Abstract
Understanding the role light quality plays on floral initiation is key to a range of pre-breeding tools, such as accelerated single-seed-descent. We have elucidated the effect of light quality on early flowering onset in cool-season grain legumes and developed predictive models for time to flowering under the optimised light conditions. Early and late flowering genotypes of pea, chickpea, faba bean, lentil and lupin were grown in controlled environments under different light spectra (blue and far red-enriched LED lights and metal halide). All species and genotypes showed a positive response to a decreasing red to far-red ratio (R:FR). In general, ratios above 3.5 resulted in the longest time to flowering. In environments with R:FR below 3.5, light with the highest intensity in the FR region was the most inductive. We demonstrate the importance of considering both relative (R:FR) and absolute (FR photons) light values for flower induction in grain legumes. Greater response to light spectra was observed in the later flowering genotypes, enabling a drastic compression of time to flowering between phenologically diverse genotypes. A novel protocol for robust in vitro germination of immature seeds was developed for lupin, a species known for its recalcitrance to in vitro manipulation. We show how combining this protocol with growth under conditions optimized for early flowering drastically speeds generation turnover. The improved understanding of the effect of light on flowering regulation and the development of robust in vitro culture protocols will assist the development and exploitation of biotechnological tools for legume breeding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past 15 years, the absence of robust doubled haploid protocols in the cool-season grain legumes has fuelled the search for novel ways to reduce generation cycle time and speed the return to homozygosis following hybridization (Croser et al. 2006; Lulsdorf et al. 2011). The literature includes techniques for rapid generation turnover comprising in vitro completion of lifecycle as well as a combination of in vivo growth with in vitro immature seed germination to truncate seed fill (Franklin et al. 2000; Ochatt et al. 2002; Surma et al. 2013; Ribalta et al. 2014; Mobini et al. 2015; Mobini and Warkentin 2016; Bermejo et al. 2016). To date, research has focused on a narrow range of early flowering cultivars, neglecting the problems inherent in obtaining single-seed-descent (SSD) progeny from hybrids with later flowering types. One exception is the study of Ribalta et al. (2016) that provides an in vitro/in vivo accelerated single seed descent protocol for genotypes of pea (Pisum sativum L.) ranging from early to very late flowering. For broad application in breeding programs, there is a clear need to devise a robust and reproducible technique (a) to accelerate flowering and (b) for healthy embryo development over a wide range of phenotypically diverse genotypes of cool-season legumes.
The goal of this research was to examine the effect of light quality on rapid in vivo floral onset in diverse genotypes of pea, chickpea (Cicer arietinum L.), narrow-leaf lupin (henceforth called lupin) (Lupinus angustifolius L.), lentil (Lens culinaris Medik.) and faba bean (Vicia faba L.), and to develop predictive models for time to flowering under the optimised light conditions. We used lupin, a species known for its recalcitrance to in vitro manipulation (Bayliss et al. 2004; Surma et al. 2013), to demonstrate how combining growth under optimized conditions for early flowering with in vitro germination of immature seed can halve generation turnover time compared to conventional SSD systems.
The influence of photoperiod and temperature on the rate of progress towards flowering has been well studied in the grain legumes and can be evaluated using linear models that describe the flowering behavior in response to these factors (Summerfield et al. 1985, 1991; Erskine et al. 1994). Light quality also plays a key role in the regulation of time to flowering in plants (Weller et al. 2001); however, its effect is still unclear but exploitable. Light modulates a wide range of plant photomorphogenic responses, including germination, stem elongation, leaf expansion and flowering (Moe and Heins 1990; Spalding and Folta 2005). Plants perceive a much different spectrum of light than do humans, making it critical to understand the amount of plant-usable light different sources emit. Traditional light technologies, such as metal halide, fluorescent or high pressure sodium, provide fixed emission spectra composed of many bands in the wavelength range from 320 to 800 nm without the possibility of modifying illumination parameters (spectrum and pulse characteristics) (Naznin and Lefsrud 2014). The recent rise of affordable light emitting diode (LED) lighting systems enables accurate manipulation of a third factor, light quality. LED optics can direct photons at the die level, delivering a more uniform and precise beam of usable light, with considerably less light spillage than traditional light sources (Massa et al. 2008; Stutte 2009; Cope and Bugbee 2013). In addition, LED arrays can be selected to target wavelengths required by plants to elicit a specific photomorphogenic response (Naznin and Lefsrud 2014).
The action of light on plant growth and development is mediated by photoreceptors. Phytochromes and cryptochromes are the major photoreceptor families involved in light perception in plants. Phytochromes, located in the leaves, are the receptors sensitive to light in the red (R) and far-red (FR) regions of the visible spectrum, and they play a well-established role in regulating time to flowering (Weller and Ortega 2015). The phytochromes come in two chemical forms; phytochrome FR (Pfr) and phytochrome R (Pr). The Pfr and Pr forms are mutually interconvertible by appropriate irradiation, with Pfr considered as the physiologically active form of the phytochrome (Rüdiger and Thümmler 2012). Changes in light quality alter the phytochrome photoequilibrium thus affecting plant physiological responses (Stutte 2009). Flowering acceleration is promoted by FR and blue lights and is mediated by Pfr and blue light photoreceptors Cryptochromes 1 and 2. The main effect of R light on flowering repression is mediated by Pr (Ausín et al. 2005).
The cool-season legumes are considered to be facultative long-day (LD) plants whose flowering is promoted by a LD condition. Research in the model LD species pea suggests that FR enriched light mediates flowering through the photoperiod pathway and in LD plants extended exposures to light with a low R to FR ratio (R:FR) is most effective for early flower induction (Runkle and Heins 2001; Weller et al. 2001; Cummings et al. 2007). In general, light with high R:FR (e.g. fluorescent lamps) suppresses stem elongation and promotes lateral branching, whereas light with a low R:FR (e.g. incandescent lamps) strongly enhances stem elongation and inhibits lateral branching (Moe and Heins 1990). Changes in environmental conditions have a significant effect on plant photomorphogenic responses but spectral shifts (in particular the R:FR) clearly play a major role in plant growth and development. We reason that optimisation of the R:FR ratio in the artificial light source (providing the photoperiod requirement) will enable us to trigger early floral evocation and accelerate flowering in the five target LD legume species, chickpea, pea, lentil, lupin and faba bean. The ability to trigger flowering across a broad range of species and flowering type genotypes and the development of models for predicting the flowering behaviour, will open new possibilities to exploit plant morphogenesis to develop new breeding tools.
Complex protocols have been developed for the in vitro rescue of enfeebled hybrid embryos of a number of cool-season grain legumes (Palmer et al. 2002; Mallikarjuna 2003; Clarke et al. 2006). More recently, we and others have exploited in vitro immature seed culture in selfed material to speed generation turnover in grain legumes, including pea, lentil and faba bean (Ochatt et al. 2002; Ochatt and Sangwan 2010; Surma et al. 2013; Ribalta et al. 2014, 2016; Mobini et al. 2015). However, progress in Lupinus biotechnology is still limited. Lupins are generally recalcitrant to tissue culture manipulation; moreover, their regeneration in vitro is highly genotype-specific and requires considerable technical expertise (Wolko et al. 2011). Recently, Surma et al. (2013) presented preliminary results on the optimisation of immature seed in vitro culture conditions for Lupinus species aimed at the development of a rapid generation system. Low survival rate after ex vitro acclimation was identified as a key issue in the development of an efficient protocol for Lupinus species. We predict that combining in vivo growth under light quality conditions optimised for rapid floral initiation with in vitro immature seed culture at a precise developmental stage will lead to a robust and relatively simple method to accelerate generation turnover in lupins.
Materials and methods
The research was undertaken within the controlled plant growth facilities at the University of Western Australia, Perth (lat: 31°58′49″S; long: 115°49′7″E). To study the effect of light quality on floral initiation, phenologically contrasting pairs of early (e) and late (l) flowering cultivars of five cool-season grain legumes were selected. The included genotypes were: PBA Twilight (e) and Kaspa (l) for pea; Rupali (e) and PBA Hattrick (l) for chickpea; PBA Blitz (e) and Northfield (l) for lentil; 1952-1 (e) and Icarus (l) for faba bean; and Mandelup (e) and Tanjil (l) for lupin. These lupin genotypes were also used to develop an in vivo/in vitro generation acceleration protocol.
Effect of light quality on time to flowering in vivo
Plants were grown in various environments with different light spectra (Fig. 1; Table 1), including blue LED from L series Valoya (Helsinki, Finland) lights (Environment 1), far-red enriched LED lights (AP67 spectrum) from L series (Environment 2) and B series (Environment 3) Valoya lights, metal halide lamps (MF-100LE, 1000 W, Eye Lighting; Environment 4), and natural light in February/March (Environment 5). In all environments, the temperature was set at 24/20 °C day/night. In Environments 1 to 4 a photoperiod of 20 h was selected, as per Ribalta et al. (2014). Seeds were sown in plastic 0.4 L pots filled with steam pasteurised potting mix (UWA Plant Bio Mix—Richgro Garden Products Australia Pty Ltd). Plants were watered daily and fertilised weekly with a water soluble N-P-K fertiliser (19-8.3-15.8) with micronutrients (Poly-feed, Greenhouse Grade, Haifa Chemicals Ltd.) at a rate of 2 g/pot. Time to flower initiation (days from sowing to the anthesis stage of first flower) was recorded under the different growth conditions. Anthesis stage was deemed to have occurred when petals extended beyond the sepals.
Light spectrum profiles [wavelength (nm) vs. photon flux density (PFD), µmol m−2 s−1] across the five environments used in our study. E1: blue LED (Valoya L series); E2: blue + far red LED (Valoya L series); E3: blue + far red (Valoya B series); E4: metal halide; E5: natural light at 12 pm. Black-dotted vertical lines indicate the broad band red (R, 600–700 nm) and far-red (FR, 700–780 nm) wavelengths with % of total light spectrum values for each region indicated within each box. White-dotted vertical lines indicate the narrow band R (650 ± 5 nm) and FR (730 ± 5 nm) regions used for calculating the ratio of R:FR light
Light quality measurements
Light measurements were made with a Sekonic C7000 SpectroMaster spectrometer (Sekonic Corp., Tokyo, Japan). Light intensity under natural conditions (Environment 5) was measured during the months of February/March at 1200 h. These values were averaged over three scans in the range of 400–780 nm: blue (B, 400–500 nm), red (R, 600–700 nm) and far red (FR, 700–780). Relative (%) and absolute (intensity, µmol m−2 s−1) values in the ranges of B, R and FR were calculated (Cope and Bugbee 2013). Red to far-red ratio (R:FR) calculations followed the method by Runkle and Heins (2001): photon irradiance between 655 and 665 nm/photon irradiance between 725 and 735 nm.
In vivo/in vitro generation acceleration protocol in lupin
Using lupin as an example, we show the benefits of the combination of far-red floral initiation and immature seed in vitro culture on the acceleration of generation cycles. Lupin plants representing early (Mandelup) and late (Tanjil) flowering phenology were grown in our optimal environment (Environment 3; Fig. 1; Table 1) and flowers were individually tagged at anthesis. Pods containing immature seeds were harvested at 20, 22, 24 and 26 days after pollination (DAP) and surface-sterilised in 70 % (v/v) ethanol for 1 min, followed by 2 min in sodium hypochlorite (21 g/l), and three rinses in sterile deionised water. Pods were opened under sterile conditions and immature seeds (1–4 seeds per pod), without integuments, were cultured in 200 ml polycarbonate vessels sealed with a screw cap (Sarstedt Australia Pty Ltd, Adelaide) containing 25 ml of autoclaved modified B5 medium (Gamborg et al. 1968) supplemented with 2 % sucrose (Sigma), 0.6 % agar (Type M, Sigma) and a pH of 5.6. Seeds were incubated at 25 °C in the dark for the first 24 h and then transferred to Environment 3. After 7 days of in vitro culture, seedlings were transplanted to new polycarbonate vessels filled with a combination of steam pasteurised potting mix and perlite (1:1) and grown under the same conditions as the parent plants. Plants were hardened to in vivo conditions by placing them under a transparent polycarbonate lid to maintain high humidity for 1 week. Two weeks after transplanting, 10 seedlings per genotype from the 22, 24 and 26 DAP developmental stages were transferred to 0.4 L pots under the same growing conditions. Time taken to anthesis of the first flower was recorded. A minimum of 50 seeds per genotype per treatment were cultured. For all treatments, the percentage of germination after 4 days of in vitro culture, survival rate after 10 days in vivo and time to flowering were recorded. Embryos were considered germinated when both radicle and shoot emergence was observed.
Experimental design and analysis
The experimental design was completely randomized and all treatments were repeated at least twice. Statistical analysis of the environment effect (Table 2) was performed by ANOVA, means were grouped in significance levels using Tukey’s test, and standard deviation values calculated using IBM SPSS Statistics 22.0 software.
For each genotype, the relative importance of the R:FR ratio and the FR photon load on flowering induction in environments E1–E4 (E2–E4 for Icarus) was assessed by building optimised linear models. The individual effect of these factors and their interaction were analysed in RStudio (Version 0.99.484, RStudio, Inc.) using the ‘lm’ and ‘anova’ functions. The models were optimised by building them in a stepwise fashion. First, each of the main effects (R:FR and absolute FR photons) was modelled separately against days to flowering using the ‘lm’ function. The model providing the most significant (lowest) P-value for this main effect was selected and then the remaining effect was added to create a purely additive model. The ‘anova’ function was then used as a nested model comparison to test the null hypothesis that the new model (with both effects) was the same as the old model (with a single effect). If the resulting P-value for this nested model comparison was <0.05, we concluded that the new model did provide a significantly improved fit to the data and so the single effect model was discarded and the additive model adopted. Where additive models were selected over single effect models, the general process was then repeated to compare the additive model to a model including interaction effects. If in any case the new model did not provide a significantly better fit to the data then the simpler model was chosen as the optimal model. The results of this stepwise model optimisation are summarised in Table 3, which includes the intercept of the optimal model, the slope and the p-values of any significant effects. For the faba genotype ‘Icarus’, flowering did not occur within the 70 days experimental period in E1 and, so the model for this genotype was built using data from only E2 to E4.
Statistical analysis of the germination percentage after 4 days of culture and survival percentage of in vitro cultured lupin seed was performed using χ 2 variance test for homogeneity of the binomial distribution using IBM SPSS Statistics 22.0 software. Unless otherwise stated, statistical tests were considered significant when P ≤ 0.05.
Results and discussion
Effect of light quality on time to flowering in vivo
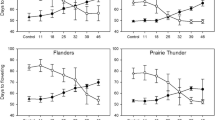
Light quality and in particular the red to far-red ratio (R:FR) had a clear effect on time to floral initiation in the five species studied (Table 2). In all species and contrasting genotypes, the latest flowering was observed when the R:FR was above 3.5 (Environment 1, E1). Among environments with R:FR below 3.5 (E2–E4), the environment with the intermediate ratio (E3 = 2.9) produced the fastest mean flowering in seven genotypes and significantly fastest flowering in four out of the 10 genotypes used in the research. These results suggest R:FR by itself was insufficient to explain the observed flowering responses.
It is well known that light quantity and quality interact to direct physiological processes (Moe and Heins 1990). Light relative values, in particular the R:FR, are commonly used to predict time to floral initiation in a number of species, including legumes (Runkle and Heins 2001; Cummings et al. 2007; Mobini et al. 2016). However, Cope and Bugbee (2013) indicated that prediction of some developmental responses can be improved with the use of absolute photon load. Smith (1994) stated that any photochemical reaction has the potential to act as a photon counter, as long as a metabolic mechanism exists for transduction of the quantity of photochemical product into a biological change. In our research, when comparing the time to flowering among environments with R:FR below 3.5 (E2–E4), the environment with the highest number of photons in the FR region of the light spectrum (E3, FR photon load = 52.9 µmol m−2 s−1) was the most inductive. These results indicate the importance of considering both relative (R:FR) and absolute (number of photons in the FR region) light values for the optimisation of floral onset in the grain legumes.
When comparing phenologically diverse genotypes, we observed greater response to light spectra in the later flowering genotypes compared to early genotypes. For example, the late flowering lupin genotype (Tanjil) flowered 21.6 days earlier and the early flowering genotype (Mandelup) flowered 12.4 days earlier in the fastest environment (E3) compared to the slowest environment (E1, Table 2; Fig. 2). This trend was consistent across the species and genotypes tested. The identification of optimised conditions for early floral induction across a range of phenotypically diverse genotypes is crucial to the development of efficient and broadly applicable breeding methods for the grain legumes species.
Regressions between A- red to far-red ratio (R:FR) and B- number of photons in the FR region (µmol m−2 s−1) and days to flowering in early (Mandelup) and late flowering (Tanjil) lupin genotypes. Open symbols = Mandelup; closed symbols = Tanjil. E1 = filled triangle, opened triangle; E2 = filled circle, opened circle; E3 = filled square, opened square and E4 = filled diamond, opened diamond
In general, the flowering response of all the genotypes studied can be expressed as a linear function of the R:FR and the FR photon load. Figure 2 shows the effect of light on days to flowering using early (Mandelup) and late (Tanjil) flowering lupin genotypes as an example. This illustrates the trend of later flowering genotypes showing a stronger response in time to flowering to the R:FR and to the number of photons in the FR region compared to the earlier genotypes.
Since both FR photon load and R:FR accelerated time to flowering, linear models were built for all genotypes studied using these factors as variables to determine their relative importance and effect (Table 3). The number of photons in the FR region had a more significant effect than the R:FR in eight out of the 10 genotypes (exceptions were pea genotype Kaspa and lupin genotype Mandelup). In five genotypes (Rupali, PBA Blitz 1952-1, Icarus and Tanjil), the FR photon load was the only significant factor (i.e. the effect of the R:FR was non-significant once the FR photon load effect was applied, model 1). In the genotypes PBA Twilight, PBA Hattrick and Northfield, the optimal model was additive (i.e. non-significant interaction between variables, models 2 and 4). As expected, increasing photon load in the FR region of the light spectrum hastened flowering in all genotypes, except in the pea genotype PBA Twilight. This effect ranged between 1.2 and 0.07 days faster flowering per unit of photons in the FR region. The proposed models provide a prediction of flowering behaviour of the different species under different R and FR conditions and reinforce the importance of absolute FR photon load on accelerating floral initiation.
The positive effect of extended photoperiods on floral initiation in long day species like the temperate grain legumes has been well documented (Nelson et al. 2010). In our experiments, a strong effect of daylength on time to flowering was observed when comparing environments with short (13–14 h, E5) and long photoperiods (20 h, E2–E4; Table 2). Despite the magnitude of the daylength effect, light quality effects are still significant and present an opportunity for further acceleration of floral initiation. Comparison of those environments with a consistent 20 h photoperiod (E1–E4) showed significant responses to light quality for all genotypes studied. Advances in LED technology are now making it economical to exploit these effects (Stutte 2009).
In vivo/in vitro generation acceleration protocol in lupin
In vitro based strategies have been shown to accelerate generation turnover in pea, lentil and faba bean (Ochatt et al. 2002; Ochatt and Sangwan 2010; Ribalta et al. 2014, 2016; Mobini et al. 2015; Bermejo et al. 2016). However, no robust protocols have been developed for lupin, a species known for its recalcitrance to in vitro culture techniques (Surma et al. 2013; Bayliss et al. 2004; Wolko et al. 2011). We employed the optimum light environment identified from our earlier results for in vivo growth, along with a novel in vitro immature seed culture technique for lupin, to demonstrate the benefit our technology can offer to accelerate generation turnover. We and others (Gallardo et al. 2006; Ochatt 2015; Ribalta et al. 2016) illustrate the importance of legume seed physiological maturity for successful precocious in vitro seed germination and fitness of germinated plants. Surma et al. (2013) presented early results on the development of an in vitro protocol for immature seed germination for Lupinus species. In this research, the culture of immature seeds resulted in high levels of in vitro germination, but with low ex vitro survival rates. Our results show in vitro culture of immature seeds 26 days after pollination (DAP) resulted in the highest level of germination after 4 days, with 100 % seedling survival (Table 4) and successful flowering for both lupin genotypes. No plants from the 22 DAP treatment survived glasshouse transfer. In pea, Ribalta et al. (2016) observed the highest germination and survival rate when embryo physiological maturity was reached at 18 DAP. In our research, delaying the harvest of immature lupin seeds from 24 to 26 DAP, resulted in significantly higher seedling survival rate. These results suggest that under our optimised conditions, lupin embryo physiological maturity is achieved between 24 and 26 DAP.
Since the seedling survival rate observed at 24 DAP was impracticably low, flowering was only recorded for the 26 DAP treatment. Floral onset occurred 36.3 ± 0.57 days after in vitro culture for the early flowering cultivar Mandelup and after 42.0 ± 1.0 days for the late flowering cultivar Tanjil. Important for rapid generation turnover, we observed minimal delay penalty caused by the in vitro culture of immature seeds 26 DAP, compared to the use of mature seed in the early flowering lupin genotype Mandelup (Table 2). However, the late flowering genotype Tanjil had a delay of 8 days compared to mature seed. Nevertheless, growing plants under our most appropriate in vivo conditions for rapid floral initiation combined with in vitro culture of immature seeds 26 DAP would enable the production of over five generations per year in both early and late flowering lupin genotypes. This approximately doubles what is achieved with conventional single-seed-descent systems (Croser et al. 2015). To our knowledge, this is the first report of a robust methodology to accelerate generation turnover in phenotypically diverse lupin genotypes.
Conclusions
The effect of light quality on early floral onset in phenotypically diverse genotypes of five cool-season grain legumes was examined. In general, low R:FR ratios resulted in faster flowering induction. However, the ratio by itself was insufficient to explain the observed flowering responses. In view of these results, the effect of FR photon load on floral induction was investigated. In environments with R:FR ratios below 3.5, the environment with the highest number of photons in the FR region was the most inductive. In addition, a stronger flowering response to altered light quality was observed in later flowering genotypes compared to early genotypes, enabling a drastic compression of time to flowering between phenologically diverse genotypes. The importance of considering both relative (R:FR ratio) and absolute (photons in the FR region) light values for flower induction in cool-season grain legumes was demonstrated and models for the prediction of flowering behavior in response to light quality were developed.
A novel protocol for robust in vitro culture of immature seeds at a precise developmental stage was developed for lupin, a species known for its recalcitrance to in vitro manipulation. We show how combining this in vitro protocol with growth under our optimal conditions for early flowering drastically speeds generation turnover. The improved understanding of the effect of light quality on flowering regulation and the development of robust in vitro protocols for immature seed germination will assist the development and exploitation of biotechnological tools for legume breeding.
References
Ausín I, Alonso-Blanco C, Martinez-Zapater JM (2005) Environmental regulation of flowering. Int J Dev Biol 49:689–705
Bayliss KL, Wroth JM, Cowling WA (2004) Pro-embryos of Lupinus spp. produced from isolated microspore culture. Aust J Agric Res 55:589–593
Bermejo C, Gatti I, Cointry E (2016) In vitro culture to shorten the breeding cycle in lentil (Lens culinaris Medik). Plant Cell Tissue Organ Cult. In press, doi:10.1007/s11240-016-1065-7
Clarke HJ, Wilson JG, Kuo I, Lülsdorf MM, Mallikarjuna N, Kuo J, Siddique KHM (2006) Embryo rescue and plant regeneration in vitro of selfed chickpea (Cicer arietinum L.) and its wild annual relatives. Plant Cell Tissue Organ Cult 85:197–204
Cope KR, Bugbee B (2013) Spectral effects of three types of white light-emitting diodes on plant growth and development: absolute versus relative amounts of blue light. HortScience 48:504–509
Croser JS, Lulsdorf MM, Davies PA, Clarke HJ, Bayliss KL, Mallikarjuna N, Siddique KHM (2006) Toward doubled haploid production in the Fabaceae: Progress, constraints, and opportunities. Crit Rev Plant Sci 25:139–157
Croser J, Ribalta F, Navarro MP, Munday C, Nelson K, Edwards K, Castello M, Bennett R, Erskine W (2015) Accelerated Single Seed Descent (aSSD)–a novel breeding technique to speed attainment of homozygosity. Proceedings 2nd international symposium on agricultural technology (ISAT2015), Bangkok, Thailand, 30 June
Cummings IG, Reid JB, Koutoulis A (2007) Red to far-red ratio correction in plant growth chambers—growth responses and influence of thermal load on garden pea. Physiol Plant 131:171–179
Erskine W, Hussain A, Tahir M, Bahksh A, Ellis RH, Summerfield RJ, Roberts EH (1994) Field evaluation of a model of photothermal flowering responses in a world lentil collection. Theor Appl Genet 88:423–428
Franklin G, Pius PK, Ignacimuthu S (2000) Factors affecting in vitro flowering and fruiting of green pea (Pisum sativum L.) Euphytica 115:65–74
Gallardo K, Kurt C, Thompson R, Ochatt S (2006) In vitro culture of immature M. truncatula grains under conditions permitting embryo development comparable to that observed in vivo. Plant Sci 170:1052–1058
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Lulsdorf MM, Croser JS, Ochatt S (2011) Androgenesis and doubled-haploid production in food legumes. In: Pratap A, Kumar J (eds) Biology and breeding of food legumes. CABI Publishing, Cambridge, pp 159–177
Mallikarjuna N (2003) Wide hybridization in important food legumes. In: Improvement strategies of Leguminosae biotechnology. Springer, Dordrecht, pp 155–171
Massa GD, Kim H-H, Wheeler RM, Mitchell CA (2008) Plant productivity in response to LED lighting. HortScience 43:1951–1956
Mobini SH, Warkentin TD (2016) A simple and efficient method of in vivo rapid generation technology in pea (Pisum sativum L.). In Vitro Cell Dev Biol. in press, doi:10.1007/s11627-016-9772-7
Mobini SH, Lulsdorf M, Warkentin TD, Vandenberg A (2015) Plant growth regulators improve in vitro flowering and rapid generation advancement in lentil and faba bean. In Vitro Cell Dev Biol 51:71–79
Mobini S, Lulsdorf M, Warkentin T, Vandenberg A (2016) Low red: far-red light ratio causes faster in vitro flowering in lentil. Can J Plant Sci. doi:10.1139/CJPS-2015-0282
Moe R, Heins R (1990) Control of plant morphogenesis and flowering by light quality and temperature. Acta Hortic 272:81–89
Naznin MT, Lefsrud MG (2014) Impact of LED irradiance on plant photosynthesis and action spectrum of plantlet. Proceedings SPIE optical engineering and applications. Int Soc Opt Photonics 19:921602–921605
Nelson MN, Berger JD, Erskine W (2010) Flowering time control in annual legumes: Prospects in a changing global climate. CAB Rev Perspect Agric Vet Sci Nutr Nat Res 5:49–62
Ochatt SJ (2015) Agroecological impact of an in vitro biotechnology approach of embryo development and seed filling in legumes. Agron Sustain Dev 35:535–552
Ochatt SJ, Sangwan RS (2010) In vitro flowering and seed set: acceleration of generation cycles. In: Davey MR, Anthony P (eds) Plant cell culture: essential methods. John Wiley & Sons, Ltd., Chichester, pp 97–110
Ochatt SJ, Sangwan RS, Marget P, Ndong YA, Rancillac M, Perney P (2002) New approaches towards the shortening of generation cycles for faster breeding of protein legumes. Plant Breed 121:436–440
Palmer JL, Lawn RJ, Adkins SW (2002) An embryo-rescue protocol for Vigna interspecific hybrids. Aust J Bot 50:331–338
Ribalta FM, Croser JS, Erskine W, Finnegan PM, Lulsdorf MM, Ochatt SJ (2014) Antigibberellin-induced reduction of internode length favors in vitro flowering and seed-set in different pea genotypes. Biol Plant 58:39–46
Ribalta FM, Pazos-Navarro M, Nelson K, Edwards K, Ross JJ, Bennett RG, Munday C, Erskine W, Ochatt SJ, Croser JS (2016) Precocious floral initiation and identification of exact timing of embryo physiological maturity facilitate germination of immature seeds to truncate the lifecycle of pea. Plant Growth Regul. doi:10.1007/s10725-016-0211-x
Rüdiger W, Thümmler F (2012) The phytochrome chromophore. In: Kendrick RE, Kronenberg GHM (eds) Photomorphogenesis in plants. Kluwer Acad Publ, Dordretch, pp 51–67
Runkle ES, Heins RD (2001) Specific functions of red, far red, and blue light in flowering and stem extension of long-day plants. J Am Soc. Hort Sci 126:275–282
Smith H (1994) Sensing the light environment: the functions of the phytochrome family. In: Photomorphogenesis in plants. Springer, Dordrecht, pp 377–416
Spalding EP, Folta KM (2005) Illumination topics in plant photobiology. Plant Cell Environ 28:39–53
Stutte GW (2009) Light-emitting diodes for manipulating the phytochrome apparatus. HortScience 44:231–234
Summerfield RJ, Roberts EH, Erskine W, Ellis RH (1985) Effects of temperature and photoperiod on flowering in lentils (Lens culinaris Medic.) Ann Bot 56:659–671
Summerfield RJ, Roberts EH, Ellis RH, Lawn RJ (1991) Towards the reliable prediction of time to flowering in six annual crops. I. The development of simple models for fluctuating field environments. Exp Agric 27:11–31
Surma M, Adamski T, Swiecicki W, Barzyk P, Kaczmarek Z, Kuczynska A, Krystkowiak K, Mikolajczak K, Ogrodowicz P (2013) Preliminary results of in vitro culture of pea and lupin embryos for the reduction of generation cycles in single seed descent technique. Acta Soc Bot Pol 82:231–236
Weller JL, Ortega R (2015) Genetic control of flowering time in legumes. Front. Plant Sci 6:1–13
Weller JL, Beauchamp N, Kerckhoffs LHJ, Platten JD, Reid JB (2001) Interaction of phytochromes A and B in the control of de-etiolation and flowering in pea. Plant J 26:283–294
Wolko B, Clements JC, Naganowska B, Nelson MN, Yang Ha (2011) Lupinus. In: Kole C (ed) Wild crop relatives: genomic and breeding resources: legumecrops and forages. Springer, Berlin, pp 153–206
Acknowledgments
This work was supported by the Grains Research and Development Corporation [UWA00159]. We thank Mr B. Piasini and Mr L. Hodgson for glasshouse expertise and Ms Christine Munday and Ms Simone Wells for technical assistance.
Author contributions
JSC, MPN, FMR conducted experimental design, data analysis and manuscript writing. WE, RC and RGB were involved in strategic experimental input. KE conducted in vitro experiments. ST conducted glasshouse experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s11240-016-1127-x.
Rights and permissions
About this article
Cite this article
Croser, J.S., Pazos-Navarro, M., Bennett, R.G. et al. Time to flowering of temperate pulses in vivo and generation turnover in vivo–in vitro of narrow-leaf lupin accelerated by low red to far-red ratio and high intensity in the far-red region. Plant Cell Tiss Organ Cult 127, 591–599 (2016). https://doi.org/10.1007/s11240-016-1092-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-016-1092-4