Abstract
This study describes in vitro propagation and cryopreservation of Hovenia dulcis, a woody species used in traditional medicine. Stem and leaf explants from axenic seedlings were cultivated on Murashige and Skoog (MS) medium containing 6-benzyladenine (BA) and kinetin (KIN) alone or in combination (0.1, 0.2, 0.5 mg L−1). For in vitro propagation, rates of regeneration (percentage of responsive explants) and proliferation (multiplication capacity of explant-derived shoots) were evaluated after 30 days and five subcultures, respectively. For cryopreservation by V Cryo-plate technique, shoot tips were excised from microcuttings cultured from in vitro-grown stock plants, or excised directly from axillary shoots of stock plants. The shoot tips were precultured in 0.3 M sucrose (24 h), exposed to loading (20 min) and to PVS2 (0–150 min) before storage in liquid nitrogen. The regrowth was assessed by plating of shoot tips on recovery medium (MS with BA + KIN), with or without a sterile filter paper over the culture medium. Cryopreservation was evaluated by survival (4-weeks) and recovery (8-weeks). The highest regeneration by direct organogenesis (100%) were reached on medium with BA + KIN (0.5 mg L−1 each). Shoots maintained multiplication capacity, showing the highest proliferation (87%) in the presence of BA. Shoot elongation and rooting were achieved on growth regulator-free MS. The most efficient cryopreservation protocol (68% survival and 62% recovery) applied exposure to PVS2 (120 min), and recovery on medium containing BA + KIN (0.5 mg L−1 each) with filter paper. The propagation and cryopreservation of H. dulcis may contribute to its conservation and that of other woody species.
Key message
This study aimed to establish an in vitro propagation methodology and the first cryopreservation protocol for Hovenia dulcis, a woody species of commercial, medicinal and nutraceutical values.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of natural products for disease prevention and health care has been described throughout the history of human civilization (Dias et al. 2012). Among natural products, plants have been the most successful sources of potential drugs due to their bioactive compounds produced mainly through secondary metabolism (Dar et al. 2017). However, owing to the variations in natural growth conditions, these compounds are frequently produced in low quantities and at a non-homogeneous level. In addition, the harvest can be time-consuming and environmentally damaging. Another difficulty for the commercial exploitation of secondary metabolites by conventional propagation methods in tree species is their slow development (Groover 2017; An 2019).
Consequently, in vitro culture techniques are used as alternative for the propagation of plant species, which are difficult to raise through conventional methods (Engelmann 2011b). In vitro propagation represents an opportunity to obtain plants in large-scale in a reduced space of time (An 2019). It also enhances the quality and quantity of desired plant metabolites by altering growth factors, such as medium, carbon source, and plant growth regulators, thereby overcoming the limitations that occur in the natural environment caused by seasonal and climatic changes (Espinosa-Leal et al. 2018). Furthermore, plant tissue cultures provide new conservation approaches, which contribute to widening the availability of options for storing plant germplasm (González-Arnao et al. 2017).
In vitro plant collections is an alternative to short-term preservation, however, the maintenance of in vitro material under active growing can lead to loss by culture contamination or even occur epigenetic or genetic alterations (Kulus et al. 2020). Therefore, once the material is in vitro established, it becomes important to apply long-term conservation approaches to suitable maintenance of the plant genetic resources (Wang et al. 2020b). Cryopreservation, the storage of cells, tissues or organs at the ultra-low temperature of liquid nitrogen (− 196 °C), is the preferable method for the long-term conservation of the biological material. Once cryopreserved, samples are stored safely for unlimited periods due to the interruption of biochemical conditions and most physical processes (Engelmann 2011b; Panis 2019).
A range of cryopreservation techniques are now available for long-term conservation of plant genetic resources, such as woody plant germplasm (Lambardi and Shaarawi 2016; Prudente et al. 2016; Rantala et al. 2019). On the other hand, updated information on technical development and progress in new cryopreservation procedures is still quite limited for forest tree species (Li et al. 2018) as compared to those available for agricultural and horticultural crops, for example, shallot (Allium cepa var. aggregatum) (Wang et al. 2020a), apple (Malus domestica) (Volk et al. 2020) and potato (Solanum tuberosum L. ssp. tuberosum) (Kaczmarczyk et al. 2011).
Hovenia dulcis Thunberg (Rhamnaceae), popularly known as Japanese raisin tree, Japanese grape and chicken-claw pear, is a deciduous tree native to Oriental Asia. The species is used in traditional medicine against intestinal infections and possess anti-asthmatic, antipyretic, diuretic properties, as well as agent to detoxification of alcohol (Lim 2013). It has been reported that extracts from different parts of H. dulcis have shown hepatoprotective (Park et al. 2019), antioxidant (Wang et al. 2012), antineoplastic (Morales et al. 2017), neuroprotective (Li et al. 2005), and antigiardial (Gadelha et al. 2005) effects. In addition, extracts obtained from in vitro cultures have also shown antioxidant (Ribeiro et al. 2015) and antitumor (Castro et al. 2002) properties.
The species H. dulcis was introduced and cultivated in several regions of temperate and subtropical areas owing to its valuable wood, ornamental features and edible fruits (Guidini et al. 2017). Despite its commercial and, mainly, medicinal interest, it is considered an invasive species in forest ecosystems in South America (Schmidt et al. 2020). In Brazil, according to Hendges et al. (2012), it is an invasive species, competing with native species for space, light and nutrients, thus reducing the availability of these resources. These characteristics show the importance of in vitro cultivation of the species, allowing its commercial exploitation without environmental impacts.
Taking into account these aspects, the present research describes a two-step study of in vitro propagation and cryopreservation of H. dulcis shoot tips. In the first part, in vitro propagation was established, evaluating the regeneration rate of different explants from seedlings obtained in vitro, as well as the multiplication capacity (proliferation rate) over time in culture of explant-derived shoots. In the second part, cryopreservation of in vitro propagated plants was established by vitrification using aluminium cryo-plates (V Cryo-plate technique). This long-term conservation technique has advantages such as very rapid cooling and warming rates, which protect explants from freezing damage (Yamamoto et al. 2011). V Cryo-plate has been successfully applied in plant cryopreservation, including fruits species such as Fragaria x ananassa (Yamamoto et al. 2012), species of genera Prunus (Vujović et al. 2015) and Vitis (Bettoni et al. 2019c), and medicinal species such as Cleome rosea (Cordeiro et al. 2015, 2017), Cleome spinosa (Vilardo et al. 2019), Petiveria alliacea (Pettinelli et al. 2017, 2020) and Passiflora suberosa (Vianna et al. 2019).
Considering the commercial interest in the family Rhamnaceae, this study contributes to biotechnological research related to in vitro propagation and long-term conservation by V Cryo-plate cryopreservation technique of other woody species of commercial, medicinal and nutraceutical importance, especially those species belonging to the Rhamnaceae family.
Materials and Methods
In vitro propagation
Plant material
Ripe fruits of Hovenia dulcis Thunberg were collected in Teresópolis, Rio de Janeiro State, Brazil (22°26′23″S 42°58′36″W). A voucher specimen (HRJ 1426) was deposited in the Herbarium of Rio de Janeiro State University, Rio de Janeiro State, Brazil. The seeds were germinated under in vitro conditions according to Castro et al. (2005). Briefly, seeds were mechanical scarified with sandpaper No. 120 and disinfected in a laminar flow with 5% (w/v) sodium hypochlorite (NaOCl) solution and 0.05% Tween 80 (v/v) for 45 min. Then, seeds were rinsed three times with sterile distilled water. The seeds were inoculated in flasks (6.0 × 8.0 cm) containing 30 mL of MS medium (Murashige and Skoog 1962) devoid of growth regulators (MS0), supplemented with 0.09 M sucrose and solidified with 8 g L−1 agar (Sigma-Aldrich®). The medium pH was adjusted to 5.8 prior to autoclaving at 121 °C for 15 min. The flasks were maintained in a growth chamber at 26 ± 2 °C and 16 h photoperiod provided by cool white fluorescent tubes (45 µmol m−2 s−1).
Bud induction and shoot regeneration
Forty-day-old seedlings (Castro et al. 2005) from H. dulcis were used as source of stem (hypocotyl and epicotyl with 0.8 cm in length) and leaf (1.0 cm2) explants that were inoculated on MS medium solidified with 8 g L−1 agar and supplemented with 6-benzyladenine (BA) and kinetin (KIN) in different concentrations (0, 0.1, 0.2, and 0.5 mg L−1) used alone or in combination. The medium pH was adjusted to 5.8 prior to autoclaving at 121 °C for 15 min. The cultures were maintained in flasks (6.0 × 8.0 cm) with 30 mL of culture medium that remained in the same physical conditions described above in relation to seed germination. The hypocotyl and epicotyl explants were inoculated according to organ polarity, and the leaf explants were inoculated with their abaxial surface in contact with the culture medium. After 30 days in culture, the regeneration rate (percentage of explants that induced shoots) and the mean number of shoots per explant were evaluated.
Primary explant–derived shoot cultures
Shoots (≥ 0.5 cm in length) obtained from stem explants after 30 days in culture were isolated and subcultured onto a fresh medium (same composition those used to primary explant cultures) at 30-day intervals during five subcultures. The percentage of explant-derived shoots with multiplication capacity (proliferation rate) and the mean number of shoots were evaluated after each subculture and represented in the results by the average of the five subcultures.
Elongation and rooting
To evaluate the elongation and rooting, shoots (≥ 1.0 cm in length) developed after five subcultures, using the in vitro propagation protocol defined as the most efficient, were transferred to MS0 medium. The cultures were maintained for 45 days under the same physical conditions described before. After this period, were evaluated the shoot height, the number and length of roots and the rooting percentage.
Cryopreservation
Plant material
The in vitro propagated plants established in the first part of this study were used as source of shoot tips for cryopreservation. The shoot tips were excised from microcuttings cultured from the in vitro-grown stock plants, or directly from axillary shoots of stock plants. Microcuttings (1.0 cm in length) with one to two nodes with one to two nodes were isolated from 60-day-old in vitro stock plants and inoculated in culture flasks (6.0 × 8.0 cm) with 30 mL of MS0 medium with a density of five microcuttings per flask and cultured under the same conditions as the in vitro stock cultures. After 3 weeks, the shoot tips (~ 2.0 mm in length) were isolated and used in the cryopreservation experiments. Shoot tips (~ 2.0 mm in length) isolated directly from in vitro propagated plants that showed break of dormancy of axillary buds were also used in cryopreservation. These shoot tips were obtained from axillary shoots developed along the entire stem, regardless of the bud position. To maintain the stock plants, at each 60 days in culture, the apical segments (1‒2 cm in length) were isolated and transferred to fresh medium supplemented with the best combination of cytokinins previously established in the in vitro propagation studies.
Preculture and cryopreservation by V Cryo-plate technique
The shoot tips were precultured on MS medium supplemented with 0.3 M sucrose, 8 g L−1 agar at pH 5.8 on Petri dishes (90 × 15 mm) at room temperature (26 ± 2 °C) for 24 h in darkness. Then, precultured shoot tips were adhered to aluminium cryo-plates No. 3 (Niino et al. 2014). A droplet (2 µL) of 3% (w/v) sodium alginate (low viscosity, Sigma-Aldrich® A2158) solution in calcium-free MS basal medium (supplemented with 0.09 M sucrose), both at pH 5.8, was placed in each well of the cryo-plate,. The precultured shoot tips were placed individually into each well and covered with a droplet (2 µL) of sodium alginate solution, followed by polymerization by the addition of calcium chloride solution (100 mM CaCl2 in MS medium containing 0.09 M sucrose) on the surface of the cryo-plates. After 15 min, at room temperature, polymerization was completed, and the CaCl2 solution was removed with a pipette and gently tapping the cryo-plates on filter paper. Shoot tips adhering to the cryo-plates were treated for 20 min at room temperature with loading solution (0.4 M sucrose and 2.0 M glycerol in MS medium; Nishizawa et al. 1993) and then exposed to vitrification solution PVS2 (30% [w/v] glycerol, 15% [w/v] DMSO, 15% [w/v] ethylene glycol, and 0.4 M sucrose [13.7% (w/v)] in MS medium; Sakai et al. 1990) at 0 °C for 0, 15, 30, 60, 90, 120 or 150 min. The cryo-plates were transferred to uncapped 2–mL cryovials filled with liquid nitrogen (LN) and maintained in LN for at least 15 min. As control, shoot tips were precultured, adhered to the cryo-plates, and exposed to loading, PVS2, and unloading solutions, but without immersion in LN (− LN). For rewarming, the cryo-plates were immersed in unloading solution (MS medium containing 1.2 M sucrose) for 15 min at room temperature.
The shoot tips were removed from the cryo-plates using a scalpel blade and transferred to the following recovery medium formulations:
-
1.
MS medium solidified with 8 g L−1 agar and supplemented with BA + KIN (0.2 mg L−1 BA + 0.2 mg L−1 KIN or 0.5 mg L−1 BA + 0.5 mg L−1 KIN) at pH 5.8 or.
-
2.
Sterilized filter paper (Whatman nº1) disc laid over MS medium solidified with 8 g L−1 agar (to provide a more gradual rehydration of the material) supplemented with 0.5 mg L−1 BA + 0.5 mg L−1 KIN at pH 5.8.
Cultures were maintained for 1 week at 26 ± 2 ºC in the dark. They were then transferred to light intensity 20 µmol m−2 s−1, for 2 weeks, before being exposed to light under standard conditions (45 µmol m−2 s−1). The shoot tips inoculated on filter paper were transferred to the same culture conditions, but without filter paper after the dark period.
The solutions used on cryopreservation assays were adjusted to pH 5.8. Loading, PVS2 and Unloading solutions were sterilized by filtration using a vacuun filter system (Nalgene®). The culture media, sodium alginate solution and calcium chloride solution were autoclaved as described above.
Figure 1 shows the V Cryo-plate protocol applied to cryopreservation of shoot tips of H. dulcis.
Assessment of survival and recovery
The parameters evaluated to determine the efficiency of the cryopreservation were: (1) survival (percentage of shoot tips presenting green color and early growth) 4 weeks after rewarming and (2) recovery (percentage of shoot tips that developed into normal shoots ≥ 0.5 cm in length) 8 weeks after rewarming. Survival and recovery were expressed based on the total number of shoot tips treated. Due to the capacity for multi-shooting proliferation observed in the in vitro plants of H. dulcis, it was also evaluated the average number of new shoots produced per recovered plant, 16 weeks after cryopreservation, was also evaluated.
Statistical analysis
For in vitro propagation assays, 20 explants were used per treatment (five explants per flask). To evaluate the elongation and rooting steps, the experiment consisted of 50 shoots (five shoots per flask). For cryopreservation assays, 10 shoot tips per cryo-plate were used per treatment. Data were analyzed using the statistical software GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA). Statistical analysis was performed using analysis of variance (ANOVA), and the means were compared by Tukey test at 5% probability (p ≤ 0.05). All assays were performed using three repetitions and the results were presented as means ± standard error.
Results
Bud induction and shoot regeneration
Adventitious buds were induced by direct organogenesis from stem explants of H. dulcis between the second and third week in culture. The propagation capacity was incremented on media supplemented with cytokinins and no morphogenic response was obtained from leaf explants.
Regeneration rates from hypocotyl explants did not show statistically significant differences for most treatments applied, although the highest response did not exceed 35% (Table 1). However, explants inoculated on media supplemented with the highest concentration of BA (0.5 mg L−1) combined with KIN showed bud initiation, but without shoot development. Taking into account the mean number of shoots per explant, usually the explants maintained in the presence of BA alone and on media supplemented with 0.1 mg L−1 BA combined with KIN reached the highest propagation capacity (2.33 ± 0.94) (Table 1).
Epicotyl explants inoculated on media with KIN only did not exceed regenerated rates of 15%, while supplementation with BA only was more effective for bud induction. Explants cultivated on medium with 0.1 mg L−1 BA reached a regeneration rate of 85% (Table 1). However, the highest percentages of epicotyl explants that induced shoots were 95 and 100% when explants were inoculated on media supplemented with 0.5 mg L−1 BA combined with 0.2 mg L−1 or 0.5 mg L−1 KIN. Explants maintained on these media also reached the highest mean number of shoots per explant (2.06 and 2.08, respectively) (Table 1).
Proliferation rate from primary explant–derived shoots
Newly developed shoots obtained from hypocotyl and epicotyl explants maintained their proliferation capacity during five subcultures. Shoots cultivated on media supplemented with BA alone or in combination with KIN showed the highest proliferation rates as compared to cultures maintained in the presence of KIN alone or MS0 (Table 2).
The hypocotyl explant-derived shoots showed an increase in multiplication capacity as compared to the original hypocotyl explants since the proliferation rates (Table 2) were higher than the regeneration rates in all treatments (Table 1). Moreover, supplementation with BA only resulted in higher proliferation rates (70‒87%) when compared to shoots cultivated on media with the addition of BA and KIN (48‒77%) (Table 2).
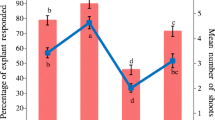
Epicotyl explant-derived shoots maintained high multiplication capacity (Table 2; Fig. 2a). The highest proliferation rate (87%) and mean number of shoots (3.2 ± 0.24) occurred in explants inoculated on 0.5 mg L−1 BA together with 0.5 mg L−1 KIN. As previously observed for cultures initiated with the original epicotyl and hypocotyl explants, the epicotyl explant-derived shoots showed higher multiplication capacity than hypocotyl explant-derived shoots (Table 2).
Elongation and rooting
Shoots propagated on medium supplemented with 0.5 mg L−1 BA + 0.5 mg L−1 KIN were transferred to MS0 medium. After 45 days in culture on the cytokinin-free medium an increase in shoot height ranging from 55 to 65% was observed. The culture on MS0 also induced rhizogenesis in H. dulcis. The development of roots was reached by 85% of shoots after 45 days. The radicular system was constituted by one to five thin, white and hairy roots with 4.0 to 10.0 cm in length and few branched (Fig. 2b).
Since in vitro propagated plants showed a normal phenotypical aspect, they were used as source material during the second step of this work: the establishment of protocols for long-term conservation by cryopreservation.
Cryopreservation by V Cryo-plate technique
Shoot tips precultured on media with 0.3 M sucrose followed by inoculation on culture medium with the same composition used in the maintenance of in vitro propagated plants (MS with 0.5 mg L−1 BA + 0.5 mg L−1 KIN), regenerated normal plantlets. This result demonstrated that the preculture step did not reduced the recovery capacity (data not shown).
Survival and recovery of cryopreserved shoot tips isolated from microcuttings
Shoot tips (Fig. 3a) that adhered to the cryo-plates (Fig. 3b) were cryopreserved and inoculated on the recovery medium with the addition of BA and KIN at 0.5 mg L−1. The non-cryopreserved shoot tips (− LN) had the survival ranging from 86 to 100% and the recovery ranging from 53 to 93% along PVS2 exposure times. There were no differences in the survival and recovery levels of − LN shoot tips that were exposed to 0‒150 min to PVS2 (Table 3).
Cryopreservation of shoot tips of Hovenia dulcis. a Isolated shoot tip. b Shoot tips adhered on the cryo-plates. c Post-cryopreservation recovery plants after 4 weeks, presenting callus formation (arrows). d Hyperhydric plants after cryopreservation. e Plants regenerated from cryopreservation after 16 weeks. Bar (a) = 1.0 mm. Bars (b, c, d, e) = 1.0 cm
Cryopreserved shoot tips (+ LN) that were not exposed to PVS2, or exposed for only 15 min, did not survive, becoming whitish after the first week of culture on recovery medium, even when kept in the dark (Table 3). The survival and recovery levels of LN-exposed (+ LN) shoot tips increased as the PVS2 exposure time was increased from 30 to 120 min and decreased at 150 min. Shoot tips dehydrated in PVS2 for 120 min had the highest level of survival of the 87% and recovery of the 45% after LN exposure. The shoot tips started to grow 2 weeks after cryopreservation, showing green color and leaf primordia development. As previously observed in the in vitro propagated plants, multiple shoots were induced on the cryopreserved shoot tips over time in culture.
Some shoot tips showed callogenesis after transference to the recovery medium (Fig. 3c). They were considered in the quantification of survival since callus is a morphogenic response of living cells and capable of redifferentiation. However, they were not considered for the recovery rate since they did not develop a plant. In addition, some plants developed from cryopreserved shoot tips showed a hyperhydric aspect between the first and the second week after recovery (Fig. 3d). However, this characteristic was no longer observed after the first subculture. 4 months after the cryopreservation process, healthy plants were obtained with an aspect similar to that of the in vitro propagated plants.
Reduction in the concentration of cytokinins in the recovery medium (0.2 mg L−1 BA + 0.2 mg L−1 KIN) did not inhibit the occurrence of callogenesis or hyperhydricity. Taking into account the results previously observed, only exposure times to PVS2 from 60 to 150 min were used in this experiment. Non-cryopreserved shoot tips reached survival rates between 83 and 96% (Table 4). The highest recovery capacity was achieved for shoot tips exposed to PVS2 for 120 min (72%), however, it was statistically non-significant than in other treatments. For cryopreserved shoot tips maximum values of both survival (63%) and recovery (40%) were reached after 150 min of PVS2 exposure (Table 4). Unexpectedly, reduction in the concentration of cytokinins in the recovery medium caused an increase in callogenesis or hyperhydricity in plants regenerated from cryopreserved shoot tips and also in some plants developed from non-cryopreserved shoot tips (data not shown). However, as previously observed, the hyperhydric aspect was not maintained throughout the subcultures, and the plants recovered a normal phenotypic aspect.
When filter paper was used over the recovery medium for non-cryopreserved shoot tips, high survival rates (96–100%) were observed for all exposure times to PVS2. Moreover, there was no influence of the dehydration duration on the recovery rates (60–92%) (Table 5). For cryopreserved material, the exposure to PVS2 for over 60 min resulted in high survival (62–77%). The highest recovery rates (43–62%) were found after 120–150 min dehydration (Table 5).
The use of filter paper resulted in regenerated plants that started to grow at the beginning of the second week of culture on recovery medium, showing the development of leaf primordia of dark green color. During their growth, these plants showed morphology similar to that of in vitro propagated plants. After 4 monthly subcultures (Fig. 3e), these plants maintained their capacity to produce multiple shoots, reaching a number of 2–5 (− LN) and 4–7 (+ LN) shoots per post-cryopreservation recovered plant (Table 5).
Survival and recovery of cryopreserved shoot tips isolated directly from axillary shoots of stock plants
The most suitable cryopreservation protocol applied to shoot tips from microcuttings, was selected to be used with shoot tips excised directly from developed axillary shoots.
The non-cryopreserved shoot tips reached survival rates above 91% for all PVS2 exposure times, and there was no influence of dehydration duration in the vitrification solution on the recovery rate (62–80%) (Table 6). For cryopreserved shoot tips, maximal survival (75%) and recovery (45%) values were achieved after exposure to PVS2 for 120 min (Table 6).
1 week after cryopreservation, the shoot tips developed dark green leaf primordia. The regenerated plants showed normal development and maintained their capacity for multi-shooting proliferation, reaching a number of 3–5 (− LN) and 3‒6 (+ LN) shoots per recovered plant after the cryopreservation process (Table 6).
Discussion
This study evaluated the in vitro propagation of H. dulcis using stem and leaf explants from axenic seedlings and also established the cryopreservation of in vitro-derived shoot tips.
In vitro propagation
According to Castro et al. (2014), in vitro germination allows the establishment of efficient methods to supply a stock of plants in excellent phytosanitary conditions to be used as a source of explants for plant tissue culture. In addition, seedlings are useful as a source of plant material with higher morphogenetic potential.
Important steps in the establishment of in vitro germplasm collections are the induction and multiplication of shoots (Manole-Paunescu 2014; Li et al. 2018). Nevertheless, the development of in vitro protocols for woody species is a challenge because of such factors as recalcitrance and prolonged juvenile stage (Read and Bavougian 2012). Additionally, the production of phenolic compounds during the process of explant excision can lead to oxidation and loss of viability (Bonga et al. 2010).
The shoot propagation of H. dulcis from stem explants was achieved by direct organogenesis. This propagation pathway reduces the risk of somaclonal variations when compared to indirect organogenesis (Zayova et al. 2010). Moreover, the propagation capacity was incremented on media supplemented with cytokinins, highlighting the presence of BA. Among the cytokinins used in in vitro propagation, BA has been mentioned as the most efficient for regenerating shoots for many species (Yuniastuti et al. 2016; Aros et al. 2017; Pereira et al. 2018; Serafim et al. 2018).
The difference in propagation capacity observed between hypocotyl and epicotyl explants, as well as the non-morphogenetic response that occurred in leaf explants, may be related to differences in tissue and cell differentiation, as well as the endogenous balance of auxins and cytokinins (George et al. 2008).
The in vitro propagation of H. dulcis was previously evaluated through protocols of indirect organogenesis (Jeong et al. 2009), indirect somatic embryogenesis (Yang et al. 2013) and direct organogenesis (Echeverrigaray et al. 1998; Park et al. 2006). In these studies, use of the cytokinins BA and KIN also proved to be efficient for inducing morphogenic response. However, no studies have ever evaluated multiplication capacity over time in culture of explant-derived shoots, such as that performed in the present work. These newly developed shoots maintained multiplication capacity by direct organogenesis throughout the subcultures, when compared to the original explants, demonstrating the viability of the protocol in mass production of plants.
The transfer of the propagated shoots of H. dulcis to a cytokinin-free MS medium induced stem elongation and rooting. Although cytokinins are known to regulate morphogenesis in plant tissue culture, stimulating shoot proliferation, their presence can inhibit the elongation process (Schaller et al. 2015). Our results corroborate previous studies with H. dulcis (Jeong et al. 2009) when shoots obtained by indirect organogenesis were elongated and rooting was performed on growth regulator-free medium. However, supplementation with auxins was necessary to induce these morphogenic responses in three other studies on the species (Echeverrigaray et al. 1998; Park et al. 2006; Yang et al. 2013), as well as species of the genus Zizyphus (Goyal and Arya 1985; Rathore et al. 1992; Mathur et al. 1995) from the Rhamnaceae family.
Cryopreservation
Once the in vitro propagation of H. dulcis was established, the long-term conservation of shoot tips using the V cryo-plate technique was investigated, resulting in the first work related to cryopreservation on the genus Hovenia. The in vitro conservation is an important complementary approach to conserve woody species, especially when considering its slow life cycle and the fact that many tree species have recalcitrant seeds, which makes them difficult to store in seed banks (Lambardi and Shaarawi 2016; Corredoira et al. 2017).
Shoot tips are the most used in vitro materials for woody plant cryopreservation. Shoot tips are composed of meristematic tissues, which are characterized by cells with small or absent vacuoles, thin walls, no intercellular space and cells dividing at a constant rate. The shoot tips show a high potential for osmotic tolerance, an important characteristic for success in the establishment of cryopreservation protocols (Normah et al. 2019). Shoot tip cryopreservation of wood species has been carried out by mainly applying vitrification-based techniques (Corredoira et al. 2017; Bettoni et al. 2019a; O’Brien et al. 2020). The V cryo-plate procedure used here to H. dulcis has a few applications in wood species, as reported in Diospyros kaki Thunb. (Matsumoto et al. 2015), Prunus spp. (Vujović et al. 2015), Vitis (Bettoni et al. 2019c) and Morus spp. (Tanaka et al. 2019). The use of this technique facilitates the handling of the material, making the procedures faster since the adhesion of the materials to the cryo-plates reduces the chance of mechanical injuries. Moreover, since the cryo-plates are made of aluminium, a metal with high heat conductivity, the procedures of cooling and rewarming are faster (Niino et al. 2019; Wang et al. 2020b).
Most vegetative explants subjected to cryopreservation contain high levels of free water in their cells and are, therefore, highly sensitive to damage caused by ice formation during the cooling process (Engelmann 2011a). Thus, the biological material is usually dehydrated to protect it from the damage caused by conversion of intracellular liquid water to ice (Corredoira et al. 2017). The first dehydration step applied to shoot tips of H. dulcis was preculture performed in a solidified MS medium with 0.3 M sucrose for 24 h. Sugars are involved in the mechanisms of plant resistance to desiccation and can, for example, replace the water in membranes or induce the process of intracellular vitrification at room temperature (Dumet et al. 1994; Benson 2008). Although sucrose is the most commonly used sugar in cryopreservation protocols, glucose, maltose, mannitol and sorbitol can also be used (Folgado et al. 2015).
After the adhesion of shoot tips to the cryo-plates, the material was submitted to an osmoprotection stage by immersion in a loading solution, which has high concentrations of sucrose (0.4 M) and glycerol (2.0 M), the function of which is to increase the tolerance of biological material to cryoprotective solutions (Nishizawa et al. 1993). Although several cryoprotective solutions have already been established (Engelmann 2013), we selected PVS2 as the vitrification solution and used it at 0ºC. PVS2 acts on some mechanisms that protect plant cells from injuries caused by cooling, replacing the cellular water and altering the cooling behavior of the water remaining in the cells, a process known as vitrification. Longer exposure periods to PVS2 are usually accompanied by an adjustment to the temperature at which the solution is used, owing to its high penetration and toxicity at room temperature (Menon et al. 2012).
For H. dulcis, dehydration with PVS2 for 120 min resulted in the maximal recovery rate (62%) of cryopreserved shoot tips. High exposure time to PVS2 used at 0 °C were also required for other woody species, such as Carica papaya L., which showed 90% recovery after 80 min of exposure to PVS2 (Tsai et al. 2009), Ziziphus jujuba, a species belonging to the Rhamnaceae family, that reached 75% recovery after 90 min of exposure to PVS2 (Wang et al. 2015) and for 12 Vitis species, that reached at least 43% recovery after 90 min of exposure to PVS2 (Bettoni et a. 2019b). On the other hand, for other woody species the most efficient cryopreservation protocols applied low exposure times to the vitrification solution. For Araucaria angustifolia the highest recovery rate (35%) was achieved after 15 min of immersion in PVS2 (Prudente et al. 2016). Exposure to PVS2 for 20‒25 min was the most efficient procedure to recovery after cryopreservation to Persea americana Mill. (73%) (O’Brien et al. 2020) and Garcinia mangostana (50%) (Ibrahim and Normah 2013). These results demonstrated that the tolerance to dehydration with PVS2 varies among species, kind of explant, PVS2 temperature and according to differences in tissue water exchange properties. These characteristics are primary determinants of the osmotic dehydration rate (Vicente et al. 2012).
During the first month after cryopreservation, were observed in some shoot tips of H. dulcis characteristics of hyperhydricity and callogenesis like twisted and transparent leaf, and proliferation of a translucent mass of cells. These aspects are often characteristic, but not limited, to cultures maintained on media with high concentrations of cytokinins and have been reported in some plant materials recovering from cryogenic procedures (Maślanka et al. 2013; Lynch et al. 2014; Kulus 2018; Kulus et al. 2018).
Because of the occurrence of hyperhydricity and callogenesis in cryopreserved shoot tips of H. dulcis, reduction in the concentration of cytokinins in the recovery medium was evaluated. However, the low concentration of BA and KIN (0.2 mg L−1) did not influence the occurrence of hyperhydric aspect and callogenesis after cryopreservation. In addition, lower rates of survival and recovery were obtained in this condition. In contrast, cryopreserved shoot tips of Thymus moroderi presented the highest regeneration rates and lowest level of hyperhydricity on medium supplemented with low BA concentration (0.06 mg L−1) (Marco-Medina et al. 2010). Although the addition of BA to the recovery medium has shown benefits to recovery, the optimal BA concentration vary with the botanical material used, as well as with the cryogenic procedure (Wang et al. 2003).
Cryopreserved shoot tips of H. dulcis reached the highest recovery rates in the presence of cytokinins (BA and KIN) at 0.5 mg L−1. In this condition, the hyperhydric aspect and callogenesis disappeared along the subcultures, and the plants recovered a normal phenotypic aspect. Similarly, in cryopreserved nodal segments and shoot tips of Cydonia oblonga, some regenerated shoots appeared hyperhydrated, but subsequently exhibited a normal morphology within 4 weeks after rewarming (Lynch et al. 2014). Physiological disorders such as hyperhydricity are closely linked to stress processes related to cell membrane damages (Rojas-Martínez et al. 2010). During the cryopreservation steps, plant tissues are exposed to several stress conditions that can result in intracellular ice crystals formation, excessive desiccation, triggering apoptosis or even cause irreversible damage to tissues (Uchendu et al. 2010). However, as observed in H. dulcis, the normal phenotypic aspect after successive subcultures, suggests that the damages caused during cryopreservation has been properly repaired.
Rehydration of cryopreserved materials is a slow and gradual process, which initiates by exposing the material to the unloading solution, followed by the transfer to a recovery medium. The use of physical barriers, such as filter paper, allows water to slowly diffuse from the culture medium into the material, contributing to the restoration of normal water status and avoidance of possible injuries caused by rapid rehydration of the material (Reed 2008; Benson and Harding 2012; Normah et al. 2019). Several studies used filter paper during the recovery step, but they did not compare the effect of the presence/absence of this support. Filter paper is often used on top of the recovery medium of cryopreserved shoot tips (Mandal and Dixit-Sharma 2007; Antony et al. 2011; Pathirana et al. 2016; Mathew et al. 2018; Sulong et al. 2018), and it is also associated with the use of high sucrose concentration (Sant et al. 2008; Marco-Medina et al. 2010; Condello et al. 2011; Panis 2019; Vollmer et al. 2019) or with decreasing concentrations of sucrose in the medium (Panta et al. 2014). The cryopreserved shoot tips of H. dulcis cultivated in the presence of filter paper placed on top of the recovery medium showed higher recovery rates (62%) when compared to protocols without the use of this support (45%). The use of supports onto which the recovered tissue is suspended was also investigated by Ford et al. (2000) for cryopreserved embryogenic tissues of Pinus patula. The authors demonstrated that the use of supports improve the recovery rates, but a combination of filter paper and polyester grids performed better than the tissue recovering on filter paper alone.
The present study also evaluated the cryopreservation of shoot tips excised directly from axillary shoots of stock plants. In H. dulcis, some axillary buds show active growth and develop into axillary shoots on the stem on mother plants. This process may be stimulated by the presence of cytokinins in the culture medium of in vitro propagated plants. Cytokinins promote axillary shoot formation by opposing apical dominance regulated by auxins (Beyl 2000; Park et al. 2006). In the present study, axillary shoots were an alternative source of shoot tips and were compared with shoot tips excised from microcuttings.
Microcuttings cultured from the in vitro-grown stock plants made prior to the excision of shoot tips have been suggested to produce a large number of relatively homogeneous and adequate shoot tips in terms of size, physiological state and growth, increasing the chance of positive and uniform responses in cryogenic procedures (Charoensub et al. 2003; Kulus and Zalewska 2014; Wang et al. 2014a; Bettoni et al. 2019b; Kulus 2020). On the other hand, the use of axillary buds excised directly from the mother plants has also been proposed as a way of increasing the number of explants for cryopreservation (Halmagyi et al. 2005; Pathirana et al. 2016).
Shoot tips from H. dulcis microcuttings produced the highest recovery rates after cryopreservation, compared to shoot tips isolated directly from stock plants. Some studies have evaluated the differences between these two sources of shoot tip. Similar to H. dulcis, cryopreservation of shoot tips sampled from Chrysanthemum morifolium (Wang et al. 2014b) and Vitis vinifera (Marković et al. 2014) microcuttings showed higher regrowth compared with shoot tips excised directly from in vitro propagated plants. The authors highlight active microcutting growth and axillary buds under the effect of apical dominance in complete plants as important aspects for post-cryopreservation responses. Studies where explants derived from apical shoot tips and axillary buds are compared observed differences related to their physiological stages, metabolic profiles, and water content (Halmagyi et al. 2005; Lee et al. 2011; Pathirana et al. 2016).
The results obtained with H. dulcis in the present work confirm the observations reported in other studies and show the feasibility of both microcuttings and axillary shoots as source of shoot tips for cryopreservation. The use of microcuttings can provide more uniform material. The use of axillary buds can save time since the microcutting cultivation step is suppressed. Therefore, the choice of the most suitable explant source must be made for each species under study, taking into account the advantages that each explant source presents.
Conclusions
The studies carried out with H. dulcis allowed the establishment of efficient protocols for in vitro propagation on medium supplemented with the cytokinins BA and KIN and the cryopreservation of the species applying the V Cryo-plate technique. Considering the commercial interest in the family Rhamnaceae, this study may contribute to biotechnological research related to in vitro propagation and long-term conservation of other woody species of great commercial, medicinal and nutraceutical importance, especially those species belonging to the Rhamnaceae family.
Abbreviations
- BA:
-
6-Benzyladenine
- KIN:
-
Kinetin
- PVS2:
-
Plant Vitrification Solution 2
- MS:
-
Murashige and Skoog (1962) Medium
- LN:
-
Liquid Nitrogen
- V Cryo-plate:
-
Vitrification Cryo-plate technique
References
An C (2019) In Vitro propagation of commonly used medicinal trees in Korea. J For Environ Sci 35:272–280
Antony JJJ, Keng CL, Rathinam X, Marimuthu S, Subramaniam S (2011) Effect of preculture and PVS2 incubation conditions followed by histological analysis in the cryopreserved PLBs of Dendrobium Bobby Messina orchid. Aust J Crop Sci 5:1557–1564
Aros D, Vásquez M, Rivas C, Loreto M (2017) An efficient method for in vitro propagation of Alstroemeria pallida Graham rhizomes. Chil J Agri Res 77:95–99
Benson EE (2008) Cryopreservation theory. In: Reed BM (ed) Plant cryopreservation: a practical guide. Springer, New York, pp 15–32
Benson EE, Harding K (2012) Cryopreservation of shoot tips and meristems: an overview of contemporary methodologies. In: Loyola-Vargas VM, Ochoa-Alejo N (eds) Plant cell culture protocols, methods in molecular biology. Humana Press, Totowa, pp 191–226
Bettoni JC, Souza JA, Volk GM, Dalla Costa M, da Silva FN, Kretzschmar AA (2019a) Eradication of latent viruses from apple cultivar ‘Monalisa’shoot tips using droplet-vitrification cryotherapy. Sci Hortic 250:12–18
Bettoni JC, Kretzschmar AA, Bonnart R, Shepherd A, Volk GM (2019b) Cryopreservation of 12 Vitis species using apical shoot tips derived from plants grown in vitro. HortScience 54:976–981
Bettoni JC, Bonnart R, Shepherd AN, Kretzschmar AA, Volk GM (2019c) Modifications to a Vitis shoot tip cryopreservation procedure: effect of shoot tip size and use of cryoplates. Cryoletters 40:103–112
Beyl CA (2000) Getting started with tissue culture—media preparation, sterile technique, and laboratory equipment. In: Trigiano RN, Gray DJ (eds) Plant tissue culture concepts and laboratory exercises, 2nd edn. CRC Press, Boca Raton, pp 21–38
Bonga JM, Klimaszewska KK, Von Aderkas P (2010) Recalcitrance in clonal propagation, in particular of conifers. Plant Cell Tiss Org Cult 100:241–254
Castro TC, Pelliccione VLB, Figueiredo MR, Soares RODA, Bozza MT, Viana VRC, Albarello N, Figueiredo SLF (2002) Atividade antineoplásica e tripanocida de Hovenia dulcis Thunb. cultivada in vivo e in vitro. Braz J Pharmacog 12:96–99
Castro TC, Barbosa KC, Albarello N, Figueiredo SFL (2005) Characterization of pseudofruits, fruits, seeds and plantules obtained from in vivo and in vitro germination of Hovenia dulcis (Rhamnaceae) medicinal species. Rev Cubana Plant Med 10:1–17
Castro TC, Simões-Gurgel C, Ribeiro IG, Coelho MGP, Albarello N (2014) Morphological aspects of fruits, seeds, seedlings and in vivo and in vitro germination of species of the genus Cleome. J Seed Sci 36:326–335
Charoensub R, Phansiri S, Yongmanitchai W, Sakai A (2003) Routine cryopreservation of in vitro-grown axillary apices of cassava (Manihot esculenta Crantz) by vitrification: importance of a simple mononodal culture. Sci Hortic 98:485–492
Condello E, Caboni E, Andrè E, Piette B, Druart P, Swennen R, Panis B (2011) Cryopreservation of apple in vitro axillary buds using droplet-vitrification. CryoLetters 32:175–185
Cordeiro LS, Simões-Gurgel C, Albarello N, Engelmann F (2015) Cryopreservation of in vitro-grown shoot tips of Cleome rosea Vahl (Cleomaceae) using the V Cryo-Plate technique. In Vitro Cell Dev Biol Plant 51:688–695
Cordeiro LS, Simões-Gurgel C, Albarello N, Engelmann F (2017) Cleomaceae (Cleome rosea Vahl ex DC.), shoot tips, V-cryo-plate method. In: Niino T, Matsumoto T, Yamamoto S-I, Maki S, Tanaka D, Engelmann F (eds) Manual of cryopreservation methods using cryo-plate, 1st edn, vol 1. Plant Tissue Culture and Cryopreservation Group (PTTCCryoG), Jalisco, pp 62–63
Corredoira E, Martínez MT, Sanjosé MC, Ballester A (2017) Conservation of hardwood forest species. In: Ahuja MR, Jain SM (eds) Biodiversity and conservation of woody plants, sustainable development and biodiversity, 1st edn. Springer, Cham, pp 421–453
Dar RA, Shahnawaz M, Qazi PH (2017) Natural product medicines: a literature update. J Phytopharmacol 6:340–342
Dias DA, Urban S, Roessner U (2012) A historical overview of natural products in drug discovery. Metabolites 2:303–336
Dumet D, Engelmann F, Chabrillange N, Dussert S, Duval Y (1994) Effect of various sugars and polyols on the tolerance to desiccation and freezing of oil palm polyembryonic cultures. Seed Sci Res 4:307–313
Echeverrigaray S, Mossi AJ, Munari F (1998) Micropropagation of raisin tree (Hovenia dulcis Thunb.) through axillary bud culture. J Plant Biochem Biot 7:99–102
Engelmann F (2011a) Cryopreservation of embryos: an overview. In: Thorpe TA, Yeung EC (eds) Plant embryo culture. Methods in molecular biology (methods and protocols), vol 710. Humana Press, Totowa, pp 155–184
Engelmann F (2011b) Use of biotechnologies for the conservation of plant biodiversity. In Vitro Cell Dev Biol Plant 47:5–16
Engelmann F (2013) Cryopreservation of clonal crops: a review of key parameters. II Int Symp Plant Cryopreserv 1039:31–39
Espinosa-Leal CA, Puente-Garza CA, García-Lara S (2018) In vitro plant tissue culture: means for production of biological active compounds. Planta 248:1–18
Folgado R, Panis B, Sergeant K, Renaut J, Swennen R, Hausman JF (2015) Unravelling the effect of sucrose and cold pretreatment on cryopreservation of potato through sugar analysis and proteomics. Cryobiology 71:432–441
Ford CS, Jones NB, Van Staden J (2000) Optimization of a working cryopreservation protocol for Pinus patula embryogenic tissue. In Vitro Cell Dev Biol Plant 36:366–369
Gadelha APR, Vidal F, Castro TC, Lopes CS, Albarello N, Coelho MGP, Figueiredo SFL, Monteiro-Leal LH (2005) In vitro susceptibility of Giardia lamblia to Hovenia dulcis extracts. Parasitol Res 97:399–407
George EF, Hall MA, De Klerk G-J (2008) Plant propagation by tissue culture. Springer, Dordrecht, 501p
González-Arnao MT, Dolce N, González-Benito ME, Castillo Martínez CR, Cruz-Cruz CA (2017) Approaches for in vitro conservation of woody plants germplasm. In: Ahuja M, Jain S (eds) Biodiversity and conservation of woody plants. Sustainable development and biodiversity, vol 17. Springer, Cham, pp 355–419
Goyal Y, Arya HC (1985) Tissue culture of desert trees: II. Clonal multiplication of Zizyphus in vitro. J Plant Physiol 119:399–404
Groover A (2017) Age-related changes in tree growth and physiology. eLS. Wiley, Chichester, pp 1–7
Guidini CC, Pinto-Maglio CAF, Lombello RA (2017) Karyotype, rDNA localization and meiotic behavior of Hovenia dulcis Thunb. (Rhamnaceae). Caryologia 70:385–389
Halmagyi A, Deliu C, Coste A (2005) Plant regrowth from potato shoot tips cryopreserved by a combined vitrification-droplet method. CryoLetters 26:313–322
Hendges CD, Fortes VB, Dechoum MS (2012) Consumption of the invasive alien species Hovenia dulcis Thunb. (Rhamnaceae) by Sapajus nigritus Kerr, 1792 in a protected area in southern Brazil. Rev Bras Zoociências 14:255–260
Ibrahim S, Normah MN (2013) The survival of in vitro shoot tips of Garcinia mangostana L. after cryopreservation by vitrification. Plant Growth Regul 70:237–246
Jeong MJ, Song HJ, Park DJ, Min JY, Jo JS, Kim BM, Choi MS (2009) High frequency plant regeneration following abnormal shoot organogenesis in the medicinal tree Hovenia dulcis. Plant Cell Tiss Org Cult 98:59–65
Kaczmarczyk A, Rokka VM, Keller EJ (2011) Potato shoot tip cryopreservation. A review. Potato Res 54:45–79
Kulus D (2018) Effects of various preculture, pretreatment, and recovery conditions on the morphogenetic response of cryopreserved Lady Orange chrysanthemum shoot tips. Turk J Biol 42:76–86
Kulus D (2020) Shoot tip cryopreservation of Lamprocapnos spectabilis (L.) Fukuhara using different approaches and evaluation of stability on the molecular, biochemical, and plant architecture levels. Int J Mol Sci 21:3901
Kulus D, Zalewska M (2014) Cryopreservation as a tool used in long-term storage of ornamental species—a review. Sci Hortic 168:88–107
Kulus D, Serocka M, Mikuła A (2018) Effect of various preculture and osmotic dehydration conditions on cryopreservation efficiency and morphogenetic response of chrysanthemum shoot tips. Acta Sci Pol Hort Cult 17:139–147
Kulus D, Muhire JD, Aksoy B (2020) Growth regulation and validation of homogeneity in in vitro-derived bleeding heart by molecular markers and spectral analysis of pigments. J Plant Growth Reg. https://doi.org/10.1007/s00344-020-10204-2
Lambardi M, Shaarawi S (2016) Importance of in vitro culture for developing cryopreservation strategies of woody plants. Acta Hortic 1187:177–188
Lee Y-G, Popova E, Cui H-Y, Kim H-H, Park S-U, Bae C-H, Lee S-C, Engelmann L (2011) Improved cryopreservation of Chrysanthemum (Chrysanthemum morifolium) using droplet-vitrification. CryoLetters 32:487–497
Li G, Min BS, Zheng C, Lee J, Oh SR, Ahn KS, Lee HK (2005) Neuroprotective and free radical scavenging activities of phenolic compounds from Hovenia dulcis. Arch Pharm Res 28:804–809
Li J-W, Ozudogru EA, Li J, Wang M-R, Bi W-L, Lambardi M, Wang Q-C (2018) Cryobiotechnology of forest trees: recent advances and future prospects. Biodivers Conserv 27:795–814
Lim TK (2013) Hovenia dulcis. In: Lim TK (ed) Edible medicinal and non-medicinal plants: v. 5, fruits. Springer, Dordrecht, pp 568–577
Lynch PT, Siddika A, Mehra A, Benelli C, Lambardi M (2014) Cryopreservation of quince (Cydonia oblonga Mill.). CryoLetters 35:188–196
Mandal BB, Dixit-Sharma S (2007) Cryopreservation of in vitro shoot tips of Dioscorea deltoidea Wall., an endangered medicinal plant: effect of cryogenic procedure and storage duration. CryoLetters 28:461–470
Manole-Paunescu A (2014) Biotechnology for endangered plant conservation. In: Ahuja MR, Ramawat KG (eds) Biotechnology and biodiversity, sustainable development and biodiversity, 4th edn. Springer, Cham, pp 181–202
Marco-Medina A, Casas JL, Swennen R, Panis B (2010) Cryopreservation of Thymus moroderi by droplet vitrification. CryoLetters 31:14–23
Marković Z, Chatelet P, Preiner D, Sylvestre I, Kontić JK, Engelmann F (2014) Effect of shooting medium and source of material on grapevine (Vitis vinifera L.) shoot tip recovery after cryopreservation. CryoLetters 35:40–47
Maślanka M, Panis B, Bach A (2013) Cryopreservation of Galanthus elwesii Hook. apical meristems by droplet vitrification. CryoLetters 34:1–9
Mathew L, McLachlan A, Jibran R, Burritt DJ, Pathirana R (2018) Cold, antioxidant and osmotic pre-treatments maintain the structural integrity of meristematic cells and improve plant regeneration in cryopreserved kiwifruit shoot tips. Protoplasma 255:1065–1077
Mathur N, Ramawat KG, Nandwani D (1995) Rapid in vitro multiplication of jujube through mature stem explants. Plant Cell Tiss Org Cult 43:75–77
Matsumoto T, Yamamoto S, Fukui K, Rafique T, Engelmann F, Niino T (2015) Cryopreservation of Persimmon shoot tips from dormant buds using the D cryo-plate technique. Hort J 84:106–110
Menon A, Funnekotter B, Kaczmarczyk A, Bunn E, Turner S, Mancera RL (2012) Cryopreservation of Lomandra sonderi (Asparagaceae) shoot tips using droplet-vitrification. CryoLetters 33:259–270
Morales P, Maieves HP, Dias MI, Calhella RC, Sánchez-Mata MC, Santos-Buelga C, Barros L, Ferreira ICFR (2017) Hovenia dulcis Thunb. pseudofruits as functional foods: phytochemicals and bioactive properties in different maturity stages. J Funct Foods 29:37–45
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Niino T, Watanabe K, Nohara N, Rafique T, Yamamoto SI, Fukui K, Engelmann F (2014) Cryopreservation of mat rush lateral buds by air dehydration using aluminium cryo-plate. Plant Biotechnol 31:281–287
Niino T, Yamamoto S, Matsumoto T, Engelmann F, Arizaga MV, Tanaka D (2019) Development of V and D cryo-plate methods as effective protocols for cryobanking. Acta Hortic 1234:249–262
Nishizawa S, Sakai A, Amano Y, Matsuzawa T (1993) Cryopreservation of asparagus (Asparagus officinalis L.) embryogenic suspension cells and subsequent plant regeneration by vitrification. Plant Sci 91:67–73
Normah MN, Sulong N, Reed BM (2019) Cryopreservation of shoot tips of recalcitrant and tropical species: advances and strategies. Cryobiology 87:1–14
O’Brien C, Hiti-Bandaralage J, Folgado R, Lahmeyer S, Hayward A, Folsom J, Mitter N (2020) A method to increase regrowth of vitrified shoot tips of avocado (Persea americana Mill.): first critical step in developing a cryopreservation protocol. Sci Hortic 266:109–305
Panis B (2019) Sixty years of plant cryopreservation: from freezing hardy mulberry twigs to establishing reference crop collections for future generations. Acta Hortic 1234:1–8
Panta A, Panis B, Ynouye C, Swennew R, Roca W (2014) Development of a PVS2 droplet vitrification method for potato cryopreservation. CryoLetters 35:255–266
Park D-J, Kang Y-M, Jung H-N, Min J-Y, Kim Y-D, Karigar CS, Choi M-S (2006) Rapid micropropagation of Hovenia ducis Thunb. through in vitro stem nodal cultures. J Korean For Soc 95:155–159
Park K, Yoon HJ, Imm JY, Go GW (2019) Hovenia dulcis extract attenuates high-fat diet-induced hepatic lipid accumulation and hypertriglyceridemia in C57BL/6 mice. J Med Food 22:74–80
Pathirana R, McLachlan A, Hedderley D, Panis B, Carimi F (2016) Pre-treatment with salicylic acid improves plant regeneration after cryopreservation of grapevine (Vitis spp.) by droplet-vitrification. Acta Phsiol Plant 38:12
Pereira GA, Santaella MB, Alves LMSM, Silva EC, Flenga AIS, Santos DMA (2018) Concentrations of 6-benzylaminopurine (BAP) in micropropagation of banana ‘Farta Velhaco’(AAB). Comun Sci 9:58–63
Pettinelli JA, Soares BO, Cantelmo L, Garcia RO, Mansur E, Engelmann F, Gagliardi RF (2017) Cryopreservation of somatic embryos from Petiveria alliacea L. by different techniques based on vitrification. In Vitro Cell Dev Biol Plant 53:339–345
Pettinelli JA, Soares BO, Collin M, Mansur EA, Engelmann F, Gagliardi RF (2020) Cryotolerance of somatic embryos of guinea (Petiveria alliacea) to V-cryoplate technique and histological analysis of their structural integrity. Acta Physiol Plant 42:1–10
Prudente DO, Paiva R, Paiva PDO, Silva LC (2016) Cryopreservation of shoot tips excised from zygotic embryos of Araucaria angustifolia Kuntze. XXIX Int Hortic Congr Hortic Sustain Lives Livelihoods Landsc 1113:257–264
Rantala S, Kaseva J, Karhu S, Veteläinen M, Uosukainen M, Häggman H (2019) Cryopreservation of Ribes nigrum (L.) dormant buds: recovery via in vitro culture to the field. Plant Cell Tiss Org Cult 138:109–119
Rathore TS, Singh RP, Deora NS, Shekhwat NS (1992) Clonal propagation of Zyzhiphus species through tissue culture. Sci Hortic 51:165–168
Read PE, Bavougian CM (2012) In vitro rejuvenation of woody species. In: Lambardi M, Ozudogru EA, Jain SM (eds) Protocols for micropropagation of selected economically-important horticultural plant. Humana Press, Totowa, pp 383–395
Reed BM (2008) Plant cryopreservation: a practical guide. Springer, New York
Ribeiro IG, Gayer CRM, Castro TC, Coelho MGP, Albarello N (2015) Compact callus cultures and evaluation of the antioxidant activity of Hovenia dulcis Thunb. (Rhamnaceae) under in vivo and in vitro culture conditions. J Med Plant Res 9:8–15
Rojas-Martínez L, Visser RG, De Klerk GJ (2010) The hyperhydricity syndrome: waterlogging of plant tissues as a major cause. Propag Ornam Plants 10:169–175
Sakai A, Kobayashi S, Oiyama I (1990) Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Rep 9:30–33
Sant R, Panis B, Taylor M, Tyagi A (2008) Cryopreservation of shoot-tips by droplet vitrification applicable to all taro (Colocasia esculenta var. esculenta) accessions. Plant Cell Tiss Org Cult 92:107–111
Schaller EG, Bishopp A, Kieberc JJ (2015) The yin-yang of hormones: cytokinin and auxin interactions in plant development. Plant Cell 27:44–63
Schmidt AD, Castellani TT, Dechoum MS (2020) Biotic and abiotic changes in subtropical seasonal deciduous forest associated with invasion by Hovenia dulcis Thunb. (Rhamnaceae). Biol Invasions 22:293–306
Serafim CM, Campos AS, Melo PDS, Castro ACR, Carvalho A (2018) Types and concentrations of cytokinins in the micropropagation of Anthurium maricense. Braz J Agrarian Environ Sci 12:117–123
Sulong N, Shahabudin NF, Noor NM (2018) Critical vitrification steps towards survival of Garcinia hombroniana (Clusiaceae) shoot tips after cryopreservation. Rev Biol Trop 66:1314–1323
Tanaka D, Yamamoto S, Matsumoto T, Arizaga MV, Niino T (2019) Development of effective cryopreservation protocols using aluminium cryo-plates for mulberry. Acta Hortic 1234:263–268
Tsai SF, Yeh SD, Chan CF, Liaw SI (2009) High-efficiency vitrification protocols for cryopreservation of in vitro grown shoot tips of transgenic papaya lines. Plant Cell Tiss Org Cult 98:157–164
Uchendu EE, Leonard SW, Traber MG, Reed BM (2010) Vitamins C and E improve regrowth and reduce lipid peroxidation of blackberry shoot tips following cryopreservation. Plant Cell Rep 29:25–35
Vianna MG, Garcia RO, Mansur E, Engelmann F, Pacheco G (2019) Oxidative stress during the cryopreservation of Passiflora suberosa L. shoot tips using the V-Cryo-plate technique: determination of the critical stages of the protocol. Plant Cell Tiss Org Cult 139:369–379
Vicente S, Nieto AB, Hodara K, Castro MA, Alzamora SM (2012) Changes in structure, rheology, and water mobility of apple tissue induced by osmotic dehydration with glucose or trehalose. Food Bioprocess Tech 5:3075–3089
Vilardo AFRM, Mendonça TF, Engelmann F, Cordeiro LS, Albarello N, Simões-Gurgel C (2019) Cryopreservation of in vitro-grown shoot tips of the medicinal species Cleome spinosa (Cleomaceae) applying vitrification-based techniques. CryoLetters 40:237–246
Volk GM, Jenderek M, Chen K (2020) Cryopreservation of dormant apple buds. In: Volk GM (Eds.) Training in plant genetic resources: cryopreservation of clonal propagules. Fort Collins, Colorado: Colorado State University. https://colostate.pressbooks.pub/clonalcryopreservation/chapter/apple-dormant-bud-cryopreservation/. Accessed 26 July 2020
Vollmer R, Villagaray R, Castro M, Anglin NL, Ellis D (2019) Cryopreserved potato shoot tips showed genotype-specific response to sucrose concentration in rewarming solution (RS). Plant Cell Tiss Org Cult 136:353–363
Vujović T, Chatelet P, Ružić D, Engelmann F (2015) Cryopreservation of Prunus spp. using aluminium cryo-plates. Sci Hortic 195:173–182
Wang Q, Li P, Batuman Ö, Gafny R, Mawassi M (2003) Effect of benzyladenine on recovery of cryopreserved shoot tips of grapevine and citrus cultured in vitro. CryoLetters 24:293–302
Wang M, Zhu P, Jiang C, Ma L, Zhang Z, Zeng X (2012) Preliminary characterization, antioxidant activity in vitro and hepatoprotective effect on acute alcohol-induced liver injury in mice of polysaccharides from the peduncles of Hovenia dulcis. Food Chem Toxicol 50:2964–2970
Wang B, Li J-W, Zhang Z-B, Wang R-R, Ma Y-L, Blystad D-R, Keller EJ, Wang Q-C (2014a) Three vitrification-based cryopreservation procedures cause different cryo-injuries to potato shoot tips while all maintain genetic integrity in regenerants. J Biotechnol 184:47–55
Wang R-R, Gao X-X, Chen L, Huo L-Q, Lib M-F, Wang Q-C (2014b) Shoot recovery and genetic integrity of Chrysanthemum morifolium shoot tips following cryopreservation by droplet-vitrification. Sci Hortic 176:330–339
Wang RR, Mou HQ, Gao XX, Chen L, Li MF, Wang QC (2015) Cryopreservation for eradication of Jujube witches’ broom phytoplasma from Chinese jujube (Ziziphus jujuba). Ann Appl Biol 166:218–228
Wang M-R, Hamborg Z, Slimestad R, Elameen A, Blystad DR, Haugslien S, Wang Q-C (2020a) Assessments of rooting, vegetative growth, bulb production, genetic integrity and biochemical compounds in cryopreserved plants of shallot. Plant Cell Tiss Organ Cult. https://doi.org/10.1007/s11240-020-01820-7
Wang M-R, Lambardi M, Engelmann F, Pathirana R, Panis B, Volk GM, Wang Q-C (2020b) Advances in cryopreservation of in vitro-derived propagules: technologies and explant sources. Plant Cell Tiss Organ Cult. https://doi.org/10.1007/s11240-020-01770-0
Yamamoto SI, Rafique T, Priyantha WS, Fukui K, Matsumoto T, Niino T (2011) Development of a cryopreservation procedure using aluminium Cryo-Plates. CryoLetters 32:256–265
Yamamoto S-i, Fukui K, Rafique T, Khan NI, Castillo Martinez CR, Sekizawa K, Matsumoto T, Niino T (2012) Cryopreservation of in vitro-grown shoot tips of strawberry by the vitrification method using aluminium cryo-plates. Plant Gen Res 10:14–19
Yang J, Wu S, Li C (2013) High efficiency secondary somatic embryogenesis in Hovenia dulcis Thunb. through solid and liquid cultures. Sci World J 2013:718754
Yuniastuti E, Wardani NC, Nandariyah (2016) The effect of explant type and 6-benzyl adenine (BAP) in sapodilla (Achras zapota) micropropagation. Am J Biochem Biotechnol 12:206–213
Zayova E, Vassilevska-Ivanova R, Kraptchev B, Stoeva D (2010) Somaclonal variations through indirect organogenesis in eggplant (Solanum melongena L.). Biol Divers Conserv 3:1–5
Acknowledgements
This study was supported by the Brazilian Federal Agency for Support and Evaluation of Graduate Education/CAPES (Finance Code 001), the Brazilian Council for Scientific and Technological Development/CNPq and The Carlos Chagas Filho Research Support Foundation/FAPERJ. The authors are grateful to Adriana M. Lanziotti (TCT/FAPERJ) for lab assistance and illustrations of methodology and to Márcio M. Silva for valuable help with graphic design. Special thanks to Dr. Takao Niino (University of Tsukuba, Japan) for the supply of cryo-plates and Dr. Florent Engelmann (Institut de Recherche pour le Développement, France) for introducing the V Cryo-plate methodology to our research group.
Funding
This research is grant aided by the Brazilian Council for Scientific and Technological Development/CNPq (Process 421,538/2016-3) and The Carlos Chagas Filho Research Support Foundation/FAPERJ (E-26/010.001631/2014 and E-26/010.001019/2016).
Author information
Authors and Affiliations
Contributions
AMS, TCC, LSC, NA, CSG conceived and planned the experiments. AMS, TCC, LSC, TA carried out the experiments. LSC, NA, CSG supervised the work. All authors contributed to the interpretation of the results, wrote, read and approved the manuscript.
Corresponding author
Additional information
Communicated by: Qiao-Chun Wang.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saavedra, A.M., de Castro, T.C., da Silva Cordeiro, L. et al. In vitro propagation and cryopreservation of the medicinal species Hovenia dulcis Thunb. (Rhamnaceae). Plant Cell Tiss Organ Cult 144, 577–591 (2021). https://doi.org/10.1007/s11240-020-01980-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-020-01980-6