Abstract
Development of modern agriculture and biotechnology is closely connected with the use of novel and effective genetic engineering methods. Presently, non-viral nanoparticle-mediated plant transformation methods gain more attention because of their stability, safety, and convenience of performance. In this work, new polymeric dimethylaminoethyl metacrylate (DMAEM)-based polymers were synthesized and investigated for their properties in gene delivery. Formation of stable complexes between TN 83/6, TN 84/5, DLM-9-DM and LM-8-DM polymers and plasmid DNA, as well as the DNA protection by the PDMAEM polymers against nuclease degradation were confirmed by electrophoresis in agarose gel. In addition, model organisms Allium cepa and Nicotiana tabacum L. were studied to evaluate cytotoxic effect of the PDMAEM carriers. The created PDMAEM-based carriers were effective in delivery of plasmid DNA into moss and tabacco protoplasts (obtaining stable transformants of Ceratodon purpureus moss, as well as in transient expression of the reporter yfp gene product in N. tabacum protoplasts). Thus, novel PDMAEM-based polymers were shown to be promising carriers for delivery of DNA into plant cells, and carriers possess high potential for further applications in this field.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The development of efficient transformation systems has led to a remarkable progress in biotechnology and agriculture. The generation of transgenic plants has allowed for new insights into gene functions. Several methods are currently available for introducing exogenous DNA into plant cells. Agrobacterium tumefaciens-mediated transformation is widely used for the genetic transformation of plants. The transformation efficiency can be attributed to the direct infection of the plant with Agrobacterium without going through protoplast isolation and regeneration steps in the transformation process (Potrykus and Spangenberg 1995). However, transformation efficiency of the Agrobacterium-mediated method of plant transformation strongly depends on a susceptibility of the host plant (Gelvin 2003; Zhang et al. 1999). Polyethylene glycol (PEG)-mediated DNA uptake by protoplasts has been proposed as an alternative transformation method for both higher plants (Armstrong et al. 1990; Shen et al. 2014; Torney et al. 2007) and mosses (Zeidler et al. 1999). However, the efficiency of gene delivery based on using this method is rather low. In addition to low efficiency, PEG-mediated transformation requires complicated tissue and protoplast manipulations, including a procedure of protoplast regeneration under sterile conditions (Jing et al. 2003). The nanoparticle bombardment or the biolistic transformation is another method widely used for transformation of higher plants and mosses (Smidkova et al. 2010). Particles used for such bombardment are typically made of tungsten, gold, gold-capped mesoporous silica nanoparticles (Cove et al. 2009a; Torney et al. 2007). That method could shorten the procedure to minimum, thereby avoiding microbial contamination. However, the biolistic transformation efficiency considerably depends on the size and conformation of the exogeneous DNA (Deng et al. 2001). Furthermore, the nanoparticle size, shape and surface coating influence their biological properties. Nanoparticles could inhibit plant tissue culture system, effected the biochemical processes and/or promote the growth of culture plantlets (Sarmast et al. 2015).
The use of the nanoparticle mediated plant transformation has a potential for further improvement. Non-viral vectors have recently gained an increased attention due to their stability, safety. These vectors can be applied for a large-scale production of plant transformants.
Reports indicate the ability of carbon nanofibers and nanomaterials to deliver plasmid DNA into cells (McKnight et al. 2003, 2004; Shukla et al. 2016). The ability of surface functionalized mesoporous silica nanoparticles (MSNs) to penetrate plant cell walls opens up new ways for precisely manipulate gene expression at a single cell level by delivering DNA and its activators in a controlled manner (McKnight et al. 2004).
Our study was inspired by recent developments of the cationic polymers containing chains of the 2-(dimethylamino)ethyl methacrylate (DMAEM) for gene transfer into yeast and mammalian cells (Ficen et al. 2013; Filyak et al. 2013; Qian et al. 2014; Samsonova et al. 2011; Schallon et al. 2010; Tanasienko et al. 2015). They could build complexes with DNA and RNA via electrostatic interaction and protect DNA from DNase I degradation. Previously, we proposed the application of new linear and branched polycationic polymers containing chains of DMAEM for plasmid DNA delivery into Ceratodon purpureus moss. It was shown that linear DMAEM-based polycationic polymers are efficient for moss transformation (Finiuk et al. 2014).
Tobacco and moss were proposed as experimental models in plant transformation (Ganapathi et al. 2004; Jing et al. 2013). Bryophytes are among the simplest and oldest of known terrestrial plants. Possessing strong regeneration and short life cycle, mosses are ideal model systems for plant study (Jing et al. 2013). In a recent decade, the cultivation protocols of such mosses as Physcomitrella patens, Funaria hygrometrica, C. purpureus, and Tortula ruralis were developed for various genetic engineering studies. They have been used to analyze gene function (Brucker et al. 2005; Finiuk et al. 2014; Jing et al. 2013; Prigge and Bezanilla 2010; Richter et al. 2012), express many complex secreted eukaryotic proteins and other products of interest (Anterola et al. 2009; Decker and Reski 2008). In addition, the moss protoplasts, unlike the protoplasts of other plants, do not need phytohormones for regeneration, and they can form a callus during regeneration. They regenerate directly into the filamentous protonemal, thus, mimicking a germinating moss spore (Rosales-Mendoza et al. 2014). Therefore, we used the moss protoplasts as a useful model system for genetic engineering studies. Besides, Nicotiana tabacum mesophylic protoplasts were used as a plant model (Cove 2000; Hunter et al. 2007; Paciolla et al. 2004; Pineros and Kochian 2001).
In this work, DMAEM-based cationic polymers with different molecular structure were synthesized and their potential as transporters for exogenous DNA delivery into plant cells (tobacco and moss) was evaluated. The formation of the cationic polymer/plasmid DNA (pDNA) complexes was characterized by using electrophoresis in the agarose gel. In addition, toxiсity and mutagenicity of the cationic polymers were tested. The biocompatibility of the polymers and the efficiency of nanoparticle-mediated gene transfer into plant cells were studied using the protoplasts.
Materials and methods
Materials for synthesis of the block polymers
2-(Dimethylamino)ethyl methacrylate, 2-aminoethyl methacrylate hydrochloride (AEM), 1-vinyl-2-pyrrolidone (NVP), butyl acrylate (BA), vinyl acetate (VA), 2-hydroxyethyl methacrylate (HEMA) were from Aldrich (Milwaukee, WI, USA). Polyethylene glycol (Mn ∼ 600 g mol− 1) and poly(ethylene glycol) methyl ether (mPEG, Mn ∼ 550 g mol− 1), were from Acros Organics (Geel, Belgium). Ammonium cerium (IV) nitrate, azobisisobutyronitrile (AIBN), N,N-dimethylmethanamide (DMF) and n-hexane were from Merck (Darmstadt, Germany). 1-Isopropyl-3(4)-[1-(tert-butyl peroxy)-1-methylethyl]benzene (monoperoxine (MP)) was synthesized from tert-butyl hydroperoxide (1.1 mo L−1) and 2-(4-isopropylphenyl)-2-propanol (1.0 mol L−1) in the acetic acid solution, as described earlier (Dikyy et al. 1975).
Synthesis of DMAEM-based cationic polymers
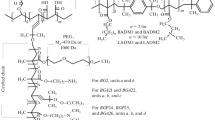
Block copolymers poly(DMAEM)-block- oligo(NVP-co-BA-co-AEM) (TN83/6) and poly(NVP-co-VA-co-HEMA)-block- oligo(NVP-co-BA-co-AEM) (TN 84/5) (Fig. 1a) were prepared according to the method described earlier (Zaichenko et al. 2008).
Block copolymers PEG-block-poly(NVP)- block-poly(DMAEM) (LM-8-DM) and poly(NVP)-block-PEG-block - poly(NVP)- block-poly(DMAEM) (DLM-9-DM) (Fig. 1b) were prepared according to the method described earlier (Miagkota et al. 2014).
Complex formation
The polymer/pDNA complexes were prepared by adding the carrier (1 μL) to the plasmid DNA (1 μg) pGLG578 (Sohn et al. 1999) at increasing concentrations (3 × 10− 3‒1%) on 8 μL of 20 мМ Tris–HCl, рН 7.4. Complexes were incubated at room temperature for 20 min. Then polymer/pDNA complexes were loaded onto 1% agarose gel (LACHEMA, Czech Republic) and run with 1× Tris–acetate (TAE) buffer containing 1 μg mL−1 of ethydium bromide. Electrophoresis was performed for 1 h at a constant voltage of 70 V. Plasmid DNA bands were visualized with an UV transilluminator (MacroVue UV-20, Hoeffer) (Fu et al. 2011).
DNase I protection assay
DNase I (0.05‒0.5 U, 2 μL) (Fermentas/ThermoFisher Scientific, USA) was added to 1 μg of naked pDNA or polymer (0.1%)/pDNA complexes and incubated at 37 °C for 30 min. The process of DNase I degradation was stopped by the addition of an ethylenediaminetetraacetic acid (EDTA) solution (Fermentas/ThermoFisher Scientific, USA) (0.5 M, 4 μL), and pDNA was released following incubation with a heparin solution (0.1%, 5 μL) (Arterium, Ukraine) at 37 °C over a period of 3.5 h. The samples were loaded onto the gel and electrophoresed to examine the integrity of pDNA (Fu et al. 2011).
Ana-telophase analysis
The Allium cepa toxicity study was performed, as described previously (Rank and Nielsen 1993). The method is based on the detection of chromosomal aberrations that occur in the root of meristem cells of A. cepa germinated on the studied polymers (10−3‒0.1%) solutions and on the distilled water as control. Seeds were germinated at 22 °C during 3 days. Cells were analyzed at the stage of anaphase and early stage of telophase at 10 × 40. Chromosomes were stained with acetoorseine (Rank and Nielsen 1993). In order to establish cyto- and genotoxicity of polymers, we have determined: mitotic index (MI, ‰) = (P + M + A + T)/(I + P + M + A + T) × 1000‰, and chromosomal aberrations (CA, %) = N/(A + T) × 100%, where P is the number of cells in prophase, M—in metaphase, A—in anaphase, T—in telophase, I—in interphase, N—number of chromosomal aberrations.
In vitro cultivation of N. tabacum
Aseptic N. tabacum plant material was grown from the sterilized seeds. For sterilization seeds were rinsed in 5% NaOCl solution for 15 min with subsequent washing out for three times in sterile deionized water. Tobacco plants were grown and micropropagated in vitro on the solid medium, containing Murashige–Skoog (МS) basal salts, 30 g L−1 sucrose and 8 g L−1 agar, рН 5.8 (Sigma, Germany).
Isolation of the tobacco protoplasts
Protoplasts of the aseptic N. tabacum plants were isolated as described by Yemets et al. (2000). Young green leaf disks of 2–2.5 sm2 area were cut into stripes and soaked into 10 mL solution containing 0.6% Cellulase Onozuka R-10 (Serva, Germany), 0.3% Driselase (Sigma, USA), 0.5 M sucrose and 5 mM CaCl2. Digestion was carried out in dark for 14 h at 28 °С. After the isolation, protoplasts were resuspended in liquid 8р medium (Kao and Michayluk et al. 1975) to the approximate density of 106 protoplasts in 1 mL of medium. This suspension was immediately used for polymer cytotoxicity assessments or genetic transformation studies.
Quantitative and qualitative analysis of the tobacco protoplasts
Morphology of protoplasts, as well as the yfp reporter gene fluorescent signal were detected using the luminescent microscope Axioskop 40 (“Carl Zeiss”, Germany) with the built-in camera. The digital processing of the microphotographs was done with the use of AxioVision LE 4.8.2.0 software (“Carl Zeiss MicroImaging GmbH”, Germany). All quantitative assessments were carried out in Neubayer chamber.
Study of the PDMAEM-based carriers cytotoxicity for tobacco protoplasts
Stock solutions of the polymers used in this study were as follows: TN 84/5, 1%, pH 7.27; DLM-9-DM, 0.5%, pH 5.0; LM-8-DM, 0.5%, pH 5.0. The effects of polymeric carriers on N. tabacum protoplasts were investigated in following range of polymers concentrations 5 × 10−5, 10−4, 10−3, 5 × 10−3, 10−2, 2.5 × 10−2 and 5 × 10−2%. Polymers were added to 1 mL aliquots of protoplasts suspension in liquid 8р medium (Kao and Michayluk et al. 1975). After addition of polymers into the protoplast suspension, the final solution was carefully mixed by the rotation. Protoplasts were co-cultivated with polymers in a sterile plastic Petri dishes for 24 h at 24 °С on the diffused light with 16/8 h photoperiod. The cytotoxicity of PDMAEM-based nanoparticles was estimated by calculating the amount of damaged and normal protoplasts using morphological criteriums (Van Doorn et al. 2011).
Tobacco protoplasts transformation with PDMAEM/pDNA complexes
The ability of PDMAEM-based carriers TN 84/5, DLM-9-DM and LM-8-DM to deliver pDNA was examined on freshly isolated tobacco protoplasts. For transformation studies, pGreen 0029 plasmid was used, which contained reporter yfp (yellow fluorescent protein) gene fused to the γ-tip (tonoplast intrinsic protein) gene sequence (Hunter et al. 2007). The general schema of the binary plasmid pGreen 0029 used in this study is shown on the Fig. 2a.
General scheme of plasmid DNA: a pGreen 0029 construct: LB and RB left and right Т-DNA borders, nos-t nopaline synthase terminators, nptII neomycine phosphotransferase gene, P35S 35S Cauliflower mosaic virus promoter, yfp yellow fluorescent protein gene, γ-tip tonoplast intristic protein gene, b pSF3 plasmid: Tocs octopine synthase terminator, gfp green fluorescent protein gene, pts1 gene of protein of peroxisomal signal of the first type, CaMV35S 35 S Cauliflower mosaic virus promoter, hyg R gene of resistance to hygromycin B
Plasmid DNA was isolated from previously transformed overnight E. coli strain DH5α culture (OD600 = 0.6) with above mentioned construct using the method of alkaline lysis (Sambrook et al. 1989). Concentration of plasmid stock solution in the deionized water used for protoplast transformation was 1 μg μL−1.
Appropriate concentrations of the nanocomposites for protoplast transformation studies were defined from the results of the above mentioned cytotoxicity assessment. These concentrations were as follows: 5 × 10−5% for LM-8-DM, 10−3% for DLM-9-DM and 5 × 10−3% for TN 84/5. The rate of protoplasts survival observed at these concentrations exceeded 70%. To obtain conjugates of polymers with plasmid DNA 20 μL of pGreen 0029, plasmid stock solution (1 μg μL−1 DNA) was mixed with 1, 2 and 5 μL of LM-8-DM, DLM-9-DM and TN 84/5 polymer stock solutions, respectively. These mixtures were incubated for 20 min at room temperature, which is important for stable complex formation. Then solutions containing PDMAEM/pDNA complexes were added into a series of 1 mL aliquots of protoplast suspension. The protoplasts were incubated with the polymers in the sterile plastic Petri dishes for 24 h at 24 °С under the diffused light with 16/8 h photoperiod. The frequency of polymers-mediated foreign gene delivery into the protoplasts was estimated by calculating the ratio of protoplasts expressing yfp gene detected as YFP signal under the luminescent microscope Axioskop 40.
Transformation of moss protoplasts
Moss C. purpureus (from the collection of the Institute of Ecology of the Carpathians, National Academy of Sciences of Ukraine, Lviv, Ukraine) was cultured at 24–26 °С, 16/8 h photoperiod in Knop medium (Hohe and Reski 2002). Plasmid DNA pSF3 (7836 base pares) was obtained at the Institute of Cell Biology, National Academy of Sciences of Ukraine) (Finiuk et al. 2014) and was used to transform moss C. purpureus. Plasmid DNA contained hyg gene conferring resistance to hygromycin B and hidden gene of protein of peroxisomal signal of the first type (pts1) fused with gfp (green fluorescent protein) gene. The genes were put under the control of the CaMV 35S promoters and octopine synthase terminators (Tocs) (Fig. 2b).
The modified PEG-mediated transformation method developed for moss P. patens was used to transform the protoplasts of C. purpureus (Cove et al. 2009b; Frank et al. 2005). Protoplasts were isolated by digestion of cell wall of protonemal tissue with 1% Driselase (Sigma, USA) for 1 h. Polymer/pDNA complexes with different polymer/pDNA ratio were used as gene carriers. In addition, 40% PEG-6000 (LobaChemie, Austria) was used as control pDNA carrier. Transformed protoplasts were placed on Petri dishes with PRMB medium containing 0.5% glucose. The regenerants were transferred on a selective medium containing hygromycin B (50 µg mL−1) after 14 days. The regenerants were subcultivated on the medium without or with hygromycin B antibiotic in order to obtain stable transformants.
Statistical analysis
All data are presented as mean ± standard error of the mean (SEM). Experiments were repeated in triplicate. Statistical analyses were performed using two-way ANOVA test. A P-value of <0.05 was considered statistically significant.
Results and discussion
Plasmid DNA/polymer complex formation
Novel PDMAEM block copolymers were designed in order to contain positively charged polymer branches needed for binding of pDNA with the negative charge of the nucleotide phosphates. Since DNA-binding capability is a prerequisite for genetic carriers in elaboration of gene delivery methods, the assembly of polymer/plasmid DNA complex was confirmed by gel retardation assay. The polycationic polymers are known to interact electrostatically with DNA. The inhibition of the electrophoretic mobility of plasmid DNA can be seen with an increase of the polymer/DNA ratios in the agarose gels. Complexes were prepared at different concentrations of polymer (3 × 10−3‒0.1%) and the retardation in the migration of the plasmid DNA during agarose gel electrophoresis was examined. The intact pDNA migrated in the lane 5 of the agarose gel, and it was not retarded being in the complex with 3 × 10−3% TN 83/6 polymer in the lane 4 of the gel (Fig. 3a). However, there was a retardation of pDNA migration in lane 3 (Fig. 3a), indicating that TN 83/6 polymer in 0.01% concentration could bind pDNA and form the complex. The formation of TN 84/5/pDNA complex took place at 0.01% concentration of the polymer (Fig. 3b).
DLM-9-DM polymer in 0.1% concentration combined with pDNA (lane 2, Fig. 3c). LM-8-DM in the same concentration binds pDNA to form complexes (lane 2, Fig. 3d). TN 83/6 and TN 84/5 block copolymers maintained more effective electrostatic binding of the carrier with negatively charged DNA molecules.
In constructing of gene carriers, the amino/phosphate groups ratio plays an important role since it is affecting a degree of the polyplexes particle size, transfection efficiency, and cytotoxicity of carriers. High ratio of amino/phosphate groups induced not only an increased cellular uptake through charge mediated interactions, but also disadvantageous higher cytotoxicity. On the other hand, a reduction of the ratio of amino/phosphate groups will lead to the particle size increase and a decrease of the transfection efficiency (Zhao et al. 2009).
DNase I protection assay
The effective binding of DNA is a key issue for its stability against enzymatic digestion by nucleases (Roy et al. 2005). The ability of the cationic polymers to protect DNA against nuclease attack is enhancing a success of gene expression. The agarose gel electrophoresis was used to monitor the sensitivity of pDNA that formed a complex with the polymer to DNase I. Intact pDNA was completely digested when incubated with DNase I (0.05 U/µg pDNA) (lane 4, Fig. 4a, b). pDNA complexed with 0.1% TN 83/6 polymer was protected against the action of the DNase I (0.05 and 0.5 U/µg pDNA) by carrier (Fig. 4a, lanes 2, 3). Plasmid DNA bound with polymer TN 84/5 remained intact after incubation with the DNase I (0.05 and 0.5 U/µg pDNA) (Fig. 4b, lanes 2, 3). Plasmid DNA complexed with 0.1% DLM-9-DM and LM-8-DM polymers was not degraded by the DNase I (0.05 and 0.5 U/µg pDNA) (lanes 2, 3, Fig. 4c, d).
Results of electrophoresis in the agarose gel of 0.1% polymers: a TN 83/6, b TN 84/5, c DLM-9-DM, d LM-8-DM and pDNA complexes after incubation for 30 min with different concentrations of DNase I. Lane 1—plasmid DNA; 2—polymer/pDNA complex incubated with DNase I at 0.05 U/μg pDNA; 3—polymer/pDNA complex incubated with DNase I at 0.5 U/μg pDNA; 4—pDNA incubated with DNase I at 0.05 U/μg pDNA
Our studies show that polymers protect pDNA against cleavage by the DNase I, which is a crucial factor for efficient gene delivery in vitro and in vivo. Such effect could be explained by (i) repulsion of Mg2+ ions (which are necessary for the enzymatic reaction) by the amino groups, (ii) a hindered access of the enzymes to the DNA that is immobilized on the nanoparticle surface, or (iii) both (He et al. 2003). So, new polymeric DMAEM-containing carriers under study can be considered as inhibitors of DNAse I activity when they are used as carriers of pDNA in DNA delivery experiments.
Study of the genotoxic properties of PDMAEM carriers
The ana-telophase test on A. cepa was used to evaluate the genotoxic properties of polymeric carriers TN 83/6, TN 84/5, DLM-9-DM, LM-8-DM. In the presence of increasing concentrations (from 10 to 3 to 0.1%) of the polymeric carriers, a non-significant drop in the MI was observed. At the same time, the polymers DLM-9-DM and LM-8-DM (0.1%) progressively decreased the MI to 118‒121‰, as compared with 167‰ for the control (Table 1).
In control (meristem cells of A. cepa that were germinated on the distilled water), the amount of chromosomal aberrations was about 3.32%. It could be explained by spontaneous mutations occurring in the meristematic cells of A. cepa. PEG-containing block polymers DLM-9-DM and LM-8-DM caused the most significant rise in the value of the chromosomal aberrations. The chromosomal aberrations significantly increased from 3.96% after treatment with 10−3% LM-8-DM to 4.96% in case of 0.1% LM-8-DM action (Table 1). The TN 83/6 and TN 84/5 carriers significantly increased the number of the chromosomal aberration only at 0.1%, which is approximately 100 times higher of the working concentrations of the polymers. The aberrations noticed due to the action of the polymeric carriers were mostly chromosome fragmentation and bridges.
The results of ana-telophase test in A. cepa showed that PEG-containing block polymers DLM-9-DM and LM-8-DM possessed higher cytotoxicity compared to the toxic effects of TN 83/6 and TN 84/5 polymeric carriers used in 0.001‒0.1% concentrations. We suggest that the presence of PEG blocks in the structure of DLM-9-DM and LM-8-DM carriers are responsible for their increased toxity. The obtained results correlated with the results described in (Van de Wetering et al. 1998).
Genetic transformation of C. рurpureus moss protoplasts
To transform the C. purpureus moss, modified PEG-mediated transformation method developed for P. patens moss was used (Cove et al. 2009b). This method is based on using polymeric carriers TN 83/6, TN 84/5, DLM-9-DM, LM-8-DM and PEG as reference carrier. The application of classical PEG-based transformation method (Cove et al. 2009b) did not enable us to obtain moss transformants (Table 2). However, the application of TN 83/6, TN 84/5, DLM-9-DM polymers as pDNA carriers at 2.5 × 10−3% allowed obtaining both transient and stable transformants of C. purpureus. As a result of transient transformation of C. purpureus protoplasts, 25 clones were picked up using TN 83/6 carrier, and 26 clones—when using TN 84/5 carrier. The application of DLM-9-DM polymer resulted in a selection of only 1 transformant clone. The polymeric carrier LM-8-DM was not capable of transferring plasmid DNA into moss protoplasts (Table 2).
Selection of stable transformants of C. purpureus was carried out on a selective medium containing hygromycin B antibiotic. As a result of stable transformation of C. purpureus protoplasts, 16 clones were picked up using TN 83/6 carrier, and 20 clones—when using TN 84/5 carrier, and 1 clone—when using DLM-9-DM polymer (Fig. 5).
In general, the results of stable transformation were comparable with the results of transient transformation. TN 83/6 and TN 84/5 carriers were more effective for both transient and stable C. purpureus transformations. TN 83/6 and TN 84/5 carriers are the diblock-copolymers consisting of the water-soluble flexible block, including chains of vinyl-pyrrolidone and monomer with initial primary amine group, except the chain of PDMAEM. One can assume that the polyplex formation occurs as a result of the electrostatic interaction between the DNA and block PDMAEM which further acquires hydrophobic properties and creates the nucleus of the nanodimensional micellar structure. Such structure was stabilized by the water-soluble block of N-vinyl-pyrrolidone chains and amino-ethyl-methacrylate with the initial prime amino group. We assume that the free amino group in the structure of TN 83/6 and TN 84/5 polymers ensures better binding of pDNA molecule with these carriers (Finiuk et al. 2014).
DLM-9-DM and LM-8-DM carriers were not effective in both transient and stable C. purpureus transformation. We suggest that the combination of PEG and DMAEM for carriers construction resulted in a decrease in the transformation efficiency. These polymers possess higher molecular weight. In addition, these polymers demonstrated less effective binding of pDNA molecule with the carriers in comparison to TN 83/6 and TN 84/5 polymers (Fig. 3). As reported by Qian et al. (2014), the application of PDMAEM carriers with lower molecular weight improves gene delivery.
Thus, we can conclude that the polycationic carriers TN 83/6 and TN 84/5 were the most effective gene transfer agents for moss protoplasts among studied carriers.
Study of cytotoxic properties of PDMAEM on N. tabacum protoplasts
Samples containing investigated polymers in different concentrations generally demonstrated decrease in levels of protoplasts viability. Nevertheless, the presence of suitable range of polymer concentrations responsible for only slight decrease of the protoplast survival in comparison to control was shown. In control, the survival ratio of protoplasts reached 91%. The most pronounced cytotoxic effect was revealed for the LM-8-DM polymer. The carrier used at concentrations in range of 5 × 10−3‒5 × 10−2% caused destructive changes in a vast majority of the exposed protoplasts. These changes resulted in a formation of large aggregates of damaged protoplasts consisting of numerous of protoplast residues (Fig. 6d). The influence of the LM-8-DM polymer at concentrations of 5 × 10−5, 10−4 and 10−3% resulted in 71, 42 and 5% protoplasts survival rates, respectively (Fig. 6e).
Effect of PDMAEM-based carriers at different concentrations on survival of tobacco protoplasts: a control sample after 24 h cultivation in 8p medium; b protoplasts after 24 h cultivation in 8p medium supplemented with the 5 × 10−2% of TN 84/5; c protoplasts after 24 h cultivation in 8p medium supplemented with the 5 × 10−2% of DLM-9-DM; d protoplasts after 24 h cultivation in 8p medium supplemented with the 5 × 10−2% of LM-8-DM; e results of effects of carriers on survival of tobacco protoplasts. Bar 20 μm
The DLM-9-DM polymer demonstrated decreased cytotoxicity levels in comparison with the LM-8-DM polymer effect. Extensive disruption of all protoplasts in samples was found only at the presence of the highest investigated concentration of the polymer—5 × 10− 2% (Fig. 6c). The rates of viable protoplasts after the treatment with the polymer at concentrations of 5 × 10−5, 10−4, 10−3, 5 × 10−3, 10−2 and 2.5 × 10−2% were of 89, 85, 68, 53, 35 and 7%, respectively (Fig. 6e). The observed survival levels of 85 and 89% were comparable with survival levels of protoplasts in the control sample. Both single dispersed damaged protoplasts and protoplasts composing aggregates demonstrated cytoplasm shrinking, which, in turn, was a common effect for the above described LM-8-DM polymer.
The observed effects of the LM-8-DM and DLM-9-DM polymers on tobacco protoplast viability are assumed to appear due to the specific chemical structure of polymer molecules. One can see on Fig. 1b that both polymers contain PEG block in their structure. Most of studies of non-viral gene delivery systems based on PDMAEM confirm that addition of PEG blocks to molecules of PDMAEM (PEGylation) substantially reduce the cytotoxicity of carriers for the mammalian cells (Agarwal et al. 2012; Guo et al. 2011). However, the application of PEG as protoplast transformation agent is associated with an increased damage of protoplasts due to PEG-induced reversible destabilization of cell membrane (Assani et al. 2005; Burris et al. 2016; Chakrabarty et al. 2008; Hashizaki et al. 2003; Masani et al. 2014), while the influence of PEGylated polymers on the tobacco protoplasts viability has not been studied yet.
A comparison of the effects of DLM-9-DM and LM-8-DM polymers indicates that the former could be up to 25 times less toxic than the latter. Namely, treatment with LM-8-DM polymer at concentration of 10−3% (Mn = 9500 g/mol, Fig. 1b) resulted in 5% protoplasts survival rate, while treatment with DLM-9-DM at concentration of 2.5 × 10−2% (Mn= 10,200 g/mol, Fig. 1b) resulted in 7% protoplasts survival rate (Fig. 6e). These findings differ from other results obtained during studies on the mammalian cells (Agarwal et al. 2012; Samsonova et al. 2011; Schallon et al. 2010; You et al. 2007), where molecular weight of PDMAEM-containing DNA carriers directly correlated with their cytotoxicity. Among possible reasons of the reported effect is the presence of a slight difference in molecular weight between DLM-9-DM and LM-8-DM polymers, while differences in their structure could have greater influence on their biological effect. Namely, our results can be attributed to quantitative variations in chemical composition of PEG-containing block copolymers. As indicated in Fig. 1b, DLM-9-DM molecule comprises higher content of PEG and NVP blocks (5.9 and 16.6%, correspondingly) than LM-8-DM polymer (5.8 and 10.5%), and lower content of DMAEM blocks (77.5% in DLM-9-DM versus 83.7% in LM-8-DM). Thus, we suggest that PEG and NVP blocks of the molecule DLM-9-DM are involved in the alleviation of the cytotoxic effect of the PDMAEM-based carrier. Our assumption is in a correspondence with the results described in (Van de Wetering et al. 1998). In the above mentioned study, the copolymers of PDMAEM and NVP were reported to possess a reduced cytotoxicity for living cells in comparison with single PDMAEM polymer.
The TN 84/5 polymer demonstrated the lowest cytotoxicity for the protoplasts compared to LM-8-DM and DLM-9-DM. Treatment with the polymer at concentrations of 5 × 10−5, 10−4, 10−3, 5 × 10−3, 10−2, 2.5 × 10−2 and 5 × 10−2% was associated with 91, 87, 82, 76, 59, 22 and 3% ratios of protoplast survival, respectively (Fig. 6b, e). The use of TN 84/5 polymer at the highest investigated concentrations—2.5 × 10− 2 and 5 × 10−2%—resulted in an extensive protoplasts deformation and cytoplasm shrinking. The aggregation in samples of protoplasts treated with TN 84/5 was much less abundant than in samples treated with LM-8-DM and DLM-9-DM.
The molecular structure of TN 84/5 is more complex than that of DLM-9-DM and LM-8-DM. It differs by the absence of PEG block and the presence of additional HEMA, BA, AEM and VA blocks. The application of TN 84/5 carrier at a concentration of 5 × 10−2% was still associated with 3% survival of protoplasts, while for DLM-9-DM and LM-8-DM polymers the highest concentrations associated with the presence of intact protoplasts in medium were 2.5 × 10−2 and 10−3%, respectively.
Due to the presence of numerous side chains in TN 84/5 molecules, the polymer has a highly branched brush-like structure. In combination with relatively low molecular weight of 6800 g/mol (in comparison with DLM-9-DM (10,200 g mol−1) and LM-8-DM (9500 g mol−1) (Fig. 1b)), these features could allow high protoplasts transformation efficiency and low cytotoxicity of TN 84/5-based carriers. Branched and modified structures together with low molecular weight were shown to be favourable factors for various biological applications of the polymeric carriers (Agarwal et al. 2012; Samsonova et al. 2011; Schallon et al. 2010; You et al. 2007). Besides, pHEMA blocks were reported to have additional fusogenic effects during interactions with cellular membranes enhancing the uptake of the polyplexes (Samsonova et al. 2011). More precise investigation of these mechanisms poses the background for the development of various innovative solutions for plant biology and biotechnology based on the polymeric carriers.
Genetic transformation of N. tabacum protoplasts
For genetic transformation of the protoplasts, the following concentrations of polymers were used: 5 × 10−5% LM-8-DM, 10−3% DLM-9-DM and 5 × 10−3% TN 84/5. Indicated concentrations corresponded to more than 70% level of protoplast survival (Fig. 6e).
The ability of different polymer carriers to deliver DNA was estimated by the frequency of transient yfp gene expression in protoplasts a day after transformation (Fig. 7). The highest protoplast transformation frequency of 16% was reported when TN 84/5 polymer was used as pDNA carrier (Fig. 7d, e). DLM-9-DM and LM-8-DM polymers were less efficient, as they were characterised by 5 and 3% transformation frequencies of tobacco protoplasts, correspondingly (Fig. 7e).
Generally, while the cytotoxicity of PDMAEM carriers decreased in the order: LM-8-DM > DLM-9-DM > TN 84/5, the frequency of protoplast transformation using PDMAEM carriers increased in the same order: LM-8-DM < DLM-9-DM < TN 84/5. Thus, the TN 84/5 polymers appeared to be the most suitable DNA carriers demonstrating the lowest cytotoxicity levels.
Among studied polymers, TN 84/5 possessed the most grafted molecular structure as the result of inclusion of five different residues, namely HEMA, AEM, BA, VA and NVP. One of possible reasons why TN 84/5 facilitated the most effective DNA delivery to protoplasts is the best DNA-binding capacity of this polymer comparing with DLM-9-DM and LM-8-DM confirmed by the results of gel retardation assay (Fig. 3). These results showed that TN 84/5 polymer was capable of binding DNA effectively at 10-fold lower concentration than the lowest effective DNA-binding concentrations of DLM-9-DM and LM-8-DM carriers (Fig. 3). DNA-binding capability of TN 84/5 polymer could result in a formation of compact carrier/DNA complexes which might easily cross the membrane of the protoplasts without a significant disruption. These results are in good correspondence with the data obtained by other authors (Agarwal et al. 2012; Samsonova et al. 2011; Schallon et al. 2010; You et al. 2007) which confirm better ability of branched polymers to bind DNA.
DLM-9-DM and LM-8-DM carriers were less effective for DNA delivery into the protoplasts. This can be due to the presence of PEG blocks with zero charge in their structure, which could prevent an effective DNA binding. However, PEGylation of PDMAEM could decrease the cytotoxicity of the polymers for the mammalian cells in culture, although such modification of structure may compromise polymers efficiency in elaboration of novel approaches for genetic transformation (Agarwal et al. 2012; Burris et al. 2016).
Conclusions
Novel oligoelectrolyte PDMAEM-based DNA carriers were synthesised, and their physico-chemical properties, such as DNA-binding capacity and protection of DNA against nuclease degradation are presented. Retardation of plasmid DNA complexed with TN 83/6, TN 84/5, DLM-9-DM, LM-8-DM carriers during its electrophoresis in the agarose gel has shown that TN 83/6 and TN 84/5 polymers possessed the best DNA binding capacity. Furthermore, TN 83/6 and TN 84/5 polymers could protect DNA against the digestion with the DNase I. The results of the ana-telophase test conducted on A. cepa did not reveal the genotoxic potential of the polymeric carriers TN 83/6, TN 84/5 used in concentration from 10 to 3 to 0.01%. By using TN 83/6, TN 84/5 and DLM-9-DM, both transient and stable transformants of C. purpureus protoplasts were obtained. Moreover, substantial variation of the polymers’ cytotoxic properties and their efficiency in DNA delivery into the tobacco protoplasts was found. Probably, the efficiency of pDNA delivery depends on chemical structure, as well as on the molecular weight of the PDMAEM carriers. It was shown that highly grafted TN 84/5 polymer containing AEM, HEMA, NVP, VA and BA residues and possessing low molecular weight was the least cytotoxic for the tobacco protoplasts and the most effective in DNA delivery in comparison with DLM-9-DM and LM-8-DM carriers. At the same time, DLM-9-DM carriers with higher molecular weight and PEG blocks in their structure, demonstrated 10 times higher cytotoxicity and lower efficiency in gene delivery, while the most cytotoxic LM-8-DM carrier was the least effective in the DNA delivery. To sum up, the results of this study show that the use of novel PDMAEM-based DNA carriers with brush-like molecular structure is a promising approach in plant biotechnology, and it has a potential for further development.
References
Agarwal S, Zhang Y, Maji S, Greiner A (2012) PDMAEMA based gene delivery materials. Mater Today 15:388–393. doi:10.1016/S1369-7021(12)70165-7
Anterola A, Shanle E, Perroud P, Quatrano R (2009) Production of taxa-4(5),11(12)-diene by transgenic Physcomitrella patens. Transgenic Res 18:655–660. doi:10.1007/s11248-009-9252-5
Armstrong CL, Petersen WL, Buchholz WG, Bowen BA, Sulc SL (1990) Factors affecting PEG-mediated stable transformation of maize protoplasts. Plant Cell Rep 9(6):335–339. doi:10.1007/BF00232864
Assani A, Chabane D, Haıcour R, Bakry F, Wenzel G, Foroughi-Wehr B (2005) Protoplast fusion in banana (Musa spp.): comparison of chemical (PEG:polyethylene glycol) and electrical procedure. Plant Cell Tissue Organ Cult 83:145–151. doi:10.1007/s11240-005-4633-9
Brucker G, Mittmann F, Hartmann E, Lamparter T (2005) Targeted sitedirected mutagenesis of a heme oxygenase locus by gene replacement in the moss Ceratodon purpureus. Planta 220:864–874. doi:10.1007/s00425-004-1411-6
Burris K, Dlugosz E, Collins G, Stewart N, Lenaghan S (2016) Development of a rapid, low-cost protoplast transfection system for switchgrass (Panicum virgatum L.). Plant Cell Rep 35:693–704. doi:10.1007/s00299-015-1913-7
Chakrabarty B, Ghoshal A, Purkait M (2008) Effect of molecular weight of PEG on membrane morphology and transport properties. J Membr Sci 309:209–221. doi:10.1016/j.memsci.2007.10.027
Cove D (2000) The moss Physcomitrella patens. J Plant Growth Regul 19:275–283. doi:10.1007/s003440000031
Cove D, Perroud P, Charron A, McDaniel S, Khandelwal A, Quatrano R (2009a) Transformation of moss Physcomitrella patens gametophytes using a biolistic projectile delivery system. Cold Spring Harb Protoc 2:45–51. doi:10.1101/pdb.prot5145
Cove D, Perroud PF, Charron A, McDaniel S, Khandelwal A, Quatrano R (2009b) The moss Physcomitrella patens. A novel model system for plant development and genomic studies. Emerging model organisms, a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Decker EL, Reski R (2008) Current achievements in the production of complex biopharmaceuticals with moss bioreactors. Bioprocess Biosyst Eng 31:3–9. doi:10.1007/s00449-007-0151-y
Deng X, Wei Z, An H (2001) Transgenic peanut plants obtained by particle bombardment via somatic embryogenesis regeneration system. Cell Res 11:156–160. doi:10.1038/sj.cr.7290081
Dikyy MA, Puchin VA, Vayda MS, Mamchur LP, Karpenko AN (1975) Obtaining new peroxide compounds p-diisopropylbenzene. Russ J Org Chem 11:1902–1907
Ficen S, Guler Z, Mitina N, Finiuk N, Stoika R, Zaichenko A et al (2013) Biophysical study of novel oligoelectrolyte based non-viral gene delivery systems to mammalian cells. J Gene Med 15:193–204. doi:10.1002/jgm.2710
Filyak Ye, Finiuk N, Mitina N, Bilyk O, Titorenko V, Hrydzhuk O et al (2013) A novel method for genetic transformation of yeast cells using oligoelectrolyte polymeric nanoscale carriers. Biotechniques 54:35–43. doi:10.2144/000113980
Finiuk N, Chaplya A, Mitina N, Boiko N, Lobachevska O, Miahkota O et al (2014) Genetic transformation of moss Ceratodon purpureus by means of polycationic carriers of DNA. Cytol Genet 48:345–351. doi:10.3103/S0095452714060048
Frank W, Decker E, Reski R (2005) Molecular tools to study Physcomitrella patens. Plant Biol 7:220–227. doi:10.1055/s-2005-865645
Fu C, Sun X, Liu D, Chen Z, Lu Z, Zhang N (2011) Biodegradable tri-block copolymer poly(lactic acid)-poly(ethylene glycol)-poly(L-lysine)(PLA-PEG-PLL) as a non-viral vector to enhance gene transfection. Int J Mol Sci 12:1371–1388. doi:10.3390/ijms12021371
Ganapathi T, Suprasanna P, Rao P, Bapat V (2004) Tobacco (Nicotiana tabacum L.)—a model system for tissue culture interventions and genetic engineering. Indian J Biotechnol 3:171–184
Gelvin SB (2003) Agrobacterium-mediated plant transformation: the biology behind the “gene-jockeying” tool. Microbiol Mol Biol Rev 1:16–37. doi:10.1128/MMBR.67.1.16-37.200
Guo S, Huang Y, Wei T, Zhang W, Wang W, Lin D et al (2011) Amphiphilic and biodegradable methoxy polyethylene glycol-block-(polycaprolactone-graft-poly(2-(dimethylamino)ethyl methacrylate)) as an effective gene carrier. Biomaterials 32:879–889. doi:10.1016/j.biomaterials.2010.09.052
Hashizaki K, Taguchi H, Itoh C, Sakai H, Abe M, Saito Y et al (2003) Effects of poly(ethylene glycol) (PEG) chain length of PEG-lipid on the permeability of liposomal bilayer membranes. Chem Pharm Bull 51:815–820. doi:10.1248/cpb.51.815
He XX, Wang K, Tan W, Liu B, Lin X, He C et al (2003) Bioconjugated nanoparticles for DNA protection from cleavage. J Am Chem Soc 125:7168–7169. doi:10.1021/ja034450d
Hohe A, Reski R (2002) Optimisation of a bioreactor culture of the moss Physcomitrella patens for mass protoplast production. Plant Sci 163:69–74
Hunter P, Craddock C, Benedetto S, Roberts L, Frigerio L (2007) Fluorescent reporter proteins for the tonoplast and the vacuolar lumen identify a single vacuolar compartment in Arabidopsis cells. Plant Physiol 145:1371–1382. doi:10.1104/pp.107.103945
Jing L, Wenjing Q, Dan S, Zhengquan H (2013) Genetic transformation of moss plant. Afr J Biotechnol 12:227–232. doi:10.5897/AJBX12.008
Kao K, Michayluk M (1975) Nutritional requirements for growth of Vicia hajastana cells and protoplasts at a very low population density in liquid media. Planta 126:105–110. doi:10.1007/BF00380613
Masani A, Noll G, Kadir G, Sambanthamurthi R, Prufer D (2014) Efficient transformation of oil palm protoplasts by PEG-mediated transfection and DNA microinjection. PLoS ONE 9:e96831. doi:10.1371/journal.pone.0096831
McKnight T, Melechko A, Griffin G, Guillorn M, Merkulov V, Serna F et al (2003) Intracellular integration of synthetic nanostructures with viable cells for controlled biochemical manipulation. Nanotech 14:551–556. doi:10.1088/0957-4484/14/5/313
McKnight T, Melechko A, Hensley D, Mann D, Griffin G, Simpson M (2004) Tracking gene expression after DNA delivery using spatially indexed nanofiber arrays. Nano Lett 4:1213–1219. doi:10.1021/nl049504b
Miagkota O, Mitina N, Nadashkevych Z, Yanchuk I, Greschuk O, Hevus O, Zaichenko A (2014) Novel peroxide containing pegylated polyampholytic block copolymers. Chem Chem Technol 8:61–66
Paciolla C, Dipierro N, Mule G, Logrieco A, Dipierro S (2004) The mycotoxins beauvericin and T-2 induce cell death and alteration to the ascorbate metabolism in tomato protoplasts. Physiol Mol Plant Pathol 65:49–56. doi:10.3389/fpls.2015.00573
Pineros M, Kochian L (2001) A patch-clamp study on the physiology of aluminum toxicity and aluminum tolerance in maize: identification and characterization of Al-induced anion channels. Plant Physiol 125:292–305. doi:10.1104/pp.125.1.292
Potrykus I, Spangenberg G (1995) Gene transfer to plants. Springer, Berlin Heidelberg
Prigge M, Bezanilla M (2010) Evolutionary crossroads in developmental biology: Physcomitrella patens. Development 137:3535–3543. doi:10.1242/dev.049023
Qian X, Long L, Shi Z, Liu C, Qiu M, Sheng J et al (2014) Star-branched amphiphilic PLA-b-PDMAEMA copolymers for co-delivery of miR-21 inhibitor and doxorubicin to treat glioma. Biomaterials 35:2322–2335. doi:10.1016/j.biomaterials.2013.11.039
Rank J, Nielsen MH (1993) A modified Allium test as a tool in the screening of the genotoxicity of complex mixtures. Hereditas 118:49–53. doi:10.1111/j.1601-5223.1993.t01-3-00049.x
Richter H, Lieberei R, Strnad M, Novák O, Gruz J, Rensing S, von Schwartzenberg K (2012) Polyphenol oxidases in Physcomitrella: functional PPO1 knockout modulates cytokinin-dependent developmentin the moss Physcomitrella patens. J Exp Bot 63:5121–5135. doi:10.1093/jxb/ers169
Rosales-Mendoza S, Orellana-Escobedo L, Romero-Maldonado A, Decker E, Reski R (2014) The potential of Physcomitrella patens as a platform for the production of plant-based vaccines. Expert Rev Vaccines 13:203–212. doi:10.1586/14760584.2014.872987
Roy I, Ohulchanskyy T, Bharali D, Pudavar H, Mistretta R, Kaur N et al (2005) Optical tracking of organically modified silica nanoparticles as DNA carriers: a nonviral, nanomedicine approach for gene delivery. Proc Natl Acad Sci USA 102:279–284. doi:10.1073/pnas.0408039101
Sambrook J, Fritsch E, Maniatis T (1989) Molecular cloning a laboratory manual, 2nd edn. Cold Spring Harbor Lab, New York
Samsonova O, Pfeiffer C, Hellmund M, Merkel O, Kissel T (2011) Low molecular weight pDMAEMA-block-pHEMA block-copolymers synthesized via RAFT-polymerization: potential non-viral gene delivery agents? Polymers 3:693–718. doi:10.3390/polym3020693
Sarmast MK, Niazi A, Salehi H, Abolimoghadam A (2015) Silver nanoparticles affect ACS expression in Tecomella undulata in vitro culture. Plant Cell Tissue Organ Cult 121:227–236. doi:10.1007/s11240-014-0697-8
Schallon A, Jérôme V, Walther A, Synatschke C, Müller A, Freitag R (2010) Performance of three PDMAEMA-based polycation architectures as gene delivery agents in comparison to linear and branched PEI. React Funct Polym 70:1–10. doi:10.1016/j.reactfunctpolym.2009.09.006
Shen J, Fu J, Ma J, Wang X, Gao C, Zhuang C et al (2014) Isolation, culture, and transient transformation of plant protoplasts. Curr Protoc Cell Biol 63:2.8.1–2.8.17. doi:10.1002/0471143030.cb0208s63
Shukla PK, Misra P, Kole C (2016) Uptake, translocation, accumulation, transformation, and generational transmission of nanoparticles in plants. In: Kole C, Kumar DS, Khodakovskaya MV (eds). Plant nanotechnology. Springer, Cham, pp 183–218. doi:10.1007/978-3-319-42154-4_8
Smidkova M, Hola M, Angelis K (2010) Efficient biolistic transformation of the moss Physcomitrella patens. Biol Plant 54:777–780. doi:10.1007/s10535-010-0141-9
Sohn JH, Choi ES, Kang HA, Rhee JS, Agaphonov MO, Ter-Avanesyan MD, Rhee SK (1999) A dominant selection system designed for copy-number-controlled gene integration in Hansenula polymorpha DL-1. Appl Microbiol Biotechnol 51:800–807. doi:10.1007/s002530051465
Tanasienko I, Yemets А, Finiuk N, Stoika R, Blume Y (2015) DMAEM-based cationic polymers as novel carriers for DNA delivery into cells. Cell Biol Int 39:243–245. doi:10.1002/cbin.10381
Torney F, Trewyn B, Lin SY, Wang K (2007) Mesoporous silica nanoparticles deliver DNA and chemicals into plants. Nat Nanotechnol 2:295–300. doi:10.1038/nnano.2007.108
Van Doorn W, Beers E, Dangl J, Franklin-Tong V, Gallois P, Hara-Nishimura I et al (2011) Morphological classification of plant cell deaths. Cell Death Differ 18:1241–1246. doi:10.1038/cdd.2011.36
Van de Wetering P, Cherng JY, Talsma H, Crommelin D, Hennink W (1998) 2-(Dimethylamino)ethyl methacrylate based (co)polymers as gene transfer agents. J Control Release 53:145–153. doi:10.1016/S0168-3659(97)00248-4
Yemets AI, Kundel’chuk OP, Smertenko AP, Solodushko VG, Rudas VA, Gleba YY, Blume YB (2000) Transfer of amiprophosmethyl resistance from a Nicotiana plumbaginifolia mutant by somatic hybridization. Theor Appl Genet 100:847–857. doi:10.1007/s001220051361
You YZ, Manickam D, Zhou QH, Oupický D (2007) Reducible poly(2-dimethylaminoethyl methacrylate): synthesis, cytotoxicity, and gene delivery activity. J Control Release 122:217–225. doi:10.1016/j.jconrel.2007.04.020
Zaichenko A, Mitina N, Shevchuk O, Rayevska K, Lobaz V, Skorokhoda T et al (2008) Development of novel linear, block and branched oligoelectrolytes and functionally targeting nanoparticles. Pure Appl Chem 80:2309–2326
Zeidler A, Hartmann E, Hughes J (1999) Transgene expression in the moss Ceratodon purpureus. J Plant Physiol 154:641–650. doi:10.1016/S0176-1617(99)80239-9
Zhang Z, Xing A, Staswick P, Clemente TE (1999) The use of glufosinate as a selective agent in Agrobacterium-mediated transformation of soybean. Plant Cell Tissue Organ Cult 56:37–46. doi:10.1023/A:1006298622969
Zhao QQ, Chen JL, Lv TF, He CX, Tang GP, Liang WQ et al (2009) N/P ratio significantly influences the transfection efficiency and cytotoxicity of a polyethylenimine/chitosan/DNA complex. Biol Pharm Bull 32:706–710. doi:10.1248/bpb.32.706
Acknowledgements
This study was supported by the Research Grant of the Molecular & Cellular Biotechnologies Program of the National Academy of Sciences of Ukraine (No. 0115U004198, 2015‒2019).
Author information
Authors and Affiliations
Contributions
NF conducted experiments on DNase I protection assay, ana-telophase analysis and moss protoplasts transformation. AB, OB were responsible for tabacco cultivation and performed experiments on tobacco protoplasts transformation and cytotoxicity of carriers for tobacco protoplasts. AZ, NM, OM were responsible for synthesis and characterization of polymeric carriers, and pDNA/polymer complex formation. OL was responsible for moss cultivation. RS, YB, AY contributed to the conception of the study and results analysis. All the authors were involved in statistical data analysis and manuscript writing.
Corresponding author
Additional information
Communicated by Ming-Tsair Chan.
Nataliya Finiuk and Anastasiia Buziashvili have equally contributed to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Finiuk, N., Buziashvili, A., Burlaka, O. et al. Investigation of novel oligoelectrolyte polymer carriers for their capacity of DNA delivery into plant cells. Plant Cell Tiss Organ Cult 131, 27–39 (2017). https://doi.org/10.1007/s11240-017-1259-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-017-1259-7