed to better increase the transformation efficiency (average about 47%) in radish using needle perforation method in Jinjudaepyung cultivar. Further studies might remain to be tested whether this in planta needle perforation method is efficiently applicable to other radish cultivars.
In summary, we report that our Agrobacterium-mediated transformation using a needle perforation and Agrobacterium tumefaciens incubation method to transform apical meristems of seedlings is a viable method to generate transgenic radish plants. This technique should be amenable to a broad array of applications such as the generation of overexpression lines, knockdown lines using RNAi, and knockout mutants using CRISPR-Cas9 genome editing. In addition, this method could be expanded and used for the transformation of other crop plants.
References
Bae H, Kim YB, Il PN et al (2012) Agrobacterium rhizogenes-mediated genetic transformation of radish (Raphanus sativus L. Cv. Valentine) for accumulation of anthocyanin. Plant OMICS 5:381–385
Baenas N, Ferreres F, García-Viguera C, Moreno DA (2015) Radish sprouts-characterization and elicitation of novel varieties rich in anthocyanins. Food Res Int 69:305–312. https://doi.org/10.1016/j.foodres.2015.01.009
Barillari J, Iori R, Broccoli M et al (2007) Glucoraphasatin and glucoraphenin, a redox pair of glucosinolates of brassicaceae, differently affect metabolizing enzymes in rats. J Agricul Food Chem 55:5505–5511. https://doi.org/10.1021/jf070558r
Beaujean A, Sangwan RS, Lecardonnel A, Sangwan-Norreel BS (1998) Agrobacterium-mediated transformation of three economically important potato cultivars using sliced internodal explants: an efficient protocol of transformation. J Exp Bot 49:1589–1595. https://doi.org/10.1093/jxb/49.326.1589
Brown PD, Tokuhisa JG, Reichelt M, Gershenzon J (2003) Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochemistry 62:471–481. https://doi.org/10.1016/S0031-9422(02)00549-6
Burow M, Halkier BA (2017) How does a plant orchestrate defense in time and space? Using glucosinolates in Arabidopsis as case study. Curr Opin Plant Biol 38:142–147. https://doi.org/10.1016/j.pbi.2017.04.009
Cartea ME, Velasco P (2008) Glucosinolates in Brassica foods: bioavailability in food and significance for human health. Phytochem Rev 7:213–229. https://doi.org/10.1007/s11101-007-9072-2
Chhajed S, Mostafa I, He Y et al (2020) Glucosinolate biosynthesis and the glucosinolate-myrosinase system in plant defense. Agronomy 10:1786. https://doi.org/10.3390/agronomy10111786
Cho MA, Min SR, Ko SM et al (2008) Agrobacterium-mediated genetic transformation of radish (Raphanus sativus L.). Plant Biotechnol 25:205–208. https://doi.org/10.5511/plantbiotechnology.25.205
Ciska E, Honke J, Kozłowska H (2008) Effect of light conditions on the contents of glucosinolates in germinating seeds of white mustard, red radish, white radish, and rapeseed. J Agricult Food Chem 56:9087–9093. https://doi.org/10.1021/jf801206g
Clough SJ, Bent AF (1998) Floral dip: a simplified method for agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743. https://doi.org/10.1046/j.1365-313X.1998.00343.x
Curtis IS (2011) Genetic engineering of radish: current achievements and future goals. Plant Cell Rep 30:733–744. https://doi.org/10.1007/s00299-010-0978-6
Curtis IS, Nam HG (2001) Transgenic radish (Raphanus sativus L. longipinnatus Bailey) by floral-dip method—plant development and surfactant are important in optimizing transformation efficiency. Transgenic Res 10:363–371. https://doi.org/10.1023/A:1016600517293
Curtis IS, Nam HG, Sakamoto K (2004) Optimized shoot regeneration system for the commercial Korean radish “Jin Ju Dae Pyong.” Plant Cell Tissue Organ Cult 77:81–87. https://doi.org/10.1023/B:TICU.0000016536.80238.ef
De Jonge J, Kodde J, Severing EI et al (2016) Low temperature affects stem cell maintenance in Brassica oleracea seedlings. Front Plant Sci 7:1–14. https://doi.org/10.3389/fpls.2016.00800
Fahey JW, Zalcmann AT, Talalay P (2001) The chemical diversity and distribution of glucosinolates and isothiocyanates amoung plants. Phytochemistry 56:5–51. https://doi.org/10.1016/S0031-9422(00)00316-2
Fimognari C, Turrini E, Ferruzzi L et al (2012) Natural isothiocyanates: genotoxic potential versus chemoprevention. Mutat Res 750:107–131. https://doi.org/10.1016/j.mrrev.2011.12.001
Foster CSP (2016) The evolutionary history of flowering plants. J Proc R Soc N S W 149:65–82
Fu JJ, Liu J, Yang LY et al (2017) Effects of low temperature on seed germination, early seedling growth and antioxidant systems of the wild Elymus nutans Griseb. J Agricult Sci Technol 19:1113–1125
Gelvin SB (2003) Agrobacterium—mediated plant transformation: the biology behind the agrobacterium—mediated plant transformation: the biology behind the “gene-jockeying” tool. Microbiol Mol Biol Rev 67:16–37. https://doi.org/10.1128/MMBR.67.1.16
Gelvin SB (2017) Integration of agrobacterium T-DNA into the plant genome. Annu Rev Genet 51:195–217. https://doi.org/10.1146/annurev-genet-120215-035320
Hegazi HH, Matsubara S (1992) Callus formation and plant regeneration from protoplast derived from cotyledons and hypocotyls of radish (Raphanus sativus L.) and other cruciferous plants. Engei Gakkai zasshi 61:63–68. https://doi.org/10.2503/jjshs.61.63
Hwang H-H, Yu M, Lai E-M (2017) Agrobacterium—mediated plant transformation: biology and applications. Arabidopsis Book 15:e0186. https://doi.org/10.1199/tab.0186
Jeong WJ, Min SR, Liu JR (1995) Somatic embryogenesis and plant regeneration in tissue cultures of radish (Raphanus sativus L.). Plant Cell Rep 14:648–651. https://doi.org/10.1007/BF00232731
Kakizaki T, Kitashiba H, Zou Z et al (2017) A 2-oxoglutarate-dependent dioxygenase mediates the biosynthesis of glucoraphasatin in Radish. Plant Physiol 173:1583–1593. https://doi.org/10.1104/pp.16.01814
Kitajima S, Miura K, Yasuda J (2020) Radish sprouts as an efficient and rapidly available host for an agroinfiltration-based transient gene expression system 92:89–92. https://doi.org/10.5511/plantbiotechnology.19.1216a
Kumar P, Augustine R, Singh AK, Bisht NC (2017) Feeding behaviour of generalist pests on Brassica juncea: implication for manipulation of glucosinolate biosynthesis pathway for enhanced resistance. Plant Cell Environ 40:2109–2120. https://doi.org/10.1111/pce.13009
Lacroix B, Citovsky V (2019) Pathways of DNA transfer to plants from agrobacterium tumefaciens and related bacterial species. Annu Rev Phytopathol 57:231–251. https://doi.org/10.1146/annurev-phyto-082718-100101
Li D, Wei X, Liu T et al (2020) Establishment of an Agrobacterium tumefaciens-mediated transformation system for Tilletia foetida. J Microbiol Methods 169:105810. https://doi.org/10.1016/j.mimet.2019.105810
Lim S, Ahn JC, Lee EJ, Kim J (2020) Antiproliferation effect of sulforaphene isolated from radish (Raphanus sativus L.) seeds on A549 cells. Appl Biol Chem. https://doi.org/10.1186/s13765-020-00561-7
Liu W, Yang Y, Liu Q (2018) Establishment of an efficient regeneration system using heading leaves of Chinese cabbage (Brassica rapa L.) and its application in genetic transformation. Hortic Environ Biotechnol 59:583–596. https://doi.org/10.1007/s13580-018-0064-5
Matveeva TV, Lutova LA (2014) Horizontal gene transfer from agrobacterium to plants. Front Plant Sci 5:1–11. https://doi.org/10.3389/fpls.2014.00326
Mi AC, Sung RM, Suk MK et al (2008) Agrobacterium-mediated genetic transformation of radish (Raphanus sativus L.). Plant Biotechnol 25:205–208. https://doi.org/10.5511/plantbiotechnology.25.205
Murashige T, Skoog F (1962) Murashige1962Revised.Pdf. Physiol Plant 15:474–497
Nugroho ABD, Han N, Pervitasari AN et al (2019) Differential expression of major genes involved in the biosynthesis of aliphatic glucosinolates in intergeneric Baemoochae (Brassicaceae) and its parents during development. Plant Mol Biol. https://doi.org/10.1007/s11103-019-00939-2
Park BJ, Liu Z, Kanno A, Kameya T (2005) Transformation of radish (Raphanus sativus L.) via sonication and vacuum infiltration of germinated seeds with agrobacterium harboring a group 3 LEA gene from B. napus. Plant Cell Rep 24:494–500. https://doi.org/10.1007/s00299-005-0973-5
Pua EC, Lee JEE (1995) Enhanced de novo shoot morphogenesis in vitro by expression of antisense 1- aminocyclopropane-1-carboxylate oxidase gene in transgenic mustard plants. Planta: Int J Plant Biol 196:69–76. https://doi.org/10.1007/BF00193219
Pua EC, Sim GE, Chi GL, Kong LF (1996) Synergistic effect of ethylene inhibitors and putrescine on shoot regeneration from hypocotyl explants of Chinese radish (Raphanus sativus L. var. longipinnatus Bailey) in vitro. Plant Cell Reports 15:685–690. https://doi.org/10.1007/BF00231925
Rao KS, Sreevathsa R, Sharma PD et al (2008) In planta transformation of pigeon pea: a method to overcome recalcitrancy of the crop to regeneration in vitro. Physiol Mol Biol Plants 14:321–328. https://doi.org/10.1007/s12298-008-0030-2
Rivera AL, Gómez-Lim M, Fernández F, Loske AM (2012) Physical methods for genetic plant transformation. Phys Life Rev 9:308–345. https://doi.org/10.1016/j.plrev.2012.06.002
Sasaki M, Nonoshita Y, Kajiya T et al (2020) Characteristic analysis of trigonelline contained in Raphanus sativus cv. Sakurajima daikon and results from the first trial examining its vasodilator properties in humans. Nutrients 12:1–11. https://doi.org/10.3390/nu12061872
Schuster J, Knill T, Reichelt M et al (2006) BRANCHED-CHAIN AMINOTRANSFERASE4 is part of the chain elongation pathway in the biosynthesis of methionine-derived glucosinolates in arabidopsis. Plant Cell 18:2664–2679. https://doi.org/10.1105/tpc.105.039339
Shah SH, Ali S, Jan SA et al (2015) Piercing and incubation method of in planta transformation producing stable transgenic plants by overexpressing DREB1A gene in tomato (Solanum lycopersicum Mill.). Plant Cell Tissue Organ Cult 120:1139–1157. https://doi.org/10.1007/s11240-014-0670-6
Sønderby IE, Geu-Flores F, Halkier BA (2010) Biosynthesis of glucosinolates—gene discovery and beyond. Trends Plant Sci 15:283–290. https://doi.org/10.1016/j.tplants.2010.02.005
Supartana P, Shimizu T, Shioiri H et al (2005) Development of simple and efficient in planta transformation method for rice (Oryza sativa L.) using agrobacterium tumefaciens. J Biosci Bioeng 100:391–397. https://doi.org/10.1263/jbb.100.391
Takahata Y, Komatsu H, Kaizuma N (1996) Microspore culture of radish (Raphanus sativus L.): Influence of genotype and culture conditions on embryogenesis. Plant Cell Rep 16:163–166. https://doi.org/10.1007/s002990050198
Ting HM, Cheah BH, Chen YC et al (2020) The role of a glucosinolate-derived nitrile in plant immune responses. Front Plant Sci 11:1–18. https://doi.org/10.3389/fpls.2020.00257
Tobeh A, Jamaati-E-Somarin S (2012) Low temperature stress effect on wheat cultivars germination. Afr J Microbiol Res 6:1265–1269
Trieu AT, Burleigh SH, Kardailsky IV et al (2000) Transformation of Medicago truncatula via infiltration of seedlings or flowering plants with agrobacterium. Plant J 22:531–541. https://doi.org/10.1046/j.1365-313X.2000.00757.x
Weigel D, Glazebrook J (2006) Transformation of agrobacterium using the freeze-thaw method. Cold Spring Harbor Protoc 2006:pdb.prot4666. https://doi.org/10.1101/PDB.PROT4666
Xu S, Lai E, Zhao L et al (2020) Development of a fast and efficient root transgenic system for functional genomics and genetic engineering in peach. Sci Rep 10:1–9. https://doi.org/10.1038/s41598-020-59626-8
Yasmeen A, Mirza B, Inayatullah S et al (2009) In planta transformation of tomato. Plant Mol Biol Rep 27:20–28. https://doi.org/10.1007/s11105-008-0044-5
Yi G, Lim S, Chae WB et al (2016) Root glucosinolate profiles for screening of radish (Raphanus sativus L.) genetic resources. J Agric Food Chem 64:61–70. https://doi.org/10.1021/acs.jafc.5b04575
Yu R, Xu L, Zhang W et al (2016) De novo taproot transcriptome sequencing and analysis of major genes involved in sucrose metabolism in radish (Raphanus sativus L.). Front Plant Sci 7:1–12. https://doi.org/10.3389/fpls.2016.00585
Zhang Y, Huai D, Yang Q et al (2015) Overexpression of three glucosinolate biosynthesis genes in Brassica napus identifies enhanced resistance to Sclerotinia sclerotiorum and Botrytis cinerea. PLoS ONE 10:1–17. https://doi.org/10.1371/journal.pone.0140491
Acknowledgements
This research was supported by the CAYSS Program of Chung-Ang University to A.N.P. and a research grant (Grant No. PJ01566203) from the Rural Development Administration (RDA) and a Grant (2021R1A5A1032428) from the National Research Foundation of Korea (NRF) funded by the Korea government (MSIT) to D-H. K.
Author information
Authors and Affiliations
Contributions
ANP and ABDN planted all plant materials and performed the molecular experiments; ANP and ABDN performed the UHPLC experiments. ANP, WHJ and JK planned the experiments; ANP, D-HK, and JK analyzed the data and wrote the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Goetz Hensel.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pervitasari, A.N., Nugroho, A.B.D., Jung, W.H. et al. An efficient Agrobacterium tumefaciens-mediated transformation of apical meristem in radish (Raphanus sativus L.) using a needle perforation. Plant Cell Tiss Organ Cult 148, 305–318 (2022). https://doi.org/10.1007/s11240-021-02190-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-021-02190-4