Abstract
We investigated the effect of carbon dioxide (CO2)-ambient (350 µmol CO2 mol−1) and CO2-enriched (1500 µmol CO2 mol−1) conditions of in vitro photoautotrophic system on two cultivars, ‘RRIM600’ and ‘RRIT413’ of rubber tree (Hevea brasiliensis) in an acclimatization process of 45 days. Survival percentage of in vitro rubber tree plantlets derived from somatic embryos under ambient CO2 was better than those under CO2-enriched conditions, especially in cv. ‘RRIT413’. Subsequently, the survival rate of ex vitro transplanted plantlets was similar to the in vitro plantlets and abnormal morphological characters such as light-green leaves (SPAD), small leaves in cv. ‘RRIT413’ acclimatized under CO2-enriched conditions were demonstrated 30 days after the plantlets were transferred into the soil. Maximum quantum yield of PSII, photon yield of PSII, stomatal conductance and transpiration rate in cv. ‘RRIT413’ acclimatized under CO2-enriched conditions were sharply declined by 39.0, 50.6, 47.1 and 45.8%, respectively as compared to those acclimatized under ambient CO2 conditions. In contrast, the in vitro acclimatized plantlets of cv. ‘RRIM600’ were un-responsive under both ambient- and enriched-CO2 conditions. In conclusion, genotypic dependent in response to CO2 enriched conditions in in-vitro acclimatization of rubber tree plantlets was evidently demonstrated as a key result to regulate plant growth and development in ex vitro environments. Interestingly, soluble sugar contents (sucrose, glucose and fructose) were increased after transplanting the plantlets of cv. ‘RRIM600’ acclimatized under CO2-enriched condition into the soil and thus, can be considered as an adaptive indicator of ex vitro adaptation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant micropropagation is a simple technique to produce a large number of uniform, genetic fidelity (true-to-type plants), and healthy plantlets. A large number of protocols for plant micropropagation i.e. organogenesis (direct or indirect organogenesis) and somatic embryogenesis are available in the literature (Rani and Raina 2000; Beruto and Debergh 2004; Haque and Ghosh 2013a, b, 2016). In vitro microclimates, i.e. enriched macro- and micro-mineral nutrients, closed vessel without ventilation, high relative humidity and low light intensity cause the morphological and physiological disorders such as hyperhydricity, stomatal disorder, loss of photosynthetic abilities and abnormal growth and development (Hazarika 2006; Kumar and Rao 2012). Subsequently, the low survival rate in transplanting process has been a major concern, especially in the woody plant species. Low survival rate (< 60%) in aquatic rotula (Martin 2003), strawberry tree (Mereti et al. 2002) and black berry (Liu and Pijut 2008), has led to the high costs required for plant micropropagation (Pence 2011). In vitro acclimatization is a procedure to train the plantlets grown in the closed vessels (Chandra et al. 2010). Photoautotrophic growth (sugar free medium) of in vitro plantlets, before transplanting it to the soil, results in a healthy plantlet production (Xiao et al. 2011; Hoang et al. 2017). However, the microenvironments such as enriched-CO2, increased air-ventilation rate, porous supporting material, reduced macronutrient supply, high light intensity, controlled relative humidity and reduced ethylene gas in the culture vessel, in photoautotrophic culture system have been well established (Seon et al. 2000; Carvalho et al. 2001; Kadleček et al. 2001). Physiological and morphological adaptations, i.e. root development, prevention of water loss by stomatal function (low transpiration rate), improved CO2 assimilation (high net photosynthetic rate), new leaf/root emerge, leaf expansion (leaf area), increased shoot and root biomass and greenish leaves of in vitro acclimatized plantlets are very important indices of the high survival and rapid growth rate of the plantlets prior to their ex vitro (Xiao et al. 2011; Hoang et al. 2017). Recently, CO2-enriched in vitro acclimatization has been reported as an effective strategy to produce healthy plantlets by adapting them to the ex vitro conditions (Shin et al. 2014; Pérez-Jiménez et al. 2015). In contrast, some plant species are very sensitive to high CO2 condition, leading to impaired physio-morphological performances of CO2-enriched in vitro acclimatization (Carvalho et al. 2002; Osário et al. 2005).
In rubber tree, there are many evidences of in vitro micropropagation via nodal cutting, and somatic embryogenesis from inner integuments and roots. In vitro seedling production of rubber tree has been well investigated with high survival rate (80%) (Sanguansermsri et al. 2015). However, the information on survival percentage of transplanted plantlets in somatic embryos derived from inner integuments, root tissues and anther initial materials of rubber tree is still lacking and needs to be investigated for commercial production (Zhou et al. 2010; Karumamkandathil et al. 2015; Nor Mayati 2015). The aim of this investigation was to improve the survival percentage and physiological and morphological adaptations of in vitro acclimatized plantlets of rubber tree under CO2-enriched conditions when transferred to greenhouse environments.
Materials and methods
Plant materials and in vitro culture
Somatic embryos derived from the inner integuments of Hevea brasiliensis cvs. ‘RRIM600’ and ‘RRIT413’ immature seeds via callus induction on MH-IN medium under darkness (Carron et al. 1989), somatic embryogenesis using MH-DEN medium under dark condition and embryos formation by MH-MAT and MH-GER under 60 ± 5 µmol m−2 s−1 photosynthetic photon flux density (PPFD) for 16 h day−1 photoperiod provide by fluorescent lamps prior to plantlet regeneration were used for the investigation (Prommee et al. 2014; Fig. 1). Somatic embryos were selected and cultured on the modified MH-PL medium for plantlet conversion. Plantlets were grown under conditions of 25 ± 2 °C air temperature, 60 ± 5% relative humidity (RH), and 60 ± 5 µmol m−2 s−1 photosynthetic proton flux density (PPFD) provided by fluorescent lamps (TLD 36W/84 Cool White 3350 Im, Philips, Bangkok, Thailand) with a 16-h day−1 photoperiod. The entire scheme of the development of the plantlets by somatic embryogenesis from the inner integuments of immature seeds has been given in Fig. 1.
A scheme of somatic embryos derived from inner integuments of rubber tree. Inner integument explants cultured on modified MH medium (a), callus induction (b), somatic embryos (c), plantlets derived from somatic embryogenesis (d), initial plantlets (e) and 3 month-old plant cv. ‘RRIM600’ in the plastic bag containing garden soil (f)
In vitro photoautotrophic acclimatization and ex vitro transplantation
Plantlets (5 ± 0.5 cm in shoot height) in the test tube (Fig. 1e) were chosen as the test plant material for in vitro photoautotrophic acclimatization. Healthy plantlets were transferred to MS (Murashige and Skoog 1962) sugar-free liquid medium (photoautotrophic conditions), using vermiculite as the supporting material in vented vessels. The culture vessels containing the plantlets were incubated in an EYELA Plant Growth Incubator (model FLI-301NH, Tokyo, Japan) in CO2-enriched culture room (1500 ± 50 µmol CO2 mol−1) or in the culture room with ambient CO2 conditions (350 ± 50 µmol CO2 mol−1) at a temperature shift of 28 ± 2 °C for 16 h in daytime and 25 ± 2 °C for 8 h in nighttime, 60 ± 5% RH, and 120 ± 5 µmol m−2 s−1 PPFD provided by fluorescent lamps with a 16-h day−1 photoperiod for 45 days. The survived plantlets were directly transplanted into the plastic bags (10 cm in diameter and 30 cm in length) containing 2 kg mixed soil (EC = 2.69 dS m−1; pH 5.5; organic matter = 10.36%; total nitrogen = 0.17%; total phosphorus = 0.07%, and total potassium = 1.19%). The plantlets planted in the plastic bag containing soil were incubated in a greenhouse at 500–1000 µmol m−2 s−1 photosynthetic photon flux density (PPFD), 10 h d−1 photoperiod, 28 ± 2 °C temperature, and 80 ± 5% relative humidity for 30 days.
Biochemical and physiological changes
Soluble sugars (sucrose, glucose and fructose) in the leaf tissues were assayed following the modified method of Karkacier et al. (2003). In brief, 50-mg of plant sample was ground in a mortar with liquid nitrogen. One millilitre of nanopure water was added and centrifuged at 12,000 rpm for 15 min. The supernatant was collected and filtered through a 0.45 µm membrane filter (VertiPure™, Vertical®). Twenty micro-litters of the filtrate was injected into a Waters HPLC equipped with a MetaCarb 87C column (300 × 7.8 mm) (Varian Inc., Palo Alto, USA) and an Agilent MetaCarb 87C guard column (Part No. A5201, Agilent Technologies, Inc., Santa Clara, CA, USA). Column was incubated in the heat jacket controlled the temperature at 85 °C. Deionized water was used as the mobile phase at a flow rate of 0.5 mL min−1. The online detection was performed using a Waters 410 differential refractrometer detector to control the temperature at 40 °C and the data was analysed by Empower® software. Sucrose, glucose and fructose (Fluka, USA) were used as the standards.
Chlorophyll content in the second fully developed leaf from the top was measured using Chlorophyll Meter (Model SPAD-520Plus, Konica Minolta, Osaka, Japan). Chlorophyll fluorescence emission was measured from the adaxial surface on the leaf using a fluorescence monitoring system (model FMS 2; Hansatech Instruments Ltd., Norfolk, UK) in the pulse amplitude modulation mode (Loggini et al. 1999; Maxwell and Johnson 2000). In brief, a leaf, kept in dark for 30 min was initially exposed to the modulated measuring beam of far-red light (LED source) with typical peak at 735 nm. Original (F0) and maximum (Fm) fluorescence yields were measured under weak modulated red light (< 85 µmol m−2 s−1) with 1.6 s pulses of saturating light (> 1500 µmol m−2 s−1 PAR) and calculated using FMS software for Windows®. The variable fluorescence yield (Fv) was calculated using the equation: Fv = Fm − F0. The ratio of variable to maximum fluorescence (Fv/Fm) was calculated as the maximum quantum yield of PSII photochemistry. The photon yield of PSII (ΦPSII) in the light was calculated as: ΦPSII = (Fm′ − F)/Fm′ after 45 s of illumination, when steady state was achieved. Net photosynthetic (Pn; µmol m−2 s−1) and transpiration rate (E; mmol m−2 s−1) were measured using a Portable Photosynthesis System with an Infra-red Gas Analyzer (Model LI 6400, LI-COR® Inc., Lincoln, Nebraska, USA). The gs and E were measured continuously by monitoring the content of the air entering and existing in the IRGA headspace chamber, according to Cha-um et al. (2007). The air-flow rate of IRGA chamber was fixed at 500 µmol s−1 and chamber temperature was set at 28 °C. The light intensity was adjusted to 1000 µmol m−2 s−1 PPFD of 6400-02B red-blue LED light source.
Survival percentage and morphological characters
Survival percentage of in vitro acclimatized plantlets under photoautotrophic growth conditions was recorded after incubation in the plant growth incubator at a temperature shift of 28 ± 2 °C for 16 h in daytime and 25 ± 2 °C for 8 h in nighttime, 60 ± 5% RH, and 120 ± 5 µmol m−2 s−1 PPFD provided by fluorescent lamps with a 16-h day−1 photoperiod for 45 days. Survival percentage of ex vitro transplanted plants was calculated 30 days after transplanting the plantlets in the green house. Plant height, number of leaves, and leaf length and width of ex vitro transplanted plants were measured.
Experiment design and statistical analysis
The experiment was arranged as 2 × 2 factorial in Completely Randomized Design (CRD) with five replicates (n = 5). Survival percentage was calculated in each treatment with five replicates (20 plantlets per replicate). The mean values obtained from four treatments were compared using Tukey’s HSD and analyzed by SPSS software.
Results and discussion
In vitro acclimatization
Overall morphological characteristics of the survived plantlets were similar irrespective of the cultivars and acclimatization conditions. Acclimatized plantlets of cv. ‘RRIT413’ grown under CO2 enriched conditions showed the toxic symptoms, i.e. leaf burn, stem die back, and death, leading to small leaves (leaf length and leaf width) after transplanting to ex vitro (Table 1). Survival percentage of in vitro acclimatized plantlets in the ambient CO2 (350 ± 50 µmol CO2 mol−1) was greater than those acclimatized in the CO2-enrichment, especially in cv. ‘RRIT413’ (Fig. 2a). Rubber tree plantlets of cv. ‘RRIT413’ was sensitive to high CO2 condition (1500 ± 50 µmol CO2 mol−1), resulting in a low survival rate (only 20.0%). Therefore, it was improved using in vitro photoautotrophic acclimatization under ambient CO2 conditions for both the cvs. ‘RRIM600’ (66.5%) and ‘RRIT413’ (75.0%).
Enriched (1500 µmol CO2 mol−1)-CO2 photoautotrophic condition for 45 days (a) and survival percentage of transplanted plants into plastic bag containing mixed soil for 30 days (b). Data presented as mean ± SE. Different letters in each column show significant difference at p ≤ 0.01 by Tukey’s HSD test
Overall growth performance i.e. new shoot initiation, leaf expansion, number of leaves and leaf area of survived rubber tree plantlets under in vitro photoautotrophic acclimatization was a key index to regulate physiological adaptation in both in vitro and ex vitro conditions, leading to lift-up survival rate of acclimatized plantlets. In woody species, CO2 enrichment of in vitro photoautotrophic acclimatization plays a critical role in overall growth elevation (Morini and Melai 2003; Vyas and Purohit 2003; Cha-um et al. 2011). Subsequently, survival percentage of the acclimatized plantlets under CO2-enriched condition is high as compared to those under ambient CO2 (Li et al. 2001; Pérez-Jiménez et al. 2015). In contrast, rubber tree plantlets were familiar to ambient CO2 condition in the in vitro photoautotrophic acclimation, especially cv. ‘RRIT413’. In carob tree also, the responses of acclimatized plantlets to the CO2 concentrations were cultivar dependent. It was found that carob tree cv. ‘Mulata’ was very sensitive to the CO2 enrichment (810 µmol CO2 mol−1) when compared with cv. ‘Galhosa’, identified by a decline of the net photosynthetic rate (Pn) (Osório et al. 2005). It was possible that high amount of CO2 (1500 ± 50 µmol CO2 mol−1) under in vitro conditions negatively affected the CO2 assimilation, and consequently retarded the plant growth and development. In Hosta ‘Blue Vision’, growth and net photosynthetic rate (Pn) of in vitro acclimatized plantlets were evidently promoted when grown under 2800 µmol CO2 mol−1 (Toler et al. 2003). The optimum intracellular CO2 concentration (Ci) that promotes Pn in rubber tree cultivars grown in the field trial was identified as “1000–1200 µmol CO2 mol−1” (Kositsup et al. 2010; Nataraja and Jacob 1999). Alternatively, an increased light intensity during in vitro acclimatization under CO2 enrichment was a minimal requirement for ex-vitro adaptation (Cavalho et al. 2002a, b; Shin et al. 2014; Toler et al. 2003).
Ex vitro adaptation
Plant morphological characters of ex vitro transplanted rubber tree cvs. ‘RRIM600’ and ‘RRIT413’ are given in Fig. 3. Rubber tree plantlets derived from in vitro acclimatization under ambient CO2 condition were represented as vigorous donor plant, as indicated by elongated stem, fully expanded, dark-green leaves and continuous branching. In contrast, the short stem, tiny and light-green leaves and lesser branching were found in plantlets acclimatized under CO2 enriched conditions, especially in cv. ‘RRIT413’, resulting in a low survival percentage (only 25.0%) (Fig. 2b). Survival percentage of plantlets derived from in vitro acclimatization under ambient CO2 condition was significantly higher in both rubber tree cultivars, ‘RRIM600’ (69.2%) and ‘RRIT413’ (60.0%) (Fig. 4b). In rubber tree, the low survival rate of the transplanted plantlets in ex vitro conditions was a major concern, especially in case of the plantlets derived from somatic embryogenesis (Zhou et al. 2010; Karumamkandathil et al. 2015) and the anther culture process (Nor Mayati 2015). In general, the in vitro seedlings of rubber tree has been reported as “easy transplanted material” with 80.0% survival percentage in the greenhouse for a month (Sanguansermsri et al. 2015). In a previous report, only two plantlets derived from clone ‘SH/RD1-B2’ and one plantlet each from the clones ‘SH/RD1-C1’, ‘SH/RD1-E2’, ‘B5/RD1-B1’ and ‘B5/RD1-E2’ survived successfully in the greenhouse, but leading to the wilting and death when cultivated for long term (Nor Mayati 2015). In present study, survival percentage of rubber tree plantlets derived from in vitro acclimatization under ambient CO2 condition was significantly greater than those under CO2 enrichment condition. In chestnut hybrid, survival rate (> 82%) of acclimatized plantlets under ambient CO2 (350 µL L−1) and enriched-CO2 (700 µL L−1) with low light (150 µmol m−2 s−1 PPF) or high light intensities (300 µmol m−2 s−1 PPF) was indifferent (Carvalho and Amâncio 2002a). In contrast, survival percentage of in vitro acclimatized plantlets of apple under CO2-enriched condition (1000 µmol CO2 mol−1) with 100 µmol m−2 s−1 PPF was 80%, which was better than those under ambient CO2 (only 50% survival) (Li et al. 2001). Moreover, 100% active growth of acclimatized Cynara scolymus plantlets under CO2-enriched conditions (800 µmol CO2 mol−1) was found, whereas only 42.3% of acclimatized plantlets under ambient CO2 condition (380 ± 40 µmol CO2 mol−1) could survive (Pérez-Jiménez et al. 2015).
SPAD (a), maximum quantum yield of PSII (Fv/Fm) (b) and quantum efficiency of PSII (ΦPSII) (c) of rubber tree derived from somatic embryo cvs. ‘RRIM600’ and ‘RRIT413’ acclimatized plantlets under ambient (350 µmol CO2 mol−1) or enriched (1500 µmol CO2 mol−1)-CO2 photoautotrophic condition for 45 days, subsequently transplanted into plastic bag containing mixed soil for 30 days. Data presented as mean ± SE. Different letters in each column show significant difference at p ≤ 0.01 by Tukey’s HSD test
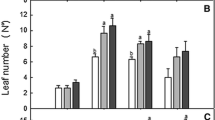
Plant height of ex vitro transplantions derived from different CO2 concentrations was indifferent. In addition, leaf characteristics of rubber tree cv. ‘RRIM600’ acclimated in vitro under enriched CO2 conditions were similar when compared to the ambient CO2 conditions (Table 1). In contrast, number of leaves, leaf length and width of rubber tree cv. ‘RRIT413’ in ex vitro transplantation declined significantly by 2.00, 2.89, and 2.17 fold, respectively, when exposed to in vitro acclimatization under CO2 enriched conditions (Table 1). Overall growth characters, number of leaves, leaf length and width of rubber tree cv. ‘RRIM600’ acclimated in vitro under CO2 enriched conditions were unaffected while those in cv. ‘RRIT413’ were declined. Similarly, new leaves of acclimatized plantlets of carob tree cvs. ‘Galhosa’ and ‘Mulata’ under CO2 enrichment emerged slowly when compared with those under ambient CO2 (Osório et al. 2005). In contrast, leaf area (in case of Cynara scolymus) and specific leaf area (in case of chestnut hybrid) of acclimatized plantlets under CO2 enrichment were improved (Carvalho and Amâncio 2002a; Pérez-Jiménez et al. 2015). The growth response of in vitro acclimatized plantlets under CO2 enrichment depends on genetic factors (Cavalho et al. 2002a, b; Osório et al. 2005).
Chlorophyll content (SPAD) in the leaf tissues of in vitro acclimatized plantlets of rubber tree cv. ‘RRIM600’ under ambient CO2 and enriched-CO2 conditions was stabilized when transplanted to the greenhouse for 30 days (Fig. 4a), resulting in the maximum quantum yield of PSII (Fv/Fm, Fig. 4b) and photon yield of PSII (ΦPSII; Fig. 4c). In contrast, chlorophyll degradation was evidently found in the in vitro acclimatized plantlets cv. ‘RRIT413’ under enriched-CO2 conditions (47.9% degradation), causing on Fv/Fm and ΦPSII to diminish by 39.0 and 50.6%, respectively (Fig. 4). Total chlorophyll pigment (SPAD),chlorophyll fluorescence (Fv/Fm and ΦPSII) and Pn in the leaf tissues of in vitro acclimatized rubber tree cv. ‘RRIT413’ was sensitive to ex vitro environments, whereas those in cv. ‘RRIM600’ were maintained. Previously, chlorophyll degradation with subsequent reduction in Fv/Fm, ΦPSII and Pn was evidently found in ex vitro transplantation of grapevine cv. ‘Touriga’ (Carvalho et al. 2002), chestnut hybrid (Carvalho and Amâncio 2002a) and carob tree cvs. ‘Galhosa’ and ‘Mulata’ (Osório et al. 2005). Photosynthetic pigments of ex vitro plantlets in tobacco acclimatization were unaffected by enriched-CO2 supply (Pospíšilová et al. 2000). In contrast, chlorophyll a and chlorophyll b in Cynara scolymus acclimatized plantlets were enhanced by CO2 enrichment (800 µmol CO2 mol−1) (Pérez-Jiménez et al. 2015).
Similarly, net photosynthetic rate (Pn), stomatal conductance (gs) and transpiration rate (E) of in vitro acclimatized plantlets cv. ‘RRIM600’ under ambient CO2 and enriched-CO2 conditions were maintained (Fig. 5). In cv. ‘RRIT413’, Pn was retained (Fig. 5a), whereas gs (Fig. 5b) and E (Fig. 5c) were sharply decreased by 47.1 and 45.8%, respectively. The photosynthetic responses of in vitro acclimatization under CO2 enrichment depend on the plant species and the degree of CO2 concentrations (Zhou et al. 2005; Shin et al. 2014). The present study demonstrated that the physiological adaptation of rubber tree cv. ‘RRIT413’ acclimatized under CO2-enriched condition was very poor, leading to low survival rate and abnormal growth characters in ex vitro environments. High CO2 conditions during in vitro acclimatization might regulate ethylene production, leading to chlorophyll degradation, dark respiration, stomatal closure, photosynthesis inhibition and leaf senescence (Reuveni and Bugbee 1997). Soluble sugar enrichment in rubber tree cv. ‘RRIM600’ acclimatized plantlets under CO2 enriched condition was a good indicator to identify “healthy plantlets”, suitable for ex vitro environments (Carvalho and Amâncio 2002b; Carvalho et al. 2002; Shin et al. 2014).
Net photosynthetic rate a, stomatal conductance b and transpiration rate c of rubber tree derived from somatic embryo cvs. ‘RRIM600’ and ‘RRIT413’ acclimatized plantlets under ambient (350 µmol CO2 mol−1) or enriched (1500 µmol CO2 mol−1)-CO2 photoautotrophic condition for 45 days, subsequently transplanted into plastic bag containing mixed soil for 30 days. Data presented as mean ± SE. Different letters in each column show significant difference at p ≤ 0.01 by Tukey’s HSD test
Soluble sugars in the leaf tissues of in vitro photoautotrophically acclimatized plantlets of cvs. ‘RRIM600’ and ‘RRIT413’ was in the order sucrose > fructose > glucose (Table 2). Interestingly, sucrose, glucose, fructose and total soluble sugar in the leaf tissues of in vitro acclimatized plantlets of cv. ‘RRIM600’ under enriched-CO2 conditions were increased by 3.6, 12.4, 6.3 and 4.9 fold, respectively (Table 1). In contrast, these sugar types were constant in the leaf tissues of in vitro acclimatized plantlets cv. ‘RRIT413’ under both ambient-CO2 and enriched-CO2 conditions. Soluble sugars such as glucose, fructose and sucrose are the primary products of photosynthesis that play a central role in providing energy, sensing/signaling networks in building blocks for plant growth and development and osmotic adjustment under fluctuation of microenvironments (Smeekens 2000; Rolland et al. 2002; Gupta and Kaur 2005; Smeekens et al. 2009).
In conclusion, survival percentage of in vitro photoautotrophic acclimatization under ambient CO2 condition was better in rubber tree cvs. ‘RRIM600’ and ‘RRIT413’, subsequently leading to rapid physiological adaptation (photosynthetic abilities) and improved growth performance in ex vitro environments. Soluble sugar enrichment in the leaf tissues of transplanted plants can be considered a biochemical indicator for the rapid adaptation of acclimatized plantlets in ex vitro conditions, especially in case of rubber tree cv. ‘RRIM600’.
References
Beruto M, Debergh P (2004) Micropropagation of Ranunculus asiaticus: a review and perspectives. Plant Cell Tiss Org Cult 77:221‒230
Carron MP, Enjalric F, Lardet L, Deschamps A (1989) Rubber (Hevea brasiliensis Müll. Arg.). In: Bajaj YPS (ed) Biotechnology in agriculture and forestry, vol 5, Trees II. Springer, Berlin, pp 222–245
Carvalho L, Amâncio S (2002a) Effect of ex vitro conditions on growth and acquisition of autotrophic behaviour during the acclimatisation of chestnut regenerated in vitro. Sci Hortic 95:151‒164
Carvalho L, Amâncio S (2002b) Antioxidant defense system in plantlets transferred from in vitro to ex vitro: effects of increasing light intensity and CO2 concentration. Plant Sci 162:33‒40
Carvalho L, Osório ML, Chaves MM, Amâncio S (2001) Chlorophyll fluorescence as an indicator of photosynthetic functioning of in vitro grapevine and chestnut plantlets under ex vitro acclimatization. Plant Cell Tiss Org Cult 67:271‒280
Carvalho L, Santos P, Amâncio S (2002) Effect of light intensity and CO2 concentration on growth and the acquisition of in vivo characteristics during acclimatization of grapevine regenerated in vitro. Vitis 41:1–6
Chandra S, Bandopadhyay R, Kumar V, Chandra R (2010) Acclimatization of tissue cultured plantlets: from laboratory to land. Biotechnol Lett 32:1199‒1205
Cha-um S, Supaibulwatana K, Kirdmanee C (2007) Glycinebetaine accumulation, physiological characterizations and growth efficiency in salt-tolerant and salt-sensitive lines of indica rice (Oryza sativa L. ssp. indica) in response to salt stress. J Agron Crop Sci 193:157‒166
Cha-um S, Chanseetis C, Chintakovid W, Pichakum A, Supaibulwatana K (2011) Promoting root induction and growth of in vitro macadamia (Macadamia tetraphylla L. ‘Keaau’) plantlets using CO2-enriched photoautotrophic conditions. Plant Cell Tiss Org Cult 106:435‒444
Gupta AK, Kaur N (2005) Sugar signalling and gene expression in relation to carbohydrate metabolism under abiotic stress in plants. J Biosci 30:761‒776
Haque SM, Ghosh B (2013a) Field evaluation and genetic stability assessment of regenerated plants produced via direct shoot organogenesis from leaf explant of an endengered ‘Asthma Plant’ (Tylophora indica) along with their in vitro conservation. Nat Acad Sci Lett 36:551‒562
Haque SM, Ghosh B (2013b) High frequency microcloning of Aloe vera and their true-to-type conformity by molecular cytogenetic assessment of two years old field growing regenerated plants. Bot Stud 54:46
Haque SM, Ghosh B (2016) High-frequency somatic embryogenesis and artificial seeds for mass production of true-to-type plants in Ledebouria revoluta: an important cardioprotective plant. Plant Cell Tiss Org Cult 127:71‒83
Hazarika BN (2006) Morpho-physiological disorders in in vitro culture plants. Sci Hortic 108:105‒120
Hoang NN, Kitaya Y, Morishita T, Endo R, Shibuya T (2017) A comparative study on growth and morphology of wasabi plantlets under the influence of the micro-environment in shoot and root zones during photoautotrophic and photomixotrophic micriopropagation. Plant Cell Tiss Org Cult 130:255‒263
Kadleček P, Tichá I, Haisel D, Čapková V, Schäfer C (2001) Importance of in vitro acclimatization and growth. Plant Sci 161:695‒701
Karkacier M, Ebras M, Uslu MK, Aksu M (2003) Comparison of different extraction and detection methods for sugars using amino-bonded phase HPLC. J Chromatog Sci 41:331‒333
Karumamkandathil R, Uthum TK, Sankaran S, Unnikrishnan D, Saha T, Nair SS (2015) Genetic and epigenetic uniformity of polyembryony derived multiple seedlings of Hevea brasiliensis. Protoplasma 252:783–796
Kositsup B, Kasemsap P, Thanisawanyangkura S, Chairungsee N, Satakhun D, Teerawatanasuk K, Ameglio T, Thaler P (2010) Effect of leaf age and position on light-saturated CO2 assimilation rate, photosynthetic capacity, and stomatal conductance in rubber trees. Photosynthetica 48:67–78
Kumar K, Rao IU (2012) Morphophysiologicals problems in acclimatization of micropropagated plants in-ex vitro conditions: a reviews. J Orn Hortic Plant 2:271‒283
Li RY, Murthy HN, Kim SK, Paek KY (2001) CO2-enrichment and photosynthetic photon flux affect the growth of in vitro-cultured apple plantlets. J Plant Biol 44:87‒91
Liu X, Pijut PM (2008) Plant regeneration from in vitro leaves of mature black cherry (Prunus serotina). Plant Cell Tiss Org Cult 94:113‒123
Loggini B, Scartazza A, Brugnoli E, Navari-Izzo F (1999) Antioxidant defense system, pigment composition, and photosynthetic efficiency in two wheat cultivars subjected to drought. Plant Physiol 119:1091‒1099
Martin KP (2003) Rapid in vitro multiplication and ex vitro rooting of Rotula aquatica Lour., a rare rhoeophytic woody medicinal plant. Plant Cell Rep 21:415‒420
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence-a practical guide. J Exp Bot 51:659‒668
Mereti M, Grigoriadou K, Nanos GD (2002) Micropropagation of the strawberry tree, Arbutus unedo L. Sci Hortic 93:143‒148
Morini S, Melai M (2003) CO2 dynamics and growth in photoautotrophic and photomixotrophic apple cultures. Biol Plant 47:167‒172
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nataraja KN, Jacob J (1999) Clonal differences in photosynthesis in Hevea brasiliensis Müll. Arg Photosyth 36:89‒98
Nor Mayati CH (2015) Effects of zeatin and kinetin on in vitro regeneration of Hevea brasiliensis RRIM 2025. J Rubber Res 18:127‒138
Osório ML, Gonçalves S, Osório J, Romano A (2005) Effects of CO2 concentration on acclimatization and physiological responses of two cultivars of carob tree. Biol Plant 49:161‒167
Pence VC (2011) Evaluating cost for the in vitro propagation and preservation of endangered plants. In Vitro Cell Dev Biol-Plant 47:176‒187
Pérez-Jiménez M, López-Pérez AJ, Otálora-Alcón G, Marín-Nicolás D, Piñero MC, del Amor FM (2015) A regime of high CO2 concentration improves the acclimatization process and increases plant quality and survival. Plant Cell Tiss Org Cult 121:547–557
Pospíšilová J, Haisel D, Synková H, Čatský J, Wilhelmová N, Plzáková Š, Procházková D, Šrámek F (2000) Photosynthetic pigments and gas exchange during ex vitro acclimation of tobacco plants as affected by CO2 supply and abscisic acid. Plant Cell Tiss Org Cult 61:125‒133
Prommee W, Sreenakkiang W, Te-chato S (2014) Somatic embryogenesis and plant regeneration from inner integument of Hevea brasiliensis. In: International conference on rubber (2014ICR), Thaksin University, Phatthalung Campus
Rani V, Raina N (2000) Genetic fidelity of organized meristem-derived micropropagated plants: a critical reappraisal. In Vitro Cell Dev Biol‒Plant 36:319‒330
Reuveni J, Bugbee B (1997) Very high CO2 reduces photosynthesis, dark respiration and yield in wheat. Ann Bot 80:539‒546
Rolland F, Moore B, Sheen J (2002) Sugar sensing and signaling in plants. Plant Cell 14:S185–S205
Sanguansermsri M, Khamthup P, Meechana K, Wongsawad M, Buddharaksa P (2015) In vitro culture of Hevea brasiliensis (rubber tree) embryo. Naresuan Phayao J 8:155–158
Seon JH, Cui YY, Kozai T, Paek KY (2000) Influence of in vitro growth conditions on photosynthetic competence and survival rate of Rehmannia glutinosa plantlets during acclimatization period. Plant Cell Tiss Org Cult 61:135‒142
Shin KS, Park SY, Paek KY (2014) Physiological and biochemical changes during acclimatization in a Dorita enopsis hybrid cultivated in different microenvironments in vitro. Environ Exp Bot 100:26‒33
Smeekens S (2000) Sugar-induced signal transduction in plants. Ann Rev Plant Biol 51:49‒81
Smeekens S, Ma J, Hanson J, Rolland F (2009) Sugar signals and molecular networks controlling plant growth. Curr Opin Plant Biol 13:1‒6
Toler JE, Adelberg JW, Bishop D (2003) Growth and net photosynthetic rates of Hosta ‘Blue Vision’ during acclimatization in bright, natural light with CO2 enrichment. In Vitro Cell Dev Biol-Plant 39:338–342
Vyas S, Purohit SD (2003) In vitro growth and shoot multiplication of Wrightia tomentosa Roem et Schult in a controlled carbon dioxide environment. Plant Cell Tiss Org Cult 75:283‒286
Xiao Y, Niu G, Kozai T (2011) Development and application of photoautotrophic micropropagation plant system. Plant Cell Tiss Org Cult 105:149‒158
Zhou YH, Guo DP, Zhu ZJ, Qian QQ (2005) Effects of in vitro rooting environments and irradiance on growth and photosynthesis of strawberry plantlets during acclimatization. Plant Cell Tiss Org Cult 81:105‒108
Zhou QN, Jiang ZH, Huang TD, Li WG, Sun AH, Dai XM, Li Z (2010) Plant regeneration via somatic embryogenesis from root explants of Hevea brasiliensis. Afri J Biotechnol 9:8168–8173
Acknowledgements
The authors wish to thank Rubber Research Institute of Thailand, Department of Agricultural, Ministry of Agricultural and Cooperative, as funding source and partially support by National Science and Technology Development Agency.
Author information
Authors and Affiliations
Contributions
The experiment design, statistical analysis and manuscript preparation were prepared by SC, somatic embryogenesis of rubber tree was provided by WP, in vitro acclimatization and transplantation was processed by TS, soluble sugar assay was done by CT and physiological and morphological data was recorded by RT. All authors were involved with editors of all versions, and agreed to the final version for publication, assuming public responsibility for the results.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Sergio J. Ochatt.
Rights and permissions
About this article
Cite this article
Tisarum, R., Samphumphung, T., Theerawitaya, C. et al. In vitro photoautotrophic acclimatization, direct transplantation and ex vitro adaptation of rubber tree (Hevea brasiliensis). Plant Cell Tiss Organ Cult 133, 215–223 (2018). https://doi.org/10.1007/s11240-017-1374-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-017-1374-5