Abstract
Nothapodytes foetida, an endangered tree of Indian origin, is a major source of the anti-cancer monoterpenoid indole alkaloid, camptothecin (CPT). Strictosidine synthase (STR) condenses tryptamine and secologanin to form strictosidine, a universal precursor of terpenoid indole alkaloids including CPT. We cloned full-length str cDNA with an open reading frame of 1059 bp from N. foetida (Nfstr) using a homology-based approach. Different tissues of N. foetida from in vitro grown cultures, as well as a mature tree, showed expression of STR, confirming the constitutive nature of the gene. In vitro tissues showed a positive correlation between STR expression and the CPT content, but tissues from wild-type mature plants did not show a similar pattern. Transgenic Ophiorrhiza rugosa plants overexpressing Nfstr showed 1.9-fold higher CPT than non-transformed plants. The results indicated that overexpression of Nfstr in target plants could improve the levels of CPT and may provide an alternative and sustainable source of camptothecin.
Key message
We report the full-length sequence and expression analysis of strictosidine synthase cDNA from Nothapodytes foetida (Nfstr). Further, the overexpression of Nfstr in Ophiorrhiza resulted in twofold enhancement in camptothecin levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Camptothecin (CPT), a water-insoluble terpenoid indole alkaloid (TIA), is a prominent anti-leukemic and anti-tumoural compound, first identified by Wall et al. (1966). Due to CPT’s ability to inhibit DNA topoisomerase I, an essential enzyme for DNA replication, the water-soluble CPT derivatives such as irinotecan, and topotecan are widely used throughout the world for the treatment of various cancers such as uterine, cervical, ovarian, colorectal cancers and small lung cell cancer (Venditto and Simanek 2010). Camptothecin has also been shown to be an effective drug in the treatment of AIDS (Priel et al. 1991) and in curing of malaria caused by Plasmodium falciparum (Bodley et al. 1998). The global demand of CPT derivatives was reported to be more than 4 billion US dollars in 2014 and is growing further with time (Shivaprakash et al. 2014) and there is a shortage of supply. Despite the rapid growth of the market, CPT analogues are still synthesized from natural CPT isolated from different parts of two plants namely Camptotheca acuminata and Nothapodytes foetida (Aiyama et al. 1988; Uma Shaanker et al. 2008; Wall et al. 1966). Among other CPT-producing plants, Ophiorrhiza spp., which are herbaceous, short-duration plants have gained a lot of attention as alternative sources for CPT production (Martin et al. 2008; Roja 2008). As a chemical synthesis of the CPT is complicated and not economical due to its complex configuration, pharmaceutical companies depend on the natural CPT isolated from plants which lead to the exploitation of these plants and their natural habitats. The ever-increasing market demand, with a limited supply of the natural CPT has resulted in the over-exploitation of these plants. Therefore, there is an urgent need to find alternative and sustainable resources for CPT.
Nothapodytes foetida (Wight) Sleumer is a forest tree species of Western Ghats of India and is a rich source of CPT. It has highest CPT content (0.1–1% dry wt.) compared to other plants (Fulzele and Satdive 2005), but the plants are getting endangered and efforts were made to propagate this plant using in vitro cell and organ cultures (Fulzele and Satdive 2003; Isah and Mujib 2015). Several researchers have used various elicitors, UV and gamma-radiations also to increase the production of CPT in the target plants (Ruan et al. 2014; Deepthi and Satheeshkumar 2016; Fulzele et al. 2015). Apart from using tissue and cell culture methods, the advent of various ‘omics’ technologies has opened a new perspective to identify essential genes involved in CPT biosynthetic pathway in host plants. Dedicated plant-specific transcriptome, proteome and metabolome databases, gene expression profiles together with various functional validation strategies have contributed significantly to enrich the toolbox for metabolic engineering of TIA biosynthesis (Bernonville et al. 2015). Recently, comprehensive metabolic and transcriptome analyses of various tissues of Nothapodytes nimmoniana unravels several putative pathway genes, transcription factors and cytochrome P450 related to camptothecin (CPT) biosynthesis (Manjunatha et al. 2016; Rather et al. 2018). The characterization of these candidate genes and transferring one or more key gene/s of the pathway into target plants, thus promises an alternative way of enhancing CPT content in the selected plant.
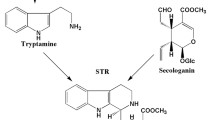
Plants synthesize camptothecin through a complicated monoterpene indole-alkaloid (MIA or TIA) pathway. However, the biosynthetic pathway and regulatory steps of CPT production in the plants are largely unclear (Yamazaki et al. 2003). The terpenoid portion of these alkaloids comes from secologanin—a secoiridoid glycoside produced from geraniol and indole portion comes from tryptamine, produced by decarboxylation of tryptophan (Stöckigt and Zenk 1977). The first committed step in monoterpene indole alkaloid pathway is catalyzed by strictosidine synthase (STR), which conjugates secologanin with tryptamine to produce strictosidine (Stöckigt and Zenk 1977; Stöckigt and Ruppert 1999). The full-length cDNA encoding STR has been cloned earlier from a few plants including Rauvolfia serpentina (Kutchan et al. 1988), Catharanthus roseus (McKnight et al. 1990), Ophiorrhiza spp. (Lu et al. 2009; Yamazaki et al. 2003) and C. acuminata (Sun et al. 2011). Overexpression of STR in transgenic C. roseus was reported to improve STR activity by tenfold and positive effect on MIA biosynthesis (Cenel et al. 1998). Recently, Cui et al. (2015) showed co-expression of str and geraniol-10-hydroxylase (g10H) genes from C. roseus in Ophiorrhiza pumila, resulted in 56% increase on the CPT yields compared to non-transgenic hairy roots.
Though relatively more is known about the biosynthesis of TIA in C. roseus, less information is available with regards to N. foetida. Huang et al. (2012) cloned and characterized three unique NADPH cytochrome P450 reductase cDNAs from N. foetida. Recently, Manjunatha et al. (2016) and Rather et al. (2018) have reported a set of putative genes associated with the biosynthesis of CPT in N. nimmoniana. In the present study, we report the full-length sequence and expression analysis of strictosidine synthase cDNA—the first such information from N. foetida. Further, the effect of heterologous overexpression of Nfstr in Ophiorrhiza rugosa, another CPT producing plant to improve the camptothecin content is also presented.
Materials and methods
Plant material
Two-month-old mature seeds of Nothapodytes foetida were collected from Mahabaleshwar, Maharashtra, India. Seeds were surface sterilized with 70% ethanol for 2 min, followed by 0.1% mercuric chloride for 20 min and germinated in the dark on Murashige and Skoog’s (MS) medium (Murashige and Skoog 1962) with 3% sucrose and 0.8% agar. Once germinated, seedlings were shifted to normal tissue culture conditions [14 h light/10 h dark at 25 ± 2 °C under white fluorescent light (Mitsubishi Osram FL40SS W/37; 12.2 µM photon m−2 s−1)] and allowed to grow for 4–6 weeks. For the plant transformation work, in vitro grown plants of Ophiorrhiza rugosa var. decumbens Deb & Mondal maintained in our laboratory were used.
Cloning of full-length strictosidine synthase (Nfstr) cDNA from N. foetida
Young leaves of the 12-week old in vitro grown plantlets of N. foetida were used for total RNA isolation using TRI reagent (SIGMA) method as described earlier (Singh et al. 2011). Purified total RNA was treated with DNase (Qiagen GambH, Hilden, Germany) to remove genomic DNA contamination if any and quantified using NanoDrop 2000™. Subsequently, two µg of total RNA was used for first-strand cDNA synthesis using random primers of Affinity Script multiple temperature cDNA synthesis kit (Agilent Technologies, USA) according to the manufacturer's protocol.
Full-length cDNA cloning of Nfstr was done using homology-based approach. The reported cDNA sequences of str from C. roseus (NCBI accession Y10182), C. acuminata (NCBI accession AES93117), Rauwolfia serpentina (NCBI accession Y00756) and Ophiorrhiza japonica (NCBI accession EU670747), which have similar biosynthetic pathways were compared and primers were designed. Further, PCR was performed with NcoI_F 5′-GCCATGGCAAACTTTTCTGAATC-3′ and BglII_R 5′-GCAGATCTCTAGCTAGAAACATAAG-3′ primers using N. foetida cDNA as template. The amplified product was sub-cloned in pTZ57R/T vector (Thermo Scientific, USA) and the sequence confirmed using automated DNA sequencing.
Sequence analysis of Nfstr
The translated sequence of putative Nfstr cDNA was searched for similarity using BLASTP 2.8.0+ (Altschul et al. 1997) against non-redundant protein (nr) sequences database at NCBI (www.ncbi.nlm.nih.gov). Further, the phylogenetic analyses of Nfstr were done using MEGA6 (Tamura et al. 2013) with the neighbour-joining tree method.
Expression analysis of Nfstr in different plant tissues and their relation with CPT levels
To correlate the levels of CPT with differential expression of str, different tissues including in vitro cultures such as callus, embryonic shoots, and complete plantlets as well leaves, roots, and seeds from a 12-year-old tree were analyzed. To compare str expression, total RNA from the selected tissues was isolated, cDNA synthesized and semi-quantitative reverse transcription PCR (RT-PCR) performed using gene-specific primers as mentioned earlier. Further, quantitative real-time PCR (qRT-PCR) was also performed using SYBR® Green Jump Start™ Taq Ready mix (Sigma, St. Louis, USA) on an Eppendorf Realplex4 (Eppendorf, GmbH, Germany) as per MIQE guidelines (Bustin et al. 2009). A typical reaction mixture contained 10 µL 2 × SYBR Green mix, 0.4 µM forward (5′-TTGAAAGCCCTTCCTATGCT-3′) and reverse primers (5′-AAGCTTTGTTCCAGAAGGGA-3′) each and 1 µL cDNA as template. Following an initial denaturation step at 94 °C for 2 min, the amplification programme was of 40 cycles of 30 s at 94 °C, 20 s at 52 °C and 20 s at 72 °C. Camptothecin estimation in these tissues was carried out using HPLC as described earlier (Fulzele and Satdive 2005).
Cloning of Nfstr in plant binary vector pCAMBIA1301
The complete Nfstr cDNA was cloned in plant binary vector pCAMBIA1301 (CAMBIA, Brisbane, Australia) using NcoI–BglII restriction sites. The resulting plasmid pSS5 has hptII as a plant selectable marker and uidA as the reporter gene, apart from Nfstr (Supplementary Fig. S1). Plasmid pSS5 was finally introduced into Agrobacterium tumefaciens EHA 105 cells using an electroporator 2510 (Eppendorf, Hamburg, Germany) as per the manufacturer's protocol (Eppendorf protocol number 4308 915.502-12/2001).
Genetic transformation of Ophiorrhiza rugosa with Nfstr
To assess the effect of Nfstr on CPT content in a target plant, the Nfstr was overexpressed in O. rugosa, a fast-growing herbaceous camptothecin producing plant. Genetic transformation of O. rugosa was carried out using leaf discs of 4-week-old in vitro plants with Agrobacterium tumefaciens harbouring pSS5 using the method described by Horsch et al. (1985). The explants after co-cultivation for 48–72 h were transferred to regeneration medium (MS with 2 mg L−1 benzyl adenine, 0.1 mg L−1 indole acetic acid, 3% sucrose and 0.25% phytagel) supplemented with, 2.5 mg L−1 hygromycin and 500 mg L−1 cefotaxime. Regenerated shoots (Fig. 1) were subsequently transferred to rooting medium (½MS with 3% sucrose, 0.8% agar, 2.5 mg L−1 hygromycin and 250 mg L−1 cefotaxime).
Molecular analyses and comparison of CPT levels in transgenic plants overexpressing Nfstr
Eight-week old well rooted in vitro plantlets of O. rugosa were selected for molecular characterization. Total genomic DNA from the leaves of control and more than two dozen independently developed putatively transformed plants was isolated (Dellaporta et al. 1983) and subjected to PCR amplification using Nfstr specific primers. Transcription of the Nfstr in six randomly selected better growing lines was confirmed by RT-PCR as described in the previous section. Finally, camptothecin content in the selected transgenic Ophiorrhiza lines was compared to assess the effect of Nfstr overexpression in improving CPT levels. As NfSTR catalyzes secologanin and tryptamine to produce strictosidine, the determination of strictosidine contents would have been a direct confirmation for NfSTR activity. However, we could not do the same due to unavailability of standard for strictosidine.
Statistical analyses
All the experiments were carried out with three replicates and repeated at least twice. For analysis, different means were subjected to Tukey’s test and one-way analysis of variance (ANOVA) using the statistical software Origin 8.1.
Results and discussion
Full-length cDNA cloning of strictosidine synthase and sequence analyses
The cDNA encoding STR from N. foetida was cloned using a homology-based approach. The sequencing results confirmed isolated full-length cDNA as a 1059 bp open reading frame (NCBI accession no. MH735146, Fig. 2) translating a protein of 352 amino acids with an expected molecular weight of 39.1 kDa. Upon comparing, translated Nfstr cDNA sequence showed it as a member of STR-synthesis superfamily. It showed a high degree of similarity with many of the reported STR including 100% identity with C. roseus STR, which shares a common initial TIA pathway with N. foetida. It is interesting, and possibly NfSTR may be one of the few proteins of TIA pathway with such a high degree of similarity/conservation between these two plants. It also showed 79% identity with STR from Rauwolfia serpentina, 72% with Tabernaemontana elegans, 57% with Ophiorrhiza pumila and O. japonica, 55% with Mitrangyna speciosa and 37% with Camptotheca acuminata. To understand the evolutionary relationships among these STRs, phylogenetic analysis of translated Nfstr cDNA was done with reported STRs and results showed a similar pattern as that of percentage homology (Fig. 3). However, an important observation was that although N. foetida, C. acuminata, and Ophiorrhiza spp synthesize CPT and share similar TIA pathway, the STRs from these plants were quite distant in the phylogeny tree.
Abundance of str in different tissues of N. foetida and its relation with CPT levels
Expression of Nfstr was observed in all the selected tissues, but the levels varied (Fig. 4). The constitutive nature of str gene has also been reported in other MIA producing plants such as Ophiorrhiza japonica, Catharanthus roseus and Cinchona ledgeriana (Aerts et al. 1990, 1992, 1994; Canel et al. 1998; Lu et al. 2009; Sibéril et al. 2001). Among the different tissues of wild-type grown tree of N. foetida, not much variation in Nfstr expression was observed, though they showed significant differences in CPT content [Fig. 4(i), (ii)]. These results suggest that in case of a mature tree, a certain level of str expression is sufficient to synthesize and maintain CPT levels. In in vitro tissues, Nfstr expression showed a gradual increase as the differentiation progressed from callus to complete plantlets, with a concomitant increase in CPT [Fig. 4(i)]. Among in vitro tissues, callus showed just traces of the CPT. Yamazaki et al. (2003) also reported the absence of CPT in callus cultures of O. pumila. This could be since biosynthesis and accumulation of secondary metabolites generally require a degree of cellular differentiation and well-organized tissue, which does not exist in callus cultures (Sakuta and Komamine 1987).
The relation between Nfstr expression and CPT levels in Nothapodytes foetida. (i) Camptothecin levels and relative expression of Nfstr in different tissues of N. foetida. For relative Nfstr expression using real-time PCR analysis, its level in regenerated tissue sample was taken as unity (one). Bars and line with different letters (A–D) and (a–d), respectively indicate significantly different values at p ≤ 0.05. (ii) Reverse-transcription PCR of cDNA prepared from total RNA of different plant tissues using actin (housekeeping gene) and Nfstr specific primers (WT wild-type)
Wild-type tissues from a 12-year-old tree also showed higher levels of CPT compared to in vitro tissues [Fig. 4(i)]. The immature seeds accumulated the highest concentration of CPT (0.25% dry wt.) followed by roots and leaves. Similar results were reported by Fulzele and Satdive (2005), Namdeo and Sharma (2012) and Rather et al. (2018) in wild type plants of N. foetida. On the contrary, young leaves of O. pumila showed higher level of CPT accumulation than other parts such as old leaves, stem and roots, but showed relatively less str expression (Yamazaki et al. 2003). It reflects the complexity in the regulation of str expression which may diverge significantly in different plant parts. This probably could be because CPT being a secondary metabolite, plays an important role in plant defense against various pathogens and predators in young tissues. Camptothecin could be a kind of phytoalexin produced as a plant defense in response to pathogen attack. The content of CPT in different plant parts may be different probably because it may be synthesized in some plant organ, later transported and stored in other plant parts. Variation in CPT accumulation levels in different plant parts was also reported in O. pumila (Yamazaki et al. 2003) and C. acuminata (Lopez-Meyer et al. 1994).
Molecular analyses/characterization of the transgenic plants and comparison of CPT levels in O. rugosa
All the selected putative transgenic O. rugosa lines when subjected to PCR with Nfstr specific primers, showed the presence of the band of interest, while no band was observed in case of control plants (Supplementary Fig. S2). Further, RT-PCR of randomly selected transgenic lines confirmed the stable integration and expression of the gene of interest, ie. Nfstr in these plants (Fig. 5). Finally, when these lines were compared for CPT levels using HPLC, most of the transgenic lines showed higher CPT content than control plant, with best line 40 showing 1.9-fold higher CPT (0.213% dw) than control plant (0.111% dw) (Figs. 6, 7). Strictosidine synthase is known to play a vital role in TIA biosynthesis leading to the production of CPT. The present studies have shown that heterologous expression of N. foetida strictosidine synthase almost doubled camptothecin production in O. rugosa. Previously, co-expression of regulatory genes such as ORCA3 and structural gene such as geraniol-10-hydroxylase (Pan et al. 2012) as well as co-expression of multiple key enzymes involved in TIA pathway (Cui et al. 2015) showed increase in accumulation of CPT. Hence, co-overexpression of Nfstr along with other genes involved in CPT biosynthesis or with regulatory genes would be helpful for further enhancement of CPT content in CPT producing plants (Supplementary Fig. S3).
Conclusions
Strictosidine synthase (STR) plays a vital role in TIA biosynthesis. Here in the present work, we cloned complete cDNA encoding STR from N. foetida and also established the potential role of Nfstr in CPT biosynthesis in the plant. Transgenic Ophiorriza rugosa plants overexpressing Nfstr showed improved levels of camptothecin and thus may find use as an alternate and sustainable resources for the CPT.
Abbreviations
- CPT:
-
Camptothecin
- dw:
-
Dry weight
- TIA:
-
Terpenoid indole alkaloid
- STR:
-
Strictosidine synthase
References
Aerts RJ, Van der Leer T, Van der Heijden R, Verpoorte R (1990) Developmental regulation of alkaloid production in Cinchona seedlings. J Plant Physiol 13:86–91
Aerts RJ, De Waal A, Pennings EJM, Verpoorte R (1992) The distribution of strictosidine synthase activity and alkaloids in Cinchona plants. Planta 183:536–541
Aerts RJ, Gisi D, De Carolis E, De Luca V, Baumann TW (1994) Methyl jasmonate vapour increases the developmentally controlled synthesis of alkaloids in Catharanthus and Cinchona seedlings. Plant J 5:635–643
Aiyama R, Nagai H, Nokata K, Shinohara C, Swada SA (1988) Camptothecin derivative from Nothapodytes foetida. Phytochemistry 27:3663–3664
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Bernonville TD, Clastre M, Besseau S, Oudin A, Burlat V, Glévarec G, Lanoue A, Papon N, Giglioli-Guivarc’h N, St-Pierre B, Courdavault V (2015) Phytochemical genomics of the Madagascar periwinkle: unravelling the last twists of the alkaloid engine. Phytochemistry 113:9–23
Bodley AL, Cumming JN, Shapiro TA (1998) Effects of camptothecin, a topoisomerase I inhibitor, on Plasmodium falciparum. Biochem Pharmacol 55:709–711
Bustin SA, BenesV GJA, Hellemans J, Hugget J, Kubista M, Mueller R, Nolan T, Pfaffi MW, Shipley GL, Vandesompele J, Wittwer CT (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622
Canel C, Lopes-Cardoso MI, Whitmer S, van der Fits L, Pasquali G, van der Heijden R, Hoge JH, Verpoorte R (1998) Effect of over-expression of strictosidine synthase and tryptophan decarboxylase on alkaloid production by cell cultures of Catharanthus roseus. Planta 205:414–419
Cui L, Ni X, Ji Q, Teng X, Yang Y, Wu C, Zekria D, Zhang D, Kai G (2015) Co-overexpression of geraniol-10-hydroxylase and strictosidine synthase improves anti-cancer drug camptothecin accumulation in Ophiorrhiza pumila. Sci Rep 5:8227
Deepthi S, Satheeshkumar K (2016) Enhanced camptothecin production induced by elicitors in the cell suspension cultures of Ophiorrhiza mungos Linn. Plant Cell Tissue Organ Cult 124:483–493
Dellaporta J, Wood JB, Hicks A (1983) A plant DNA minipreparation: version II. Plant Mol Biol Rep 14:19–21
Fulzele DP, Sadive RS (2003) Somatic embryogenesis, plant regeneration and the evaluation of camptothecin content in Nothapodytes foetida. Vitro Cell Dev Biol Plant 39:212–216
Fulzele DP, Satdive RK (2005) Distribution of anticancer drug camptothecin in Nothapodytes foetida. Fitoterapia 76:643–648
Fulzele DP, Satdive R, Kamble S, Singh S, Singh S (2015) Improvement of anticancer drug camptothecin production by gamma irradiation on callus cultures of Nothapodytes foetida. Int. J Pharma Res Allied Sci 4:19–27
Horsch RB, Fry JE, Hoffman NL, Eichholtz D, Rogers SG, Fraley RTA (1985) Simple and general method of transferring genes into plants. Science 227:1229–1231
Huang HC, Sung PH, Do YY, Huang PL (2012) Differential expression and functional characterization of the NADPH cytochrome P450 reductase genes from Nothapodytes foetida. Plant Sci 190:16–23
Isah T, Mujib A (2015) In vitro propagation and camptothecin production in Nothapodytes nimmoniana. Plant Cell Tissue Organ Cult 121:1–10
Kutchan TM, Hampp N, Lottspeich F, Beyreuther K, Zenk MH (1988) The cDNA clone for strictosidine synthase from Rauvolfia serpentine. DNA sequence determination and expression in Escherichia coli. FEBS Lett 237:40–44
Lopez-Meyer M, Nessler CL, Mcknight TD (1994) Sites of accumulation of the antitumor alkaloid camptothecin in Camptotheca acuminate. Planta Med 60:558–560
Lu Y, Wang H, Wang W, Zhongying Q, Li L, Wang J, Zhou G, Kai G (2009) Molecular characterization and expression analysis of a new cDNA encoding strictosidine synthase from Ophiorrhiza japonica. Mol Biol Rep 36:1845–1852
Manjunatha BL, Singh HR, Ravikanth G, Nataraja KN, Shankar R, Kumar S, Uma Shaanker R (2016) Transcriptome analysis of stem wood of Nothapodytes nimmoniana (Graham) Mabb. Identifies genes associated with biosynthesis of camptothecin, an anti-carcinogenic molecule. J Biosci 41(1):119–131
Martin KP, Zhang CL, Hembrom ME, Slater A, Madassery J (2008) Adventitious root induction in Ophiorrhiza prostrata: a tool for the production of camptothecin (an anticancer drug) and rapid propagation. Plant Biotechnol Rep 2:163–169
McKnight TD, Roessner CA, Devagupta R, Scott AI, Nessler CL (1990) Nucleotide sequence of a cDNA encoding the vacuolar protein strictosidine synthase from Catharanthus roseus. Nucleic Acids Res 18:4939
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Namdeo AG, Sharma A (2012) HPLC analysis of camptothecin content in various parts of Nothapodytes foetida collected on different periods. Asian Pac J Trop Biomed 2:389–393
Pan Q, Wang Q, Yuan F, Xing S, Zhao J, Choi YH, Verpoorte R, Tian Y, Wang G, Tang K (2012) Overexpression of ORCA3 and G10H in Catharanthus roseus plants regulated alkaloid biosynthesis and metabolism revealed by NMR-metabolomics. PLoS ONE 7(8):e43038
Priel E, Showwalter SD, Blair DG (1991) Inhibition of human immunodeficiency virus (HIV-1) replication in vitro by noncytotoxic doses of camptothecin, a topoisomerase I inhibitor. AIDS Res Hum Retrovir 7(1):65–72
Rather GA, Sharma A, Pandith SA, Kaul V, Nandi U, Misra P, Lattoo SK (2018) De novo transcriptomic analyses reveals putative pathway genes involved in biosynthesis and regulation of camptothecin in Nothapodytes nimmoniana (Graham) Mabb. Plant Mol Biol 96:197–215
Roja G (2008) Micropropagation and production of camptothecin from in vitro plants of Ophiorrhiza rugosa var. decumbens. Nat Prod Res 22:1017–1023
Ruan J, Zhang J, Li M, Zhu Y, Sun L, Jin H, Su H, Xu M (2014) Dependence of UV-B-induced camptothecin production on nitrate reductase-mediated nitric oxide signaling in Camptotheca acuminata suspension cell cultures. Plant Cell Tissue Organ Cult 118:269–278
Sakuta M, Komamine A (1987) Cell growth and accumulation of secondary metabolites. In: Constabel F, Vasil IK (eds) Cell culture and somatic cell genetics of plats. Cell culture in phytochemistry, vol 4. Academic Press, San Diego, pp 49–76
Shivaprakash KN, Ramesha BT, Shaanker RU, Dayanandan S, Ravikanth G (2014) Genetic structure, diversity and long term viability of a medicinal plant, Nothapodytes nimmoniana Graham. (Icacinaceae), in protected and non-protected areas in the Western Ghats biodiversity hotspot. PLoS ONE 9:e112769
Sibéril Y, Benhamron S, Memelink J, Giglioli-Guivarc’h N, Thiersault M, Boissoni B, Doireau P, Gantet P (2001) Catharanthus roseus G-box binding factors 1 and 2 act as repressors of strictosidine synthase gene expression in cell cultures. Plant Mol Biol 45:477–488
Singh S, Premsagar K, Ramachandran V, Eapen S (2011) Transgenic Nicotiana tabacum plants expressing a fungal copper transporter gene show enhanced acquisition of copper. Plant Cell Rep 30:1929–1938
Stöckigt J, Ruppert M (1999) Strictosidine—the biosynthetic key to monoterpenoid indole alkaloid. In: Barton DHR, Nakanishi K, Meth-Cohn O, Kelly JW (eds) Comprehensive natural products chemistry: amino acids, peptides, porphyrins and alkaloids, vol 4. Elsevier, Amsterdam, pp 109–139
Stöckigt J, Zenx MH (1977) Strictosidine (Isovincoside): the key intermediate in the biosynthesis of monoterpenoid indole alkaloids. J Chem Soc Chem Commun. https://doi.org/10.1039/C39770000646
Sun Y, Luo H, Sun YLC, Song J, Niu Y, Zhu Y, Dong L, Lv A, Tramontano E, Chen S (2011) Pyrosequencing of Camptotheca acuminata transcriptome reveals putative genes involved in camptothecin biosynthesis and transport. BMC Genom 12:533
Tamura K, Stecher G, Peterson D, Filipski A, Sudhir Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Uma Shaanker R, Ramesha BT, Ravikanth G, Gunaga R, Vasudeva R, Ganeshaiah KN (2008) Chemical profiling of Nothapodytes nimmoniana for camptothecin, an important anticancer alkaloid: toward the development of a sustainable production system. In: Ramawat KG, Merillon JM (eds) Bioactive molecules and medicinal plants. Springer, London, pp 197–213
Venditto VJ, Simanek EE (2010) Cancer therapies utilizing the camptothecin: a review of the in vivo literature. Mol Pharm 7:307–349
Wall ME, Wani MC, Cook CE, Palmer KH, McPhail AT, Sim GA (1966) Plant antitumor agents. I. The isolation and structure of camptothecin, a novel alkaloid leukemia and tumor inhibitor from Camptotheca acuminata. J Am Chem Soc 88(16):3888–3890
Yamazaki Y, Urano A, Sudo H, Kitajima M, Takayama H, Yamazaki M, Aimi N, Saito K (2003) Metabolite profiling of alkaloids and strictosidine synthase activity in camptothecin producing plants. Phytochemistry 62:461–470
Acknowledgements
Work communicated in the present manuscript is supported by Department of Atomic Energy, Government of India. Authors thank Head, NABTD for his encouragement and support.
Author information
Authors and Affiliations
Contributions
SS conceived and designed the study. SS, SNK, and RKS performed the experiments and analyzed the data. SS, SNK, RKS, and DPF contributed inputs, wrote and critically reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Sergio J. Ochatt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Singh, S., Kamble, S.N., Satdive, R.K. et al. Heterologous overexpression of Nothapodytes foetida strictosidine synthase enhances levels of anti-cancer compound camptothecin in Ophiorrhiza rugosa. Plant Cell Tiss Organ Cult 141, 67–76 (2020). https://doi.org/10.1007/s11240-020-01767-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-020-01767-9