Abstract
Precision biotechnologies have appeared on the horizon resulting in a plethora of possibilities to modify the genome of different organisms with relatively easy application, low cost, and high precision. These technologies make it possible to work with a very simple biological system and have great potential for medicine, and agriculture. Latin American is embracing the technology and researchers are already developing tropical products from its use. The following article explains the operation of these technologies, and some considerations about its regulation among counties in Latin America and the Caribbean region. Survey results demonstrated that seven countries (Argentina, Brazil, Colombia, Chile, Guatemala, Honduras, and Paraguay) have a clearly defined and operational legal framework for new breeding technologies. Nevertheless, the majority of countries in the region have no experience regarding these technologies and lack legal clarity. Therefore, these countries require regulatory clarity to legally differentiate those products of gene editing that are comparable to conventional breeding and those that can be legally defined as a genetically modified organism.
Key message
New precision biotechnologies could introduce advantageous traits for the improvement of crops, which could be available for the consumers in Latin America and the Caribbean region very soon. Nevertheless, governments should consider the regulatory framework of genome editing technologies and establish appropriate regulations, if necessary, without representing an obstacle to the commercialization of products derived from them.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Modern biotechnology is defined in the Article 3(i) of The Cartagena Protocol on Biosafety to the Convention on Biological Diversity (CBD) as “the application of in vitro nucleic acid techniques (including recombinant deoxyribonucleic acid (DNA) and direct injection of nucleic acid into cells or organelles), or the fusion of cells beyond the taxonomic family, that overcome natural physiological reproductive or recombination barriers and that are not techniques used in traditional breeding and selection” (The Cartagena Protocol; https://bch.cbd.int/protocol/). The continuous understanding of plant biology and development, genetic diversity, and advances in omics technologies provides new opportunities for the development of plant varieties (Seyran and Craig 2018). In this sense, modern biotechnology could contribute to solving limitations in diverse fields and to facing some challenges of climate change and food security (de la León et al. 2018; Seyran and Craig 2018). In agriculture, traditional breeding complemented with pest and disease management, and genetic engineering could offer an opportunity to increase production and nutritional quality of crops, thereby ensuring the supply of food for a growing population, projected to reach 10 billion people by 2050 (Roca et al. 2004; Izquierdo and de la Riva 2000; Seyran and Craig 2018).
The Latin America and Caribbean region (LAC) is an important center of origin and diversity for a number of organisms contributing to food security (Roca et al. 2004). Nevertheless, many countries of the LAC region are characterized by poverty, food insecurity, and dependence on food imports (Izquierdo and de la Riva 2000). Therefore, innovation and research in the agri-food systems, comprising the application of plant biotechnology, are fundamental for agricultural production, economic and social growth and for contributing towards addressing these problems. In this regard, modern biotechnology offers an alternative to many farmers in an attempt to produce more food per unit of land with fewer inputs, as well as for enabling the cultivation of areas currently not suitable for agriculture, thereby preserving the biodiversity and natural habitats (Cockcroft et al. 2004). In Latin America, investment in agricultural biotechnology for the adoption of crop improvement using genetic modification has been made by some countries (ISAAA 2017). In this sense, in 2017 ten countries in Latin America (Brazil, Argentina, Paraguay, Uruguay, Bolivia, Mexico, Colombia, Honduras, Chile, and Costa Rica) planted 79.4 million hectares of GMOs representing 42% of the worldwide area cultivated with GMO crops (ISAAA 2017).
New precision biotechnologies (grouped here under the name of new plant breeding technologies; NPBT) provide new alternatives for the improvement of crop performance, nutritional quality, and biotic and abiotic resistance (Cao et al. 2016; Lassoued et al. 2018a). Genome editing consists of producing directed, permanent, and inheritable mutations in a specific place of the genome, mediated by DNA repair systems in the cell, with the lowest probability of committing unwanted errors (off-targets) and leaving no foreign DNA sequences. New breeding technologies (NPBT) such as zinc finger nucleases (ZFN), transcriptional activator-like effector nucleases (TALEN), and clustered regularly interspaced short palindromic repeats associated with the Cas9 endonuclease (CRISPR/Cas9), oligo-directed mutagenesis (ODM), cisgenesis, RNA-directed DNA Methylation (RdDM), grafting, reverse breeding, and agro-infiltration have been used to induce specific mutations in the genome, or introduce beneficial traits, or express transgenes in a specific tissue in a wide range of crops and model plants (Table 1) (Miglani 2017; Seyran and Craig 2018).
New precision biotechnologies could introduce advantageous traits for the improvement of crops that could be accessible rapidly for the production and commercialization. Nevertheless, from the point of view of risk assessment for environmental and food and feed safety, it is still required to clarify their regulatory status, mainly with regard to global regulations on genetically modified organisms (GMOs) (Kinderlerer 2008; McHughen and Smyth 2008). According to the Article 3(g) of the Cartagena Protocol, a GMO is “any living organism that possesses a novel combination of genetic material obtained through the use of modern biotechnology” (The Cartagena Protocol; https://bch.cbd.int/protocol/). In many countries, the use of GMOs is regulated (Rosado and Craig 2017). Nevertheless, with respect to NPBT, governments should consider their regulatory status and establish, if necessary, suitable risk and safety assessment, without representing an obstacle to the commercialization of products derived from NPBTs (Seyran and Craig 2018). However, important in this context is to determine if products derived from NPBT represent new risks compare to products of traditional plant breeding or GMOs. If risk and safety assessment is needed, a stepwise case-by-case problem formulation analysis could guide the evaluation of products derived from NPBTs (Wolt 2019). Likewise, if consumers are educated about the benefits and possible risks of NPBTs, these improved crops could be progressively incorporated into the market. So far, there are some precedents for Latin American government decisions on whether a crop obtained by one or another genome-editing strategies is under the regulation usually applied to GMOs. Therefore, the purpose of this article is to describe the regulatory framework of genome editing in Latin America and the Caribbean region in order to determine the capabilities for the assessment of non-negligible negative effects for the environment and/or health of plants derived from NPBTs.
Materials and methods
Regulatory status of genome editing in Latin America and the Caribbean
In April 2018, the Inter-American Institute for Cooperation on Agriculture (IICA) organized an international workshop in genome editing, regulatory issues, communication and public acceptance at the International Center for Tropical Agriculture (CIAT) in Cali, Colombia. This workshop was designated to enable an exchange of information on how countries are approaching the regulation of products obtained using genome editing, enable innovation in support of rural incomes, minimize the risk of trade disruptions associated with the use of new production technologies, promote global food security, and advocate for science and risk-based international trade policies. Government officials from the National Commissions of Biosafety from Central America (Costa Rica, Guatemala, and Honduras), North America (Canada, United States of America, and Mexico), South America (Argentina, Bolivia, Brazil, Chile, Colombia, Ecuador, Paraguay, Peru, Uruguay), and the Caribbean (Bahamas, Belize, Dominican Republic, and Trinidad and Tobago) participated in this workshop, as well as experts from the academic and industry world in the Americas. The questionnaire developed by Schuttelaar (2015) was translated into Spanish and used as a basis for the collection of information regarding the regulatory status of genome editing in the LAC region (Supplementary Table 1). In addition, we validated the data with the legal information available at the Biosafety Clearing House (https://bch.cbd.int/) and their legal official online sites.
Results
The multiple applications of the genome editing technologies also means that there could also be several ways to regulate them. In this article, we will mention general aspects for the regulation framework for non-human genetic material in LAC countries (Table 2). We clarify that what refers to regulatory aspects will be treated with a general approach, without detailing the procedures or specific procedures of each country. It is important to mention that Argentina, Australia, Brazil, Canada, Dominican Republic, Guatemala, Honduras, Paraguay, United States of America, and Uruguay, have presented an international declaration at the World Trade Organization (WTO)Footnote 1 to avoid subjective and unjustifiable distinctions between end products derived from NPBTs and similar end products obtained through other production methods. Moreover, Argentina on behalf of some South American countries (Argentina, Brazil, Chile, Paraguay, and Uruguay) presented to the WTO on March 15th 2019 a declarationFootnote 2 by the Ministers of Agriculture of the Southern Agriculture Council (CAS) on gene editing techniques in order to simplify the interchange of information on product development and existing regulatory frameworks with a scientific basis for regional and international regulatory harmonization, as well as, to work together and with third countries to avoid obstacles without scientific basis to the trade of improved agricultural products developed by genome editing.
Argentina
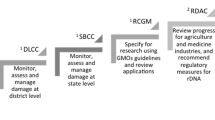
In 2015, the Ministry of Agroindustry (MAGyP) issued the ‘NBT Resolution’ 173/15 thus becoming the first country in the LAC region to have set a national legal framework for NPBT. Basically, this Resolution established the procedures to define whether a crop obtained using NPBT is or is not a GMO and therefore should be regulated under the current regulation for GMOs (Resolution No. 701/11 and 763/11). The definitions used in the Resolution 701/11, in particular the GMO definition, correspond to those of the Cartagena Protocol on Biosafety. In this sense, a GMO is “an organism that has a novel combination of genetic material obtained through the application of modern biotechnology”. In general terms, the analysis under the scope of the NBT Resolution 173/15 is concentrated on the interrogation whether a novel combination of genetic material has happened. To achieve the analysis, a new combination of genetic material is defined as “when one or more genes or DNA sequences of the genetic construct are inserted into the plant genome”. The National Advisory Commission on Agricultural Biotechnology is responsible for analyzing within 60 days, on a case-by-case basis the information supplied by the developer. The analysis is not restricted to a list of NPBTs and the applicants must submit information regarding the methodology used to breed the crop, the innovative trait introduced, evidence of the genetic changes present in the end product, evidence of elimination of the transitory transgene employed to achieve the product (if necessary), and any additional information that the Biotechnology Directorate and CONABIA consider necessary. The general process for determining whether a product derived from new breeding technologies should be considered as GMO or not is shown in the Fig. 1. An interesting feature to be highlighted in this Resolution is that developers can consult the CONABIA about the status of a putative product that is still is in the design phase. According to the Argentinian Resolution all products derived from NPBTs that resort to modern biotechnology must be submitted to prior consultation, or otherwise are considered a GMO. Since 2015, the Ministry of Agroindustry has received 18 applications from public institutions, public and private PYMEs (small and medium-sized enterprises), and multinational companies for the analysis of plants and animals products resulting from any of the NPBTs. The specific cases remains confidential. Researchers from INTA (Institute of Agricultural Technology of Argentina) used CRISPR/Cas9 to develop potatoes that do not turn brown and milk that does not affect allergic consumers (Plaza 2018, González et al. 2019b) and to increase alfalfa productivity and quality (Soto 2019). Moreover, Argentinian researchers from Kheiron Biotech, together with FLENI scientists, used CRISPR gene editing technology to improve polo horses (Argentina.gob.ar 2018).

(Adapted from Eriksson et al. 2019)
General procedure for determining if a product derived from new breeding technologies should be consider as GMO in Argentina. Briefly, if there is a new combination of genetic material in the genome, the product is considered a GMO. On the other hand, if there is not a new combination of genetic material but the development of the NBT product uses a transgene temporally, and the final product is not free of transgene, it is also considered GMO. Contrary, if the product does not contain a new combination of genetic material in the genome, the product does not fall under the GMO Resolution.
Belize
According to the participant from the National Biosafety Committee, the country has a moratorium that prevents the liberation into the environment of GMOs for cultivation or breeding purposes but not for research. At the time of the workshop, there were no research initiatives or any applications for the commercialization of crops/products derived from any of the NPBTs.
Brazil
The Brazilian National Biosafety Technical Commission (CTNBio) is by law (No. 11.105/2005) the responsible for evaluating the biosafety impact for the environment and human/animal health of new technologies. In this sense, on 15th January 2018 the Normative Resolution No. 16 (NR 16) was published in order to evaluate on a case-by-case analysis whether or not a product developed using NPBTs will be consider as a GMO or not under the scope of the Law No. 11.105/2005 and the Normative Resolution No. 5/2008. Unlike the legislation in Argentina and Chile, the NR 16 contains as an Annex a non-exhaustive list that determines which techniques may lead to a product not being classified as a GMO. These techniques are: early flowering, seed production technologies, reverse breeding, RNA-dependent DNA methylation, site-directed mutagenesis (SDN), oligonucleotides directed mutagenesis (ODM), agroinfiltration/ agroinfection, topical or systemic use of RNAi, and viral vectors. The CTNBio has simplified the risk assessment evaluation through a previous consultation mechanism and the applicant must submit a request including the following information: NPBTs used, the molecular map of the constructs employed, the gen(s) manipulated and it(s) function, the purpose uses of the end product obtained, molecular data of parental and progeny showing absence of recombinant DNA in the progeny, the product has been approved for commercialization in other countries, evidence of unintentional effects (off-target) in the end product. The CTNBio has analyzed seven applications of crops/products resulting from one of the NPBTs. These include Saccharomyces cerevisiae for ethanol and bioethanol production, waxy maize (Zea mays), hornless cow, tilapia with higher fillet yield, and breeding of bulls for bovine semen (Ambrozevicius 2018).
Chile
The procedures for import, domestic propagation, and re-export of propagated GMO plant material in the country were established through the Exent Resolution 1523/2001. In this sense, the regulatory framework allows GMO seed production exclusively for export, and research and development activities; nevertheless, the permanence and commercialization of those seeds are not allowed. Under this Resolution, a GMO is defined as “a living biological organism, capable of transferring or replicating genetic material, including the sterile organism, viruses, and viroids that possesses a new combination of genetic material that has been obtained through the application of modern biotechnology”. Discussions about NPBTs began in 2016 and as a result in July 2017 a general procedure for case-by-case analysis and based on the end product was published. In general terms, the analysis is focused on the question whether a “new combination” of genetic material has occurred. This is defined as “a stable insertion of one or more genes or DNA sequences that encode proteins, interfering RNA, double-stranded RNA, signaling peptides or regulatory sequences”. This is the key criterion that determines whether or not an organism will be considered as a GMO and therefore the Resolution 1523/2001 should be apply or nor to the new materials derived from NPBTs. The Forestry and Agricultural Protection Division of the Agricultural and Livestock Service (SAG) is responsible for analyzing in 20 days the information supplied by the developer through a consultation form. For this, the applicant must provide the comprehensive documentation including the name of the species, variety/line, description of the phenotype, developer information, the methodology and characteristics of the technique employed, and modified DNA target sequences. The techniques employed to create the “new combination” of genetic material should be provided. Finally, the applicant must indicate, whether the propagation material has been authorized by another country providing all available written information regarding the type of authorization. The decision of SAG is valid for an indefinite period, but can be canceled if new scientific findings are available. SAG has analyzed three applications of crops/products resulting from one of the NPBTs. These include annual crops, such as Camelina sativa, Glycine max, and Zea mays, and the main traits are low content of linoleic acid, high content of oleic acid, and tolerance to water stress and performance improvement, respectively (Pardo-Hernández 2018). Chilean researchers from Favet-Inbiogen are developing an efficient genome editing method through CRISPR to confer resistance against diseases in Chilean and Norwegian salmonids (Feest 2017).
Colombia
The technical control of the production and commercialization of agricultural products, animal genetic material and seeds for cultivation, in order to avoid risks that may affect agricultural health, food safety, and agricultural production in the country is under responsibility of the Colombian Agricultural Institute (ICA). In this sense, through Resolution 0946 issued on April 17th 2006, ICA has established the internal procedure for CTNBio to process the applications of GMOs for agricultural, livestock, fishing, commercial forest plantations and agro-industry purposes. Since the innovation dynamics in plant breeding allows one to obtain heterogeneous products, a previous technical analysis is necessary in order to determine if the regulation of GMOs (Decreto 4525 issued on December 6th 2005) or should not be applied. In 2017, regulators at ICA began consultations with researchers from CIAT and EAFIT University to learn about new breeding technologies. As a result, the Resolution No. 29299 was made official on August 1st 2018. The objective of this resolution was to establish a procedure for processing applications of an improved cultivar developed with techniques of innovation in plant breeding through modern biotechnology, in order to determine if the cultivar corresponds to a Genetically Modified Organisms or a conventional one. The decision as to whether a variety (end product) is classified as GMO or not is based on whether it contains foreign genetic material. According to Article 3 paragraph 4, foreign genetic material is defined as “a gene, set of genes, or DNA sequences that are part of a defined genetic construction introduced into the genome of an individual in a stable manner, through modern biotechnology techniques, overcoming the natural physiological barriers of reproduction”. In this sense, if a crop does not contain foreign DNA sequences, it will not be treated as a GMO. For this, the applicant must provide to the competent authority comprehensive documentation including the taxonomic classification of the specie, the breeding methodology employed for obtaining it, the genetic map(s) of the DNA construct(s) used in the breeding process, and in the case of DNA free editing, the protein and RNA sequences used. One must also provide a description of the achieved phenotype and its use, as well as molecular characterization showing the genetic changes present in the improved cultivar compared to the initial material and the demonstrating the absence of foreign genetic material. Once the application has been submitted, ICA will have a maximum term of 30 working days for determining if the new cultivar is considered a GMO and therefore whether or not it is within the scope of Regulation 4525. Since the publication of Resolution No. 29299, none applications for the analysis of crops/products derived from any of the NPBTs have been received at ICA. Colombian scientists from the CIAT are using genome editing with CRISPR to develop cassava (for the production of waxy starchy plants and for conferring resistance against Xanthomonas axonopodis pv. Manihotis), cacao (to create varieties with reduced cadmium absorption), and rice (to confer resistant to rice hoja blanca virus (RHB), to develop plants with pollen sterility, to increase the concentration of amylopectin in the grain, to increase the number of panicles and the number of grains per panicle, and to confer resistance against Xanthomonas oryzae) (Sánchez et al. 2019; Sierra-Robles et al. 2019; Valdés et al. 2019).
Costa Rica
At the moment of the workshop, only the University of Costa Rica (Dr. Andrés Gatica-Arias, CIBCM) was carrying out a study to analyze whether the alteration of the active site of trehalase enzyme by genome editing, specifically CRISPR/Cas9, influences tolerance/ resistance to salinity in rice, a crop of economic, social and nutritional importance for Latin American countries (Barrantes 2017). At the time of the workshop, the regulatory status of this product was not yet a topic of discussion and the country has no specific regulation for NPBTs and applications for the commercialization of crops/products derived from any of the NPBTs have not been received.
Dominican Republic
According to the participant from the Dominican Institute for Innovation in Biotechnology and Industry, the country has no specific regulation for new breeding technologies, although a National Biosafety Committee has been established and the introduction, research, testing, development, handling, transport, transit, storage, production, marketing, import, export, use and release into the environment, disposal of GMOs and their derivatives is regulated by Law 219-15. Moreover, at the time of the workshop, there were no research initiatives or any applications for the commercialization of crops/products derived from any of the NPBTs.
Ecuador
The Cartagena Protocol on Biosafety of the Convention on Biological Diversity (CBD) was ratified and published in Official Gazette No. 145 on August 12th 2003. Furthermore, and among other regulations, Article 401 of The Constitution of Ecuador from 2018 states that the country is declared free of transgenic crops and seeds. Nevertheless, seeds and genetically modified crops may be introduced only in those cases of national interest duly supported by the Presidency of the Republic and approved by the National Assembly. Moreover, the use and development, experimentation, and commercialization of modern biotechnology and its products will be strict regulated by the State. The application of risky or experimental biotechnologies is prohibited.
However, a single Article in the Constitution lacks the necessary information regarding the processes for the case-by-case analyses scenario of GMOs developed from transgenesis or NPBTs. Therefore, a regulatory framework should be implemented. Research on developing GMOs in plants has been performed mainly for the standardization of genetic transformation methodology, and only from the academic sector for crops like tomato (López et al. 2015) and species of Musa including bananas and plantains (Santos et al. 2016a, b; Villao et al. 2019) for research purposes only, using Agrobacterium-mediated transformation.
On the other hand, although the presence of transgenic crops or seeds would be limited in Ecuador, the consumption of food from GMOs or their derivatives is allowed because their proper labeling is required as indicated in Article 26 of the Organic Law of the Food Sovereignty Regime of Ecuador issued in December 2010. Furthermore, the Organic Law of Agrobiodiversity, Seeds and Promotion of Agriculture, issued on 8th June 2017, indicates that one of the guidelines of the Government is "to dictate measures to control the illegal use of seeds and transgenic crops," as well as " monitor and control the condition of the country as a territory free of seeds and transgenic crops". Similarly, Art. 56 indicate, "The entry of transgenic seeds and crops into the national territory is allowed, only to be used for research purposes. In the event that the entry is required for other purposes, the procedure established in the Constitution for that purpose must be followed.” Furthermore, the Organic Code of the Environment, issued on 12th April 2017, indicates in Art. 75 that “the biosafety norms will regulate the products of modern biotechnology, to contribute to the conservation and sustainable use of modern biotechnology”.
These regulations do not refer specifically to NPBTs. Recently, the Regulation of the Organic Code of the Environment, issued on 21 May 2019, refers to the final product (genetically improved organism) and not to the methodology involved. Therefore, Art. 230a indicates “organisms resulting from genetic improvement which are not harboring recombinant or foreign DNA in the resulting genome are excluded from risk assessment”. This could be interpreted as saying that genetically improved organisms resulting from genome editing or other NPBTs will not undergo risk assessment in the case where foreign or recombinant DNA is not present in the resulting improved organism. However, research projects in Ecuador regarding genome editing and/or other NPBTs are not yet established. Nevertheless, the government and academic sectors in Ecuador through their corresponding agriculture and/or biotechnology research centers are willing to start projects to genetically improved crops using NPBTs including species of Musa (bananas and plantains), rice, and potato.
Guatemala
According to the participant of the Ministry of Agriculture and the National Biosafety Committee (CTNBio), the country has no specific regulation for GMOs derived from modern biotechnology including NPBTs. Moreover, there were no research initiatives or any applications for the commercialization of crops/products derived from any of the NPBTs. Guatemala and Honduras signed a bilateral Resolution No. 60-2019 where they defined in Article 4.6 a novel combination of genetic material as a “stable insertion in the genome of one or more genes or DNA sequences that encode proteins, RNA, double-stranded RNA or regulatory sequences, that could not occur through conventional breeding or not found in nature”. The Agreement 271-MAGA provides the procedures to distinguish between a genetic engineer product and genome editing final product that can be considered as conventional.
Honduras
According to the participant of the Ministry of Agriculture and the National Biosafety Committee (CTNBio), the country has specific regulation for GMOs resulting from modern biotechnology (Acuerdo No. 1570-98) and a specific Agreement for genome editing C.D.-008-2019. The Honduras system is based on the final product in comparison with a conventional breeding product and allows harmonization with other countries.
Paraguay
Since 2015, according to the participant of the National Commission on Agricultural and Forestry Biosafety, the country has been discussing how to regulate or not-to regulate the crops/products resulting from any of the NPBTs. Since the workshop, progress has been made and recently the Ministry of Agriculture and Livestock (MAG) announced Resolution No. 565 dated May 13th 2019, which approves the "Form of Prior Consultation for products obtained through new techniques of genetic improvement. The National Commission on Agricultural and Forestry Biosafety is responsible for regulating this Form and the applicant must provide to the competent authority the comprehensive documentation including the name and taxonomic classification of the organism, the name of the cultivars that are intended to be introduced into the agroecosystem, the breeding methodology used for obtaining them, molecular description of the target nucleotide sequences and their functions in the organism prior to and after the application of the technique, changes in the functions of the sequences after applying the technique, the genetic map(s) of the DNA construct(s) employed in the breeding procedure, analysis of off-target effects, as well as evidence of the nonexistence of recombinant DNA. Moreover, the description of the achieved phenotype must be provided as well as information about whether there are products with a similar phenotype in the market. Additionally, an analysis of the possibility of other effects beyond the intended phenotype expected changes in the proposed uses of the organism, and changes in the management recommendations of the resulting organism should be provided. Finally, the applicant should specified if the organism has been approved by any national agency of another country, and if so, indicate the type of authorization. In this regard, Paraguay is the fifth country in Latin America that is regulating these NPBTs.
Peru
According to the participant of the Instituto de Investigaciones Agropecuarias (INIA), the country has a moratorium of 10 years established by law (Ley No. 29811) that prevents the entry and production in the national territory of living modified organisms for cultivation or breeding purposes, including aquatic ones, to be released into the environment. Moreover, at the time of the workshop, there were no research initiatives in NPBTs or any applications for the commercial use of crops/products resulting from any of the NPBTs and the country has no specific regulation for NPBTs.
Trinidad and Tobago
According to the participant of the Ministry of Planning, the country has no specific regulation for NPBTs. Moreover, there were no research initiatives or any applications for the commercialization of crops/products derived from any of the NPBTs.
Uruguay
According to the participant of the Ministry of Agriculture and the National Biosafety Committee, the country has no specific regulation for NPBTs. at the time of the workshop, were no research initiatives or any applications for the commercialization of crops/products derived from any of the NPBTs. Nevertheless, recently researchers at the Universidad de la República and Instituto Nacional de Investigación Agropecuaria (INIA) are implementing the CRISPR/Cas9 technology to edit genes in soybean to confer resistance to the herbicide glyphosate, to increase the content of saccharose, and to reduce the content of agglutinin (Coronel et al. 2019; Da Silva et al. 2019; Fleitas et al. 2019; González et al. 2019a) and in mandarine and tomato to increase the content of licopene (Arruabarrena et al. 2019).
Discussion
Modern biotechnology tools are advancing rapidly for the improvement of organisms, or the design and generation of new metabolic pathways, and biotechnological products of commercial interest (Lassoued et al. 2018a). These new biotechnological developments could imply possible benefits and risks associate with unintentional, unwanted human and animal health, ecological, and social side effects (Lassoued et al. 2018b; Fears and ter Meulen 2018; Gatica-Arias et al. 2019). Given that there is a need to legally differentiate whether or not a specific development results in a GMO or is undifferentiated from conventional breeding products, it is advisable to conduct case-by-case analyses of the products developed with the most recent biotechnologies with a clear legal definition of what is and what is not a GMO (Lassoued et al. 2018a, b). A legal understanding is of great importance because, looking back over the last decades, the experience has demonstrated that the wide regulatory assessment of plant products subject to precautionary principles used for GMOs did not necessarily contribute to building trust. On the contrary, it has contributed to increasing negative public attitudes toward transgenic products (Lassoued et al. 2018b; Herman et al. 2019). Therefore, the following question arises: Is it necessary to have a special regulatory framework for these products? In this sense, numerous models have been suggested for the regulation of products resulting from modern biotechnology, including trait-based and a technique independent model where products are categorized into diverse risk classes on a case-by-case basis analysis (Stanford model) (Huang et al. 2016; Eriksson 2019).
Moreover, various national, regional, and international mechanisms have been developed to guarantee the safe use of GMOs and therefore these guidelines could be applied for the assessment of the safety of plants and plant products derived from NPBTs (Wolt 2019). Extensive history of risk and safety assessment of GMOs has demonstrated until now no adverse impacts to human or animal health or the environment (Wolt 2019). Therefore, existing guidelines and protocols for environmental risk and safety assessment of GMOs could be applicable to NPBTs emphasizing in the phenotype as the focus of the analysis (OECD 2016; Wolt 2019). In this sense, unnecessary regulation of products developed through NPBTs should be avoided and governments should adopt a harmonized approach for the approval of these products in order to facilitate their access to farmers and consumers (Lema 2019). The lack of harmonization could result in a delay in the approval of products derived from NPBTs affecting the trade and limiting the range of commodities from which consumers can choose, as has happened in the past with GMOs in Europe, and the Hawaiian GM Papaya industry in Japan (Hundleby and Harwood 2018). In this sense, countries of the LAC region could follow the will of African Union countries to harmonize biosafety regulations and promote cooperation and mutual recognition of biosafety regulatory decisions in order to ensure more effective handling of biotechnology applications (Nkechi 2019). Given the unwillingness to adopt the GMO technology, and in some cases a slow import approval process for a given country to adopt a new technology (such as NPBTs), it must have the confidence necessary to sell its crops in domestic and foreign markets (Hundleby and Harwood 2018). Moreover, the approval by consumers and regulators determines the achievement of agricultural and food advances. Therefore, even in cases where a certain crop cannot be considered a commercial product for a particular country, it is vital that the opinions of the country do not negatively impact on other countries that can advantage from such technology (Hundleby and Harwood 2018).
However, with respect to their potential for plant and animal breeding, countries differ in how they regulate these new breeding technologies, which could be classified between genetic engineering and conventional breeding techniques (Sprink et al. 2016; Eckerstorfer et al. 2019b; Eriksson et al. 2019; Smyth 2019). The biosafety frameworks of the different countries are embedded in different laws, which define the scope of the regulations and provide definitions of products or organisms (Eckerstorfer et al. 2019b). Therefore, the current differences in national regulations on NPBT find their origin in whether changes in the phenotype of a plant (product-oriented analysis) or if the technique used to create it (product-oriented analysis) triggers its regulation and safety assessment prior to marketing (Kleter et al. 2019). In this sense, a question arises: which of the regulatory analyzes oriented to the process or product is the most appropriate for the regulation of modern biotechnology products and in particular those derived from NPBTs (Eckerstorfer et al. 2019b).
As noted by the US National Academy of Sciences (2016) emerging technologies represent a challenge to the regulatory systems by blurring the distinction between genetic engineering and plant conventional breeding; whereas it is the final product, not the process that should be regulated. In some jurisdictions, some NPBTs have been considered as modestly a modification of existing conventional plant breeding, while other countries have not determined what to do or how to proceed to regulate them (Lassoued et al. 2018a, b). It is important to emphasize that the approval of products derived from NPBTs similarly as those obtained by conventional genetic improvement does not mean that the former will not be fully regulated. On the contrary, in the European Union and in many other countries these products could be regulated under the different food laws (Wesseler et al. 2019). Nevertheless, if necessary, the biosafety frameworks of some LAC countries (Argentina, Brazil, Colombia, Chile, Guatemala, Honduras, and Paraguay) establish a risk-oriented regulatory approach and a risk assessment is performed in order to guarantee the environmental and health safety of the products derived from NPBTs prior to authorization for environmental release and commercialization. At the moment, the existing knowledge and experience with the determination of the regulatory status of NPBTs is limited. However, the LAC countries analyzed in this study have different level of regulatory experience for the contained use, confined use, unconfined use, and importation of GMOs or their derived products for food, feed, or processing purposes (Rosado and Craig 2017) which guarantees the technical and scientific capacity for risk analysis of products derived from NPBTs. In this sense, due to the possible similarities between the products derived from NPBT and conventional genetic improvement, case-by-case analyzes should be considered only if an environmental risk assessment and/or for human or animal health is required. A central point in the whole discussion regarding NPBTs is whether specific biosafety issues may be associated with their plant products (Eckerstorfer et al. 2019a). In this sense, if necessary, the risk analysis assessment could start comparing the GMO with a non-GMO counterpart with a history of safe use and based on the identified differences determine which studies are required for determining possible adverse impacts to human or animal health (e.g. toxicity and allergenicity) or the environment (e.g. pollen flow, weed resistance to herbicides, and insect resistant to Bacillus thuringiensis) (Kleter et al. 2019).
New plant breeding technologies are being used to introduce novel traits or modifying those already present in wild populations or related species in a simpler, faster, and less costly approach. Some of these traits may instead be introduced with either conventional breeding or GM technology (Eckerstorfer et al. 2019a). Therefore, risk and biosafety assessment of these plants with such novel traits represent a challenge for stakeholders, regulators, and scientists who have to be determined if plant development with a particular NPBT approach can lead to unintended changes associate with unintentional, unwanted human and animal health, and environmental damage and whether the intended use of the plants derived from NPBT may result in adverse effects related to the newly developed traits (Eckerstorfer et al. 2019a). Biosafety considerations associated with uses of NPBTs should be based on the characteristics of the particular application and risks assessments should be addressed regarding e.g. herbicide tolerance, insect resistance, disease resistance to virus, bacteria, and fungi, altered nutritional composition, morphological or reproductive plant characteristics.
For herbicide tolerance, existing experience regarding risk assessment of herbicide resistant GM plants has raised several concerns, such as dispersal and persistence of volunteers (Eckerstorfer et al. 2019a). In that context, lessons learned with herbicide-resistant traits developed by mutation such as Clearfield that end up in weeds should not be forgotten (Singh et al. 2017). In this sense, CRISPR/Cas9 technology has been used to create male sterile lines by editing the genes TMS5 and the MS8 genes in rice and maize, respectively; causing pollen sterility and avowing gene flow (Chen et al. 2018; Barman et al. 2019).
On the other hand, insects are rapidly evolving resistance against insecticide genes from the bacterium Bacillus thuringiensis (Bt) reducing the effectiveness of insect management (Tabashnik and Carrière 2017). Jin et al. (2018) demonstrated that a dominant point mutation in a tetraspanin gene associated with field-evolved resistance of cotton bollworm to transgenic Bt cotton. In this sense, CRISPR/Cas9 technology together with gene drive could be used to reduce the resistance of insects to transgenic Bt cotton by reverting the mutation in the tetraspanin gene. This highlights the necessity of performing the assessment of the traits independently of the method or technology that was used to produce the crops (Eckerstorfer et al. 2019a). Instead, trait-based and variety protection systems should consider best farming practices when authorizing a variety to achieve sustainable farming, no matter the technology used to develop it.
The analysis of the legal framework for NPBTs of fifteen different LAC countries resulted in three main outcomes. The first outcome is that all countries identified a point of reference in the definition of GMO. In this sense, “Living modified organism” means “any living organism that possesses a novel combination of genetic material obtained with modern biotechnology” (Article 3(g) of the Cartagena Protocol on Biosafety. This point of reference is important since countries like Argentina are not party to the international treaty. The relevance of this point of common understanding is that even though the countries have different legal backgrounds, the Cartagena Protocol on Biosafety prevails in order to achieve more clarity. In this sense, regulation of NPBTs must respond to the national definitions of biosafety of each country in the LAC region. In addition, most countries have established that biotechnology governance relies heavily on the principles and concepts enshrined in the Cartagena Protocol on Biosafety, and many countries apply it in a complementary way or they use it as a basis for their national legislation (Orozco 2018).
The second outcome is that in the countries with a NPBTs legal framework, the definition of "new combination of genetic material" is a point of discussion. In this sense, there are slight differences in the definition of this term in Argentina, Brazil, Colombia, Chile, and Guatemala-Honduras; however, they all conclude that there must be a stable insertion of "foreign" DNA in the genome. According to Seyran and Craig (2018), depending on the definition of GMO used, a main criterion to determine the regulatory status of a particular NPBT could be the “amount” of consequential genetic change. Nevertheless, some the changes induced by gene-editing technologies are minor enough that they could not be easily distinguish from those occurring naturally (Ledford 2019). Therefore, from the biosecurity point of view, it is challenging to imagine that products that have undergone a small quantity of specifically induced mutations in predefined positions represent more risk than those products that carry a large number of random mutations (Eriksson 2019). In this sense, the Office of the Genetic Technology Regulator (OGTR) of Australia states that the genetic modifications made without new genetic material are no dissimilar from the changes that occur in nature and, therefore, do not represent an extra risk to the environment or human health. Therefore, the Australian Government has issued a decision that says it will not regulate the use of genetic editing techniques in plants, animals and human cells that do not introduce new genetic material (Mallapaty 2019).
The third outcome is that the practical approach clarifying whether there is or is not a new combination of genetic material or foreign DNA is provided by a comparison of the final product of genome editing versus conventional breeding, and natural or induced mutations products. Some techniques, such as RdDM, are an improvement of traditional breeding, and the genetic material is not changed; some methods of gene-editing tools, such as CRISPR, TALEN, and ZFN uses site-directed nucleases (SDN 1 and 2) to induce site-specific genome changes resulting in final products that are transgene-free and might not be considered as a GMO. On the other hand, some gene editing tools use SDN3 causing gene insertions and therefore the final products have transgenes and might follow the GM rules (Araki and Ishii 2015; Sprink et al. 2016; Hundleby and Harwood 2018). Like Argentina, Brazil, Chile, Colombia, and Paraguay, other countries outside the LAC region (such as Canada, Israel, the USA, and Japan) determined that products developed using genome editing, in cases where new genetic sequences have not been introduced, should not be differently regulated than a product of conventional mutagenesis (Hundleby and Harwood 2018).
On the contrary, the Court of Justice of the European Union (Press Release No. 111/18 of 25 July, 2018) indicated that directed mutagenesis techniques (with the exception of chemical and radiation mutation breeding) should be subject to the provisions of the GMO Directive 2001/18/EU, essentially putting many NPBTs in the same regulatory basket (Hundleby and Harwood 2018; Eriksson 2019). However the scientific advisors of the European Commission stated in November that “the impossibility to distinguishing between spontaneous occurring mutation and different types of human interventions is a major issue from a regulatory point of view”, and consequently, “there is a need to improve EU GMO legislation to be clear, evidence-based, implementable, proportional and flexible enough to cope with future advances in science and technology in this area”.
The LAC region is well known for its wealth of natural resources (land, water, and biodiversity) which are vital for a developing bio-economy. In the coming years The countries of the LAC region must invest in the development of local varieties of genetically edited plants and animals, as proposed by Russia through its federal program to create 10 new crop varieties and modified animals by 2020 (Dobrovidova 2019). Nevertheless, the development of the bio-economy in the region is limited, among other reasons, by the lack of regulatory frameworks, especially in fields of rapid scientific progress, such as NPBTs (Trigo et al. 2013). Some LAC countries still have very weak biosafety regulatory instruments while others have none in place at all in place (Rosado and Craig 2017). In this regard, several countries in the LAC region, have delayed the establishment of regulatory frameworks that allow their farmers and consumers to access the NPBTs. Therefore, in the era of NPBTs, the academic and scientific community of some countries in the LAC region face a great challenge and should promote discussion of the regulatory status of NPBTs in order to respond to the next question: If the modifications are impossible to distinguish from those that can also be achieved through traditional improvement or natural mutation, is it necessary to create a new category of regulated product? Another question arises to the development of products for small farmer of developing countries, now that sequencing and genome editing techniques are affordable (Yin et al. 2017), and Institutes such as CIAT, EMBRAPA, and universities are starting to work with tropical products such as banana, pineapple, rice, beans, cassava, and local products that in the past could not reach the market because of their slow or difficult breeding: should the scientific community promote a more democratic development of traits and products based on this new technologies for small farmers? Will the regulatory system make it impossible and instead increase the gap between big and small farmers? Or will the regulatory environment affects the costs and benefits of investments in NPBTs?
Notes
Declaración de los Ministros de Agricultura del Consejo Agropecuario del Sur (CAS) sobre técnicas de edición génica. Comunicación de Argentina, Australia, Brasil, Canadá, los Estados Unidos de América, Guatemala, Honduras, Paraguay, República Dominicana y Uruguay. https://G/Sps/Gen/1658/Rev.3. Accessed 17 July 2019.
Declaración de los Ministros de Agricultura del Consejo Agropecuario del Sur (Cas) sobre técnicas de edición génica. Comunicación de Argentina. https://docs.wto.org/dol2fe/Pages/FE_Search/DDFDocuments/252340/s/G/SPS/GEN1699.pdf. Accessed 17 July 2019.
References
Ambrozevicius L (2018) Precision Breeding Innovation Techniques (PBI) “TIMP - Técnicas Inovadoras de Melhoramento de Precisão”. In: Genome editing seminar for biotechnology regulators in the Americas. Calí, Colombia, 4–5 April
Araki M, Ishii T (2015) Towards social acceptance of plant breeding by genome editing. Trends Plant Sci 20:145–149. https://doi.org/10.1016/j.tplants.2015.01.010
Argentina.gob.ar (2018) Caballos clonados con genes editados, otra hazaña de científicos argentinos. https://www.argentina.gob.ar/noticias/caballos-clonados-con-genes-editados-otra-hazana-de-cientificos-argentinos. Accessed 17 Aug 2019
Arruabarrena A, Lado J, Stange CR, González-Arcos M, Rivas CF, Vidal S (2019) Mejoramiento de precisión para promover la acumulación de licopeno en frutos de mandarina y tomate. In: X Encuentro Latinoamericano y del Caribe de Biotecnología Agropecuaria y XI Simposio REDBIO Argentina. Libro de Resúmenes. Montevideo, Uruguay, 12–15 November. https://doi.org/10.35676/INIA/ST.253
Barman HN, Sheng Z, Fiaz S et al (2019) Generation of a new thermo-sensitive genic male sterile rice line by targeted mutagenesis of TMS5 gene through CRISPR/Cas9 system. BMC Plant Biol 19:109. https://doi.org/10.1186/s12870-019-1715-0
Barrantes E (2017) UCR trabaja en nueva variedad de arroz tolerante a la sequía y la salinidad. https://semanariouniversidad.com/universitarias/ucr-trabaja-nueva-variedad-arroz-tolerante-la-sequia-la-salinidad/. Accessed 30 August 2019
Cao HX, Wenqin W, Hien TTL, Giang THV (2016) The power of CRISPR-CAS9-induced genome editing to speed up plant breeding. Int J Genomics. https://doi.org/10.1155/2016/5078796
Cartagena protocol on biosafety to the convention on biological diversity. https://bch.cbd.int/protocol/. Accessed 30 July 2019.
Chen R, Xu Q, Liu Y, Zhang J, Ren D, Wang G, Liu Y (2018) Generation of transgene-free maize male sterile lines using the CRISPR/Cas9 system. Front Plant Sci 9:1180. https://doi.org/10.3389/fpls.2018.01180
Cockcroft CE, Herrera-Estrella L, Borroto Nordelo CG (2004). Agricultural biotechnology in Latin America and the Caribbean. In Christou P, Klee H (eds) Handbook of plant biotechnology, Wiley, London, pp 1243–1291. https://doi.org/10.1002/0470869143.kc067
Coronel MP, Fleitas AL, Vidal S (2019) Desarrollo de una metodología de remplazo alélico optimizada para soja utilizando un modelo de resistencia a herbicida. In: X Encuentro Latinoamericano y del Caribe de Biotecnología Agropecuaria y XI Simposio REDBIO Argentina. Libro de Resúmenes. Montevideo, Uruguay, 12–15 November. https://doi.org/10.35676/INIA/ST.253
Da Silva DA, Bonnecarrère V, Vidal S (2019) Agregado de valor a la soja mediante el desarrollo de variedades nacionales, no transgénicas, para consumo humano. In: X Encuentro Latinoamericano y del Caribe de Biotecnología Agropecuaria y XI Simposio REDBIO Argentina. Libro de Resúmenes. Montevideo, Uruguay, 12–15 November. https://doi.org/10.35676/INIA/ST.253
de la León ODI, Thorsteinsdóttir H, Calderón-Salinas JV (2018) The rise of health biotechnology research in Latin America: a scientometric analysis of health biotechnology production and impact. PLoS ONE. https://doi.org/10.1371/journal.pone.0191267
Dobrovidova O. 2019. Russia joins in global gene-editing bonanza. Nature. https://www.nature.com/articles/d41586-019-01519-6. Accessed 17 July 2019
Eckerstorfer MF, Dolezel M, Heissenberger A, Miklau M, Reichenbecher W, Steinbrecher RA, Waßmann F (2019a) An EU perspective on biosafety considerations for plants developed by genome editing and other new genetic modification techniques (nGMs). Front Bioeng Biotechnol 7:31. https://doi.org/10.3389/fbioe.2019.00031
Eckerstorfer MF, Engelhard M, Heissenberger A, Simon S, Teichmann H (2019b) Plants developed by new genetic modification techniques—comparison of existing regulatory frameworks in the EU and non-EU countries. Front Bioeng Biotechnol 7:26. https://doi.org/10.3389/fbioe.2019.00026
Eriksson D (2019) The evolving EU regulatory framework for precision breeding. Theor Appl Genet 132:569–573. https://doi.org/10.1007/s00122-018-3200-9
Eriksson D, Kershen D, Nepomuceno A, Pogson BJ, Prieto H, Purnhagen K, Smyth S, Wesseler J, Whelan A (2019) A comparison of the EU regulatory approach to directed mutagenesis with that of other jurisdictions, consequences for international trade and potential steps forward. New Phytol 222:1673–1684
Fears R, ter Meulen V (2018) Assessing security implications of genome editing: emerging points from an international workshop. Front Bioeng Biotechnol 6:34. https://doi.org/10.3389/fbioe.2018.00034
Feest P (2017) Chile: Los primeros pasos para el uso de CRISPR/Cas9 en salmónidos. https://www.salmonexpert.cl/article/chile-los-primeros-pasos-para-el-uso-de-crispr-cas9-en-salmonidos/. Accessed 17 August 2019
Fleitas AL, Gallino JP, Señorale M, Bonnecarrere V, Vidal S (2019). Optimización de técnicas de edición genómica libres de DNA en Soja. In: X Encuentro Latinoamericano y del Caribe de Biotecnología Agropecuaria y XI Simposio REDBIO Argentina. Libro de Resúmenes. Montevideo, Uruguay. 12–15 November. https://doi.org/10.35676/INIA/ST.253
Gatica-Arias A, Valdez-Melara M, Arrieta-Espinoza G, Albertazzi-Castro FJ, Madrigal-Pana J (2019) Consumer attitudes toward food crops developed by CRISPR/Cas9 in Costa Rica. Plant Cell Tissue Organ Culture. https://doi.org/10.1007/s11240-019-01647-x
González J, Fort S, Gallino JP, Fleitas AL, Bonnecarrère V, Vidal S (2019a) Edición genómica en soja para mejoramiento de caracteres nutricionales. In: X Encuentro Latinoamericano y del Caribe de Biotecnología Agropecuaria y XI Simposio REDBIO Argentina. Libro de Resúmenes. Montevideo, Uruguay, 12–15 November. https://doi.org/10.35676/INIA/ST.253
González MN, Massa GA, Andersson M, Storani L, Décima Oneto CA, Hofvander P, Feingold SE (2019b) Potato plants (Solanum tuberosum L.) with reduced tuber enzymatic browning developed by genome editing with the CRISPR/Cas9 system. In: X Encuentro Latinoamericano y del Caribe de Biotecnología Agropecuaria y XI Simposio REDBIO Argentina. Libro de Resúmenes. Montevideo, Uruguay, 12–15 November. https://doi.org/10.35676/INIA/ST.253
Herman RA, Fedorova M, Storer NP (2019) Will following the regulatory script for GMOs promote public acceptance of gene-edited crops? Trends Biotechnol. https://doi.org/10.1016/j.tibtech.2019.06.007
Huang S, Weigel D, Beachy RN, Li J (2016) A proposed regulatory framework for genome-edited crops. Nat Genet 48(2):109–111
Hundleby P, Harwood W (2018) Impacts of the EU GMO regulatory framework for plant genome editing. Food Energy Secur 8:e00161. https://doi.org/10.1002/fes3.161
ISAAA (2017) Global Status of Commercialized Biotech/GM Crops in 2017: Biotech crop adoption surges as economic benefits accumulate in 22 years. ISAAA Brief No. 53. ISAAA: Ithaca, NY. https://www.isaaa.org/resources/publications/briefs/53/download/isaaa-brief-53-2017.pdf. Accessed 11 July 2019
Izquierdo I, de la Riva GA (2000) Plant biotechnology and food security in Latin America and the Caribbean. Electron J Biotechnol 3(1). https://www.ejbiotechnology.info/index.php/ejbiotechnology/article/view/v3n1-1/835
Jin L, Wang J, Guan F, Zhang J, Yu S, Liu S, Xue Y, Li L, Wu S, Wang X, Yang Y, Abdelgaffar H, Jurat-Fuentes JL, Tabashnik BE, Wu Y (2018) Dominant point mutation in a tetraspanin gene associated with field-evolved resistance of cotton bollworm to transgenic Bt cotton. PNAS 115(46):11760–11765. https://doi.org/10.1073/pnas.1812138115
Kinderlerer J (2008) The Cartagena protocol on biosafety. Collect Biosaf Rev 4:12–65
Kleter G, Kuiper H, Kok E (2019) Gene-edited crops: towards a harmonized safety assessment. Trends Biotechnol 37(5):443–447
Lassoued R, Hesseln H, Phillips PWB, Smyth SJ (2018a) Top plant breeding techniques for improving food security: an expert Delphi survey of the opportunities and challenges. Int J Agric Resour Gov Ecol 14(4):321–337
Lassoued R, Smyth SJ, Phillips PWB, Hesseln H (2018b) Regulatory uncertainty around new breeding techniques. Front Plant Sci 9:1291. https://doi.org/10.3389/fpls.2018.01291
Ledford H (2019) CRISPR conundrum: Strict European court ruling leaves food-testing labs without a plan. https://www.nature.com/articles/d41586-019-02162-x?utm_source=Nature+Briefing&utm_campaign=2fabe86eff-briefing-dy-20190723&utm_medium=email&utm_term=0_c9dfd39373-2fabe86eff-43587561. Accessed 17 July 2019.
Lema M (2019) (2019) Regulatory aspects of gene editing in Argentina. Transgenic Res 28:147–150. https://doi.org/10.1007/s11248-019-00145-2
López E, Proaño K, Jadán M, Mihai R (2015) Callus tissue induction and analysis of GUS reporter gene expression in tomato (Solanum lycopersicum L.) transformed with Agrobacterium tumefaciens. Roman Biotechnol Lett 20(2):10205–10211
Mallapaty S (2019) Australian gene-editing rules adopt ‘middle ground’. https://www.nature.com/articles/d41586-019-01282-8. Accessed 17 July 2019
McHughen A, Smyth S (2008) US regulatory system for genetically modified [genetically modified organism (GMO), rDNA or transgenic] crop cultivars. Plant Biotechnol J 6:2–12
Miglani G (2017) Genome editing in crop improvement: present scenario. Crop Improv 31(4):453–559
National Academies of Sciences, Engineering, and Medicine (2016) Genetically engineered crops: experiences and prospects. The National Academies Press, Washington, DC. https://doi.org/10.17226/23395
Nkechi I (2019) African Union mulls harmonized biosafety system framework. https://allianceforscience.cornell.edu/blog/2019/07/african-union-mulls-harmonized-biosafety-system-framework/). Accessed 17 July 2019
OECD (Organization for Economic Cooperation and Development) (2016) Report of the OECD workshop on environmental risk assessment of products derived from new plant breeding techniques. In: Directorate E (ed) Organization for economic cooperation and development, Paris
Orozco P (2018) Argentina and Brazil merge law and science to regulate new breeding techniques. Cornell alliance for science. https://allianceforscience.cornell.edu/blog/2018/01/argentinaand-brazil-merge-law-and-science-to-regulate-new-breedingtechniques. Accessed July 16 2019
Pardo-Hernández G (2018) Enfoque metodológico para productos vegetales desarrollados por nuevas técnica de fitomejoramiento. In: Genome editing seminar for biotechnology regulators in the Americas. Calí, Colombia, 4–5 April
Plaza S. (2018) INTA y dos alimentos del futuro: “superpapas” y leche no alergénica. https://www.lacapitalmdp.com/inta-y-dos-alimentos-del-futuro-superpapas-y-leche-no-alergenica/. Accessed 17 August 2019
Roca W, Espinoza C, Panta A (2004) Agricultural applications of biotechnology and the potential for biodiversity valorization in Latin America and the Caribbean. AgBioForum, 7(1, 2): 13–22
Rosado A, Craig W (2017) Biosafety regulatory systems overseeing the use of genetically modified organisms in the Latin America and Caribbean region. AgBioForum 20(2):120–132
Sánchez FJ, Arciniegas JP, Brand A, Vacca O, Tohme J, Becerra LA, Chavarriaga P (2019) edición de genomas en manihot esculenta Crantz para la producción de plantas waxy y para la resistencia a la bacteriosis vascular producida por Xanthomonas axonopodis pv. Manihotis. In: X Encuentro Latinoamericano y del Caribe de Biotecnología Agropecuaria y XI Simposio REDBIO Argentina. Libro de Resúmenes. Montevideo, Uruguay, 12–15 November. https://doi.org/10.35676/INIA/ST.253
Santos E, Pacheco R, Villao L, Galarza L, Ochoa D, Jordán C, Flores J (2016a) Promoter analysis in Banana. In: Mohandas S, Ravishankar KV (eds) Banana: genomics and transgenic developments for crop improvement. Springer, New York, pp 157–179
Santos E, Sánchez E, Hidalgo L, Chávez T, Villao L, Pacheco R, Flores J, Korneva S, Navarrete O (2016b) Advances in Banana Transformation through Agrobacterium tumefaciens in Ecuador: progress, challenges and perspectives. Acta Hortic 1114:197–202. https://doi.org/10.17660/ActaHortic.2016.1114.27
Schuttelaar P (2015) The regulatory status of new breeding techniques in countries outside the European Union. Version: June 2015
Seyran E, Craig W (2018) New breeding techniques and their possible regulation. AgBioForum 21(1):1–12
Sierra-Robles S, Moreno-Ramirez JL, Chavarriaga-Aguirre P, Tohme J (2019) Avances en la edición de genomas libre de ADN para variedades colombianas de Theobroma cacao. In: X Encuentro Latinoamericano y del Caribe de Biotecnología Agropecuaria y XI Simposio REDBIO Argentina. Libro de Resúmenes. Montevideo, Uruguay, 12–15 November. https://doi.org/10.35676/INIA/ST.253
Singh V, Singh S, Black H, Boyett V, Basu S, Gealy D, Gbur E, Pereira A, Scott RC, Caicedo A, Burgos NR (2017) Introgression of ClearfieldTM rice crop traits into weedy red rice outcrosses. Field Crop Res 207:13–23. https://doi.org/10.1016/j.fcr.2017.03.004
Smyth SJ (2019) Global status of the regulation of genome editing technologies. CAB Reviews 14, No. 021. https://www.cabi.org/cabreviews/review/20193130669
Soto G (2019) Alfalfa improvement through the application of NBTS. In: X Encuentro Latinoamericano y del Caribe de Biotecnología Agropecuaria y XI Simposio REDBIO Argentina. Libro de Resúmenes. Montevideo, Uruguay. 12–15 November. https://doi.org/10.35676/INIA/ST.253
Sprink T, Eriksson D, Schiemann J, Hartung F (2016) Regulatory hurdles for genome editing: process- vs. product-based approaches in different regulatory contexts. Plant Cell Rep 35:1493–1506. https://doi.org/10.1007/s00299-016-1990-2
Tabashnik B, Carrière Y (2017) Surge in insect resistance to transgenic crops and prospects for sustainability. Nat Biotechnol 35(10):926–935
Trigo EJ, Henry G, Sanders J, Schurr U, Ingelbrecht I, Revel C, Santana C, Rocha P (2013) Towards bioeconomy development in Latin America and the Caribbean. Bioeconomy Working Paper. Cali, Colombia: ALCUE-KBBE. https://agritrop.cirad.fr/567934/1/document_567934.pdf. Accessed 15 July 2019
Valdés S, Marín D, Delgado G, Lorieux M, Álvarez M, Tohme J, Chavarriaga P (2019) CRISPR/Cas9: acelerando el mejoramiento del arroz. In: X Encuentro Latinoamericano y del Caribe de Biotecnología Agropecuaria y XI Simposio REDBIO Argentina. Libro de Resúmenes. Montevideo, Uruguay, 12–15 November. https://doi.org/10.35676/INIA/ST.253
Villao L, Sánchez E, Romero C, Galarza L, Flores J, Santos E (2019) Activity characterization of the plantain promoter from the heavy metal-associated isoprenylated plant gene (MabHIPP) using the luciferase reporter gene. Plant Gene 19:100187. https://doi.org/10.1016/j.plgene.2019.100187
Wesseler J, Politiek H, David Z (2019) The economics of regulating new plant breeding technologies—implications for the bioeconomy illustrated by a survey among Dutch plant breeders. Front Plant Sci 10:1597. https://doi.org/10.3389/fpls.2019.01597
Wolt J (2019) (2019) Current risk assessment approaches for environmental and food and feed safety assessment. Transgenic Res 28:111–117. https://doi.org/10.1007/s11248-019-00140-7
Yin K, Gao C, Qiu JL (2017) Progress and prospects in plant genome editing. Nat Plants 3(8):17107
Acknowledgements
This study was financed by the “Espacio de Estudios Avanzados de la Universidad de Costa Rica” (Space for Advanced Studies at the University of Costa Rica) (UCREA; Project No. 801-B7-294). Dr. Andrés Gatica-Arias would like to thank Agustina Whelan (Biotechnology Directorate, Ministry of AgroIndustry, Argentina) and Dr. Efrén Santos-Ordoñez (ESPOL Polytechnic University, Escuela Superior Politécnica del Litoral, ESPOL, Centro de Investigaciones Biotecnológicas del Ecuador (CIBE), Ecuador) for reviewing the respective country descriptions. Dr. Pedro Rocha (Inter-American Institute for Cooperation on Agriculture (IICA), San José, Costa Rica) and Dr. Luiz Filipe Protasio Pereira (Instituto Agronómico do Paraná (IAPAR) and Empresa Brasileira de Pesquisa Agropecuária (Embrapa Café), Brazil) for the for proofreading and constructive recommendations on this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Communicated by Goetz Hensel.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gatica-Arias, A. The regulatory current status of plant breeding technologies in some Latin American and the Caribbean countries. Plant Cell Tiss Organ Cult 141, 229–242 (2020). https://doi.org/10.1007/s11240-020-01799-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-020-01799-1