Abstract
Jatropha curcas has considerable potential for production of biodiesel and secondary metabolites with medicinal uses. Herein, J. curcas cell suspension cultures were established to study the effect of jasmonic acid (JA) elicitation on triterpene production and oxidative responses. Cell cultures grown in dark conditions reached maximum biomass accumulation at the 12th day of culture (14.3 ± 0.45 g DW L−1) with a specific growth rate μ = 0.131 d−1. Elicitation with JA (200 or 400 μM) on 4-days-old cell cultures caused reduction in biomass and triterpene contents. In contrast, application of 200 μM JA at the 7th day of culture triggered triterpene accumulation by three times (1180 ± 12.3 μg g−1 DW, at day 2) with respect to control, without significant changes in biomass and viability. After 2 days of elicitation, betulin increased up to 7.3-fold (from 110.6 ± 20.7 to 808.7 ± 55.4 μg g−1 DW), while betulinic acid reached the maximum amount at day 6 after elicitation (245.6 ± 3.7 to 835 ± 41.5 μg g−1 DW). Lupeol presented a moderate increase (167.9 ± 51.0–288.8 ± 7.3 μg g−1 DW) along 8 days after elicitation. In correlation with triterpene production, JA application induced oxidative responses evaluated by an increase in the H2O2 levels up to three times and of malondialdehyde by 59 %. At day 4 after elicitation, catalase showed higher increase (122 %) than peroxidases (63 %) and ascorbate peroxidase (26 %). Incorporation of radioactive labels from (R,S)-[2-14C]mevalonic acid in triterpenes and sterols confirmed its role as metabolic precursor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Jatropha curcas (Euphorbiaceae), commonly known as “physic nut” or “piñoncillo”, is native to Central America, although is widely distributed in many tropical regions of the world including Mexico. This plant with multiple attributes has a significant potential for biodiesel production and contains a variety of remarkable metabolites with medicinal and therapeutic uses (Kumar and Sharma 2008). Most J. curcas accessions are toxic due to the presence of phorbol esters. Nevertheless, non-toxic specimens have been found in some areas of Mexico (Valdés-Rodríguez et al. 2014). This plant has proven to withstand various types of biotic and abiotic stresses due to their defense mechanisms, which include the presence of several secondary metabolites, mainly diterpenes, triterpenes, and flavonoids (Kumar and Sharma 2008). Despite their importance, studies on the factors that induce the production of such secondary metabolites have been insufficiently explored. Particularly for J. curcas, it has been reported the presence of triterpenes as lupeol, amyrin and phytosterols in the stem bark and seeds (Adebowale and Adedire 2006; Falodun et al. 2011). Pentacyclic triterpene acids have received much attention due to their pharmacological properties as anti-HIV, anti-inflammatory, and anti-tumor agents (Laszczyk 2009). Their in vitro production under controlled conditions by different plant cell cultures has been documented (Srivastava et al. 2011; Pandey et al. 2015). Cell suspension cultures provide a sustainable source of natural substances, including metabolites with high commercial value such as the pentacyclic triterpenes (Hu and Zhong 2008; Lambert et al. 2011). The most effective strategy to increase the production of secondary metabolites in cell cultures is through an elicitation process. The elicitor signal perception initiates a signal transduction network that leads to activation or de novo biosynthesis of transcription factors, which regulate the expression of biosynthetic genes involved in plant secondary metabolism (Zhao et al. 2005). Jasmonic acid (JA) and its methyl ester derivative methyl jasmonate (MeJA) are lipid-based hormone signals that regulate a wide variety of physiological process in plants. Likewise, it is known that exogenous JA can act as an elicitor in plants (Zhao et al. 2005; Wasternack and Hause 2013). In diverse plant cell cultures, jasmonates have been used to induce the production of pentacyclic triterpenes, mainly oleanolic acid and ursolic acid (Norrizah et al. 2012), as well as β-amyrin (Broeckling et al. 2005), betulinic acid (Pandey et al. 2015) and other triterpenes (James et al. 2013). In the presence of elicitors, there is an immediate cellular response to trigger plant defense signals with increased accumulation of reactive oxygen species (ROS) such as hydrogen peroxide (H2O2), superoxide anions (O2 −) and hydroxyl free radicals (HO·). Different cell compartments may activate different defensive systems to reduce ROS excess, using antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), peroxidases (POD), and glutathione reductase (GR), among others, as well as non-enzymatic components such as ascorbate, glutathione, carotenoids, and phenolic compounds. The main sources of ROS production are chloroplast and peroxisomes in the light and mitochondria in the dark (Foyer and Noctor 2005). Oxidative stress is defined as a serious imbalance between ROS production and the antioxidant defenses. This situation can cause cellular damage and an increase in the secondary metabolites (Zhao et al. 2005). Hence, it has been also reported that application of MeJA induced the ROS burst in dark-grown cultured cells of parsley (Kauss et al. 1994) and Taxus chinensis (Wang and Wu 2005), as well as in protoplasts of Arabidopsis (Sasaki-Sekimoto et al. 2005). However, studies about the linkage between jasmonates and ROS signaling that modulate the secondary metabolites production are still limited.
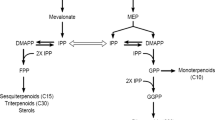
In several plant species, it has been postulated that sterols and triterpenes are biosynthesized through the mevalonate pathway, with 2,3-oxidosqualene as a common intermediate for these two pathways. Subsequently, 2,3-oxidosqualene is converted to cyclic compounds by different kinds of cyclases to afford a wide variety of triterpenoid skeletons. Then, oxidation, substitution, and glycosylation, also mediated by an assortment of enzymes, give rise to diverse types of functionalized triterpenes. Due to their complexity, many enzymes involved in the later steps of their biosynthesis are not yet known or characterized (Fukushima et al. 2011; Lambert et al. 2011). Additionally, little direct experimental evidence to confirm mevalonate as biosynthetic precursor of specific triterpenes has been reported (Akashi et al. 1994; Flores-Sánchez et al. 2002).
In this work, the effect of JA elicitation on triterpene production and its relation with the oxidative responses and antioxidant enzyme activities in J. curcas cell suspension cultures were investigated. In addition, evidence of mevalonate as a precursor in the triterpene biosynthesis of J. curcas is presented.
Materials and methods
Plant material and cell suspension cultures
Leaf explants of Jatropha curcas (non-toxic accession) grown in pots containing soil were surface-sterilized with 70 % (v/v) ethanol and sodium hypochlorite. Callus cultures were induced in Gamborg B5 (Gamborg et al. 1968) medium supplemented with 20 g sucrose L−1, 5.4 μM naphthalene acetic acid (NAA), 8.3 μM picloram, and 2 % (w/v) phytagel. Cell suspension cultures were established from 20-days-old callus cultures. Callus tissues (2 g) were transferred to Erlenmeyer flasks (250 mL) containing 50 mL Jc-pale medium (Gamborg B5 with 2.7 μM NAA, 4.1 μM picloram and 20 g sucrose L−1). The cultures were maintained on a rotatory shaker at 110 rpm and 25 ± 2 °C, under dark conditions. Cells were subculture every 7 days using a 1:4 dilution of cells into fresh medium. Subcultures were carried out during the exponential growth phase.
Growth kinetics
Erlenmeyer flasks (250 mL) containing 50 mL Jc-pale medium were inoculated with 5 g fresh weight (FW) of 7-day-old J. curcas cells. Samples were collected at 2-day intervals during a 16-day period. Each point of the growth curve is represented by the mean of three independent determinations. Cell viability was determined using fluorescein diacetate according to Huerta-Heredia et al. (2009). To determine growth kinetics, cells were separated by suction filtration, lyophilized and weighted for measuring the dry cell weight (DW). Specific growth rate (μ) was calculated by the slope of plotting the natural logarithm of biomass versus time. Glucose concentration was measured in the culture supernatants using a YSI 2700 Select biochemical analyzer (Yellow Springs Instruments).
JA elicitation
JA (Sigma Aldrich) was dissolved in ethanol and then a stock solution of 100 mM with 30 % (v/v) ethanol was prepared and sterilized by microfiltration through 0.45 µm filters. The elicitor was individually added to 4- and 7-days-old cell suspension cultures of J. curcas growing in Erlenmeyer flasks at the same conditions as described above, giving final JA concentrations of 200 and 400 μM. Equal volumes of 30 % (v/v) ethanol were added to the control cultures. Cell suspension cultures of J. curcas were harvested after 2 days of JA addition. The experiments were performed in triplicate. A subsequent experiment was performed adding JA 200 μM on 7-days-old cell suspension cultures, growing under the same conditions as described above. Cells were harvested at 0, 2, 4, 6, and 8 days after elicitation to determine the triterpene contents, oxidative responses, and antioxidant enzyme activities.
Triterpene extraction and quantification
J. curcas cells were frozen with liquid nitrogen, pulverized using a mortar, and lyophilized. Dried cells (0.1 g) were extracted twice with ethyl acetate. The organic layers were combined and evaporated to dryness under vacuum. The residues were dissolved in 600 μL methanol. The solutions were filtered through 0.25 μm nylon membranes and injected (30 μL) into an HPLC system (Varian Chromatograph ProStar 333) equipped with a photodiode array detector (Varian, Walnut Creek, CA) using a reversed-phase C18 column (Waters Spherisorb 5 mm, ODS2 of 250 mm length × 4.6 mm i.d.). Elution was carried out with a 90:10 mixture of methanol–water at a flow rate of 0.7 mL min−1, detecting at 205 nm. The retention times of the triterpenes were betulinic acid 9.8 min, betulin 13.4 min, and lupeol 22. 5 min.
Determination of H2O2 content
H2O2 level was determined according to Velikova et al. (2000). Samples (0.5 g FW) were frozen, ground with liquid nitrogen, and homogenized with 1 mL 0.1 % (w/v) trichloroacetic acid. The homogenate was centrifuged at 15,000 rpm for 15 min and 0.5 mL of the supernatant was added to 0.5 mL 10 mM potassium phosphate buffer (pH 7.0) and 1 mL of 1 M potassium iodide. Absorbance was read at 390 nm and the H2O2 concentration was calculated from a standard curve.
Determination of lipid peroxidation
Levels of lipid peroxidation were measured as the amount of malondialdehyde (MDA) that reacted with thiobarbituric acid (TBA) to form the TBA–MDA complex. Cells (0.3 g FW) were homogenized in 2 mL 0.1 % (w/v) trichloroacetic acid in a prechilled mortar at 4 °C. The homogenate was centrifuged at 14,000 rpm for 10 min at 4 °C and 1 mL of the supernatant was added to a test tube containing 1 mL of 0.5 % (w/v) TBA, and 5 μL of 3.75 % (w/v) butylhydroxytoluene. Samples were then vigorously mixed, heated at 95 °C for 30 min, and cooled on ice. Absorbance determinations were done at 532 and 600 nm. OD600 values were subtracted from the MDA-TBA complex values at 532 nm. The concentration of MDA was calculated from a calibration curve obtained by using 1,1,3,3-tetramethoxypropane, a precursor of MDA, at concentrations of 0–5 μM. MDA was expressed as μmol per g DW.
Protein extraction and enzyme activity assays
Cells (1 g FW) were ground with liquid nitrogen in a mortar with 2 % (w/w) polyvinylpolypyrrolidone and homogenized in 1 mL of extraction buffer (50 mM phosphate buffer pH 7.0 with 1 mM EDTA). The homogenate was centrifuged at 14,000 rpm for 15 min at 4 °C and the supernatant was used as the enzyme source. Protein concentration was determined spectrophotometrically using the Peterson (1977) method with bovine serum albumin as the standard protein. The ascorbate peroxidase (APX) activity was determined by monitoring the decrease in absorbance of ascorbic acid at 290 nm per 3 min according to Silva et al. (2010). The enzyme solution consisted of 50 mM potassium phosphate pH 7.0, 0.5 mM ascorbic acid, 30 mM H2O2 and 0.1 mL of enzyme extract, in a total volume of 2 mL incubated at 25 °C. The enzyme activity was calculated using the molar extinction coefficient ε290 = 2.8 mM−1 cm−1 expressed as units of enzyme activity (oxidation of 1 μmol of ascorbate per minute). The catalase (CAT) activity was determined by measuring the rate of H2O2 disappearance at 240 nm. The reaction mixture contained 1.8 mL of 50 mM potassium phosphate buffer (pH 7.0), 0.1 mL of 2 % H2O2 (v/v) and 0.1 mL of protein extract. The reaction was run at 25 °C for 3 min, after adding the enzyme extract. The rate of decrease in absorbance at 240 nm (ε240 = 39.4 mM−1 cm−1) was used to calculate the enzyme activity. One unit of catalase was defined as the amount of enzyme required for the decomposition of 1 μmol H2O2 per minute. Peroxidase (POD) activity was measured by following the H2O2-dependent oxidation of guaiacol at 470 nm, using ε470 = 26.6 mM−1 cm−1 for tetraguaiacol according to Pütter (1974). The total reaction mixture (3 mL) contained 100 mM sodium phosphate buffer (pH 7.0), 20.1 mM guaiacol, and 12.3 mM H2O2. The reaction was initiated by addition of 0.1 mL of protein extract and its progress was directly measured for 3 min by the increment in absorbance at 470 nm at 30 s intervals. POD activities were defined as the amount of enzyme that produced 1 μmol of tetraguaiacol per minute. The CAT, APX and POD activities are reported as units of enzyme per milligram of protein (U mg−1 protein).
(R,S)-[2-14C]mevalonic acid feeding
Four Erlenmeyer flasks (25 mL) containing 5 mL Jc-pale medium were inoculated with 1 g FW of cells and incubated at 110 rpm in dark conditions at 25 ± 2 °C. After 6 days of culture JA (200 μM) was applied to two flasks at same time. All flasks were fed with 0.33 mM (R,S)-[2-14C]mevalonic acid, 0.9 MBq. Cells were harvested after 168 h of application and washed twice with water. Then, the labeled metabolites were extracted twice with 2 mL chloroform. The organic layers were transferred to new tubes and dried under a nitrogen flow. Samples were dissolved in 100 μL of methanol and one aliquot (10 μL) was used for counting the incorporated radioactivity using a liquid scintillation spectrometer (Beckman Instruments, Inc.). The remaining material was employed for TLC analysis on Silica G-25 UV 254 plates. The eluent for TLC analysis was hexane–acetone 4:1. After drying, the TLC plates were β-scanned in a Molecular Imager apparatus (Bio-Rad). Compound identifications were done by comparing their retention factor (R f ) with cold standards for betulinic acid (R f 0.29), β-sitosterol/stigmasterol (R f 0.56), and lupeol (R f 0.68) after revealing with an anisaldehyde-sulfuric acid reagent.
Statistical analysis
Results were analyzed by one-way ANOVA using the SPSS software version 21.0 (SPSS Inc., Chicago IL). Significant differences between the means of parameters were determined by using the Tukey’s test (P < 0.05).
Results and discussion
Growth characteristics of J. curcas cell suspension cultures
J. curcas cell suspension cultures were successful initiated from friable callus achieved from leaf explants (Fig. 1a), using the same basal culture medium and sucrose content, but supplemented with the half concentration of growth regulators (2.7 μM NAA and 4.1 μM picloram), which allowed to obtain homogeneous cell cultures (Fig. 1b). Under the experimental growth conditions, cell viability was maintained during the cultured period at 90 % (Figs. 1c, d). Kinetics of the cell growth through 16 days showed that cells presented a typical growth curve (Fig. 2a). Cultures remained in the lag phase only 1 day and, after that, the biomass continuously increased to reach the maximum accumulation of 14.3 ± 0.45 g DW L−1, which was achieved on the 12th day. During the exponential phase, the cells presented a specific growth rate (μ) of 0.131 d−1. Within this period, glucose in the medium (Fig. 2b) was totally consumed which coincided with the onset of the stationary phase of growth. Triterpene production varied depending on the phase of the cell growth cycle (Fig. 2c), starting on the 2th day of growth. Although the highest concentration (490 ± 24 μg g−1 DW) was accumulated when cell cultures entered in the deceleration and stationary phase of growth, within 8-and 10-days (Fig. 2a) and remained around this concentration until the end of culture at the 16th day (Fig. 2c). Therefore, triterpene production seems to be mainly growth-associated. Many reports reflect the increasing interest in developing several types of in vitro culture from the energy crop J. curcas, as callus, somatic embryos, and micropropagated plantlets. In contrast, there are only a few reports of homogeneous J. curcas cell suspension cultures (Soomro and Memon 2007). In this work, the established cell cultures were able to produce pentacyclic triterpenes, which have their maximum concentration in the stationary phase of growth (Fig. 2c), consisting in mostly lupeol and betulin, which are 75 % of the total content, together with betulinic acid (ca. 25 %). Similarly, in several cell suspension cultures from different plant species, the maximum production of triterpenes is reached at the beginning of the deceleration of growth or during the stationary phases (Srivastava et al. 2011). Numerous metabolites have been isolated from J. curcas whole plants, among which are some isoprenoids, principally triterpenes as α-amyrin, lupeol, and taraxasterol from oil seeds (Adebowale and Adedire 2006) in addition to oleanolic acid and lupeol from stem bark (Falodun et al. 2011), but no reports have been published on the triterpene production in in vitro cultures.
Effect of JA concentration and time of elicitation
The cell growth and product yield responded differently to elicitation timing and dosage, which may be attributed to the differences among plant cell species, cell lines within species, and cellular physiological state (Zhao et al. 2005). In order to select the appropriate concentration and time of elicitation for inducing triterpene production, two JA concentrations, 200 or 400 μM, were applied to the cultures at the 4th or at the 7th day of growth (Table 1). Elicitation on 4-day-old cell cultures caused reduction in biomass by 25 %, as well as a decrease in triterpene production, even with the lowest concentration of JA (200 μM). In contrast, addition of 200 μM JA at the 7th day of elicitation triggered a significant increase in triterpene accumulation of 3.5-fold (1185.0 ± 5.3 μg g−1 DW) with respect to the control (338.9 ± 18.1 μg g−1 DW) without significant changes in biomass (Table 1), while the viability was maintained over 86 %. Treatments on day 7 with 400 μM JA concentration stimulated a noticeable increase in triterpenes (31 %), but a slight decline in biomass of 17 % was observed. Our result indicated that the response of triterpene production to the elicitor was closely related to its cellular physiological state in the early o later exponential phase of growth. Consequently, the JA concentration of 200 μM, applied to 7-days-old J. curcas cell suspension cultures, was selected for further elicitation studies. Jasmonic acid or its derivatives play important roles in the process of signal transduction pathways that regulate plant defense mechanisms (Wasternack and Hause 2013). In most studies, the increase in production of triterpenoids by JA in cell cultures and plant tissues mainly reveals the involvement of these compounds in plant defense mechanisms (Lambert et al. 2011; Fan et al. 2013). It has been reported that the time of elicitation is one of the key factors that affect cell growth and production of secondary metabolites in plant cell suspension cultures. Thus, in Taxus cell cultures, the repression of the growth through inhibition of cell cycle progression by MeJA elicitation occurs accompanied by the increased accumulation of paclitaxel (Patil et al. 2014). In Panax notoginseng cell cultures, elicitation with jasmonate derivates during the early exponential phase of growth up-regulate squalene synthase and squalene epoxidase, two common enzymes of triterpene and sterol biosynthesis, but down-regulate the sterol related cycloartenol synthase transcription, which implies that oxidosqualene can be directed from sterol to ginsenoside biosynthesis (Hu and Zhong 2008). Similar results about the decrease in free phytosterols and the increase in triterpene production were observed when MeJA was applied in the late or in the exponential phase of Centella asiatica cell suspension cultures (James et al. 2013).
Triterpene production in JA elicited cell cultures
The effect of 200 μM JA on intracellular accumulation of triterpenes was examined during 8 days after elicitation (Fig. 3a). After 2 days, JA stimulated a 3-fold increase in triterpenes (1180 ± 12.3 μg g−1 DW) with respect to the control (396 ± 16.3 μg g−1 DW). These triterpenes remained in high concentrations until the 6th day of elicitation, and after that they decreased to 658 ± 13.3 μg g−1 DW, which represented 26 % more than the control at that day. In addition, it was observed that JA induced a differential profile in triterpene production. Thus, betulin concentration was highly accumulated up to 7.3 times after 2 days of elicitation (from 110.6 ± 20.7 in the control to 808.7 ± 55.4 μg g−1 DW) and 5.1 times at day 4 (Fig. 3b). Interestingly, betulinic acid was increased by 3.5-fold (from 245.6 ± 3.7 to 835 ± 41.5 μg g−1 DW) at day 6 after elicitation, in contrast to betulin which reached its lower concentration at the same day. Lupeol presented a moderate increase from 167.9 ± 51.0 to 288.8 ± 7.3 μg g−1 DW, within the 8 days after elicitation (Fig. 3b). It has been reported that betulin is formed from lupeol (Fukushima et al. 2011) and its biosynthesis is regulated through lupeol synthase and stimulated by fungal elicitors via oxidative stress (Fan et al. 2013). Furthermore, diverse strategies have been done to achieve high yields of betulinic acid from plants, which include the utilization of in vitro cell and tissue cultures, and the biotransformation from betulin by microorganisms (Liu et al. 2011; Pandey et al. 2015). Recently, it has been reported that MeJA induced the production of betulinic acid in callus cultures of three species of Ocimum (Pandey et al. 2015). It is worth to mention that the current overproduction of lupane-type pentacyclic triterpenes as lupeol, betulin and betulinic acid by JA application in J. curcas cells raises its biotechnological potential. Lupeol is widely studied by presenting antimicrobial, anti-inflammatory and anticancer activity (Gallo and Sarachine 2009). Betulinic acid has antitumor properties by activating the mitochondrial pathway of apoptosis in cancer cells (Dzubak et al. 2006; Fulda and Kroemer 2009), besides inhibiting the replication of HIV virus (Evers et al. 1996). In addition, betulin isolated from Betula species has important anti-inflammatory and cardio-protective effects (Dzubak et al. 2006).
a Time course of triterpene production in J. curcas cell suspension cultures supplied with JA 200 μM. b Profile of pentacyclic triterpenes after elicitation with JA 200 μM. The elicitor was added to 7-days-old cell cultures. Each data point represents the mean of three replications and bars are ±1 SE. Mean values with asterisk are significantly different according to Tukey’s multiple range test at the 5 % level
Effect of JA on oxidative responses and antioxidant enzyme activities
The effects of JA on H2O2 and MDA production were studied during the 8 days after elicitation (Table 2). The cells increased H2O2 and MDA levels up to 3 times (0.34 ± 0.02 to 1.10 ± 0.01 mmol g−1 DW) and 59 % (36.48 ± 1.98 to 69.17 ± 3.18 μmol g−1 DW) respectively, at the first 6 days after elicitation with respect to control. Levels remained high (P < 0.05) during the 8 days of assessment, while the biomass concentration and viability stayed without noteworthy changes (Table 2). In control cell cultures, a small range of difference in the levels of H2O2 (0.19 ± 0.01 to 0.32 ± 0.0 mmol g−1 DW) and MDA (24.21 ± 3.52 to 32.45 ± 4.84 μmol g−1 DW) were maintained within the 8 days of elicitation (Table 2). In JA elicited J. curcas cell cultures, the elevated level of the two important oxidative stress biomarkers H2O2 and MDA could show the imbalance in redox homeostasis, thus revealing the occurrence of oxidative stress. A significant response of plant cells to elicitor treatments is the stimulation of the ROS burst (Zhao et al. 2005). Therefore, H2O2 may function as a signal activating defense genes and, as part of the coordinate antioxidant response, could enhance phytoalexin production (Ramos-Valdivia et al. 2012). Likewise, it has been reported that treatment with JA or MeJA can lead to a marked increase of ROS production (Suhita et al. 2004; Zhang and Xing 2008) as well as high MDA levels which can be associated with an improved production of secondary metabolites (Chong et al. 2005).
To obtain some insights of the cellular antioxidant responses caused by JA elicitation, the activities of CAT, APX and POD were evaluated. The activities of CAT and POD increased rapidly (122 and 63 %, respectively) at the second day of elicitation and their activities were maintained until the 4th day (Table 3). On the other hand, APX activity increased by 26 %, but until the 4th day of elicitation (Table 3). In this way, JA provoked differential effects in CAT, POD and APX specific activities, which were capable to scavenge and neutralize the adverse consequences in cell membrane stability provoked by the high H2O2 levels (Table 2). After elicitation, catalases showed the highest increase in activity with respect to POD and APX. Catalases exist as multiple isozymes including mitochondrial isoforms (Zhang and Xing 2008) located mostly in peroxisomes and glyoxysomes that efficiently scavenge H2O2 due to they do not require a reducing substrate to act (Foyer and Noctor 2005). Nevertheless, the high levels of lipidic peroxidation in J. curcas cell cultures during the 8 days after elicitation implicate that the classical antioxidant systems are not sufficient to avoid oxidative stress. The slightly increase of APX activity in elicited cells suggests that there may be limitations in the ascorbate–glutathione cycle for an efficient H2O2 detoxification (Foyer and Noctor 2005). Moreover, the lack of increase in the response of antioxidant enzyme activities in elicited cells at the 6th day (Table 3) may indicate that the antioxidant role could be being played by other antioxidant entities, as shown by the increase of betulinic acid that occurred at that same day (Fig. 3b).
Mevalonate precursor of the elicited induced triterpenes
In order to investigate the participation of mevalonate as the biosynthetic precursor of triterpenes, elicited and control J. curcas cell cultures were incubated with [2-14C]-mevalonate. The percentage of incorporation of this radioactive precursor into sterols and triterpenes is shown in Fig. 4. In the control cell cultures, the distribution of radioactivity from [2-14C]-mevalonate was 4 % in β-sitosterol/stigmasterol and 16.8 % in pentacyclic triterpenes (5.8 % lupeol and 11 % betulinic acid). Elicited cells incorporated a higher percentage of radioactivity into sterols (7.7 %) and triterpenes (29.9 %, distributed in 9.9 % lupeol and 20 % betulinic acid) than in the control cultures. Incorporation of radioactive label from mevalonate into J. curcas triterpenes, as well as in sterols, confirmed that mevalonate is their precursor, similarly to that reported for cells of Uncaria tomentosa (Flores-Sánchez et al. 2002) and Taraxacum officinale (Akashi et al. 1994). It has been postulated that sterols and triterpenes are biosynthesized from mevalonate through 2,3-oxidosqualene, which is the last common intermediate for these two pathways (Fukushima et al. 2011; Lambert et al. 2011). Lupeol and betulinic acid were strongly labelled in the elicited cultures, in agreement with the stimulation of triterpene biosynthesis by JA.
Conclusion
Elicitation of J. curcas cell cultures with 200 μM of JA during the late exponential phase of growth boosts by 3-fold a differential triterpene production and oxidative stress responses together with an enhancement in the antioxidant enzyme activity without affecting biomass accumulation and viability. The triterpene production increases the usefulness of this culture system for production of pharmacologically important compounds such as betulin, betulinic acid, and lupeol.
References
Adebowale KO, Adedire CO (2006) Chemical composition and insecticidal properties of the underutilized Jatropha curcas seed oil. Afr J Biotechnol 5:901–906. doi:10.5897/AJB05.424
Akashi T, Furuno T, Takahashi T, Ayabe SI (1994) Biosynthesis of triterpenoids in cultured cells, and regenerated and wild plant organs of Taraxacum officinale. Phytochemistry 36:303–308. doi:10.1016/S0031-9422(00)97065-1
Broeckling CD, Huhman DV, Farag MA, Smith JT, May GD, Mendes P, Dixon RA, Sumner LW (2005) Metabolic profiling of Medicago truncatula cell cultures reveals the effects of biotic and abiotic elicitors on metabolism. J Exp Bot 56:323–336. doi:10.1093/jxb/eri058
Chong TM, Adullab MA, Fadzillabb NM, Lai OM, Laijs NH (2005) Jasmonic acid elicitation of anthraquinones with some associated enzymic and non enzymic antioxidant responses in Morinda elliptica. Enzyme Microb Technol 36:469–477. doi:10.1016/j.enzmictec.2004.11.002
Dzubak P, Hajduch MV, Hustova A, Kvasnica M, Biedermann D, Markova L, Urban M, Sarek J (2006) Pharmacological activities of natural triterpenoids and their therapeutic implications. Nat Prod Rep 23:394–411. doi:10.1039/B515312N
Evers M, Poujade C, Soler F, Ribeil Y, James C, Lelièvre Y, Geguen JC, Reisdorf D, Morize I, Pauwels E, De Clercq E, Hénin Y, Bousseau A, Mayaux JF, Le Pecq JB, Dereu N (1996) Betulinic acid derivatives: a new class of HIV type 1 specific inhibitors with a new mode of action. J Med Chem 39:1056–1068. doi:10.1021/jm950670t
Falodun A, Nworgu ZAM, Osayemwenre E (2011) Smooth muscle relaxant evaluation of Jatropha curcas Linn (Euphorbiaceae) and isolation of triterpenes. Niger J Physiol Sci 26:133–137
Fan G, Zhai Q, Li X, Zhan Y (2013) Compounds of Betula platyphylla cell suspension cultures in response to fungal elicitor. Biotechnol Biotechnol Equip 27:3569–3572. doi:10.5504/BBEQ.2012.0114
Flores-Sánchez IJ, Ortega-Lopez J, Montes-Horcasitas MC, Ramos-Valdivia AC (2002) Biosynthesis of sterols and triterpenes in cell suspension cultures of Uncaria tomentosa. Plant Cell Physiol 43:1502–1509
Foyer CH, Noctor G (2005) Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17:1866–1875. doi:10.1105/tpc.105.033589
Fukushima EO, Seki H, Ohyama K, Ono E, Umemoto N, Mizutani M, Saito K, Murakana T (2011) CYP716A Subfamily members are multifunctional oxidases in triterpenoid biosynthesis. Plant Cell Physiol 52:2050–2061. doi:10.1093/pcp/pcr146
Fulda S, Kroemer G (2009) Targeting mitochondrial apoptosis by betulinic acid in human cancers. Drug Discov Today 14:885–890. doi:10.1016/j.drudis.2009.05.015
Gallo BC, Sarachine MJ (2009) Biological activities of lupeol. Int J Biomed Pharma Sci 3:46–66
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158. doi:10.1016/0014-4827(68)90403-5
Hu FX, Zhong JJ (2008) Jasmonic acid mediates gene transcription of ginsenoside biosynthesis in cell cultures of Panax notoginseng treated with chemically synthesized 2-hydroxyethyl jasmonate. Process Biochem 43:113–118. doi:10.1016/j.procbio.2007.10.010
Huerta-Heredia AA, Marín-López R, Ponce-Noyola T, Cerda-García-Rojas CM, Trejo-Tapia G, Ramos-Valdivia AC (2009) Oxidative stress induces alkaloid production in Uncaria tomentosa root and cell cultures in bioreactors. Eng Life Sci 3:211–221. doi:10.1002/elsc.200800118
James JT, Tugizimana F, Steenkamp PA, Dubery IA (2013) Metabolomic analysis of methyl jasmonate induced triterpenoid production in the medicinal herb Centella asiatica (L) Urban. Molecules 18:4267–4281. doi:10.3390/molecules18044267
Kauss H, Jeblick W, Ziegler J, Krabler W (1994) Pretreatment of parsley (Petroselinum crispum L.) suspension cultures with methyl jasmonate enhances elicitation of activated oxygen species. Plant Physiol 105:89–94. doi:10.1104/pp.105.1.89
Kumar A, Sharma S (2008) An evaluation of multipurpose oil seed crop for industrial use (Jatropha curcas L.): A review. Ind Crop Prod 28:1–10. doi:10.1016/j.indcrop.2008.01.001
Lambert E, Faizal A, Geelen D (2011) Modulation of triterpene saponin production: in vitro cultures, elicitation, and metabolic engineering. Appl Biochem Biotechnol 164:220–237. doi:10.1007/s12010-010-9129-3
Laszczyk M (2009) Pentacyclic triterpenes of the lupane, oleanane and ursane group as tools in cancer therapy. Planta Med 75:1549–1560. doi:10.1055/s-0029-1186102
Liu J, Fu ML, Chen QH (2011) Biotransformation optimization of betulin into betulinic acid production catalysed by cultured Armillaria luteo-virens Sacc ZJUQH100-6 cells. J Appl Microbiol 110:90–97. doi:10.1111/j.1365-2672.2010.04857.x
Norrizah JS, Suhaimi YM, Rohaya A, Roslan N (2012) Ursolic acid and oleanolic acid production in elicited cell suspension cultures of Hedyotis corymbosa. Biotechnology 11:238–242. doi:10.3923/biotech.2012.238.242
Pandey H, Pandey P, Singh S, Gupta R, Banerjee S (2015) Production of anti-cancer triterpene (betulinic acid) from callus cultures of different Ocimum species and its elicitation. Protoplasma 252:647–655. doi:10.1007/s00709-014-0711-3
Patil RA, Lenka SK, Normanly J, Walker EL, Roberts SC (2014) Methyl jasmonate represses growth and affects cell cycle progression in cultured Taxus cells. Plant Cell Report 33:1479–1492. doi:10.1007/s00299-014-1632-5
Peterson GL (1977) A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem 83:346–356. doi:10.1016/0003-2697(77)90043-4
Pütter J (1974) Peroxidases. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol 2. Verlag Chemie-Academic Press, New York, pp 685–690
Ramos-Valdivia AC, Huerta-Heredia AA, Trejo-Tapia G, Cerda- García-Rojas CM (2012) Secondary metabolites as non-enzymatic plant protectors from oxidative stress. In: Anjum NA, Umar S, Ahmad A (eds) Oxidative stress in plants: causes, consequences and tolerance. International Publishing House, New Delhi, pp 413–441
Sasaki-Sekimoto Y, Taki N, Obayashi T, Aono M, Matsumoto F, Sakurai N et al (2005) Coordinated activation of metabolic pathways for antioxidants and defense compounds by jasmonates and their roles in stress tolerance in Arabidopsis. Plant J 44:653–668. doi:10.1111/j.1365-313X.2005.02560.x
Silva EN, Ferreira-Silva SL, Fontenele AV, Ribeiro RV, Viégas RA, Silveira JAG (2010) Photosynthetic changes and protective mechanisms against oxidative damage subjected to isolated and combined drought and heat stresses in Jatropha curcas plants. J Plant Physiol 167:1157–1164. doi:10.1016/j.jplph.2010.03.005
Soomro R, Memon R (2007) Establishment of callus and suspension culture in Jatropha curcas. Pakistan J Bot 39:2431–2441
Srivastava P, Sisodia V, Chaturvedi (2011) Effects of culture conditions on synthesis of triterpenoids in suspension cultures of Lantana camara L. Bioprocess Biosyst Eng 34:75–80. doi:10.1007/s00449-010-0448-0
Suhita D, Raghavendra AS, Kwak JM, Vavasseur A (2004) Cytoplasmic alkalization precedes reactive oxygen species production during methyl jasmonate- and abscisic acid-induced stomatal closure. Plant Physiol 134:1536–1545. doi:10.1104/pp.103.032250
Valdés-Rodríguez OA, Pérez-Vazquez A, Muñoz-Gamboa C (2014) Drivers and consequences of the first Jatropha curcas plantations in Mexico. Sustainability 6:3732–3746. doi:10.3390/su6063732
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Protective role of exogenous polyamines. Plant Sci 151:59–66. doi:10.1016/S0168-9452(99)00197-1
Wang JW, Wu JY (2005) Nitric oxide is involved in methyl jasmonate induced defense responses and secondary metabolism activities of Taxus cells. Plant Cell Physiol 46:923–930. doi:10.1093/pcp/pci098
Wasternack C, Hause B (2013) Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann Bot 111:1021–1058. doi:10.1093/aob/mct067
Zhang L, Xing D (2008) Methyl jasmonate induces production of reactive oxygen species and alterations in mitochondrial dynamics that precede photosynthetic dysfunction and subsequent cell death. Plant Cell Physiol 49:1092–1111. doi:10.1093/pcp/pcn086
Zhao J, Davis LC, Verpoorte R (2005) Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv 23:283–333. doi:10.1016/j.biotechadv.01.003
Acknowledgments
This research was financed by CINVESTAV-IPN and CONACYT-Mexico (grant 222097). FZM thanks CONACYT-Mexico for a doctoral fellowship (163338). Authors wish to thank Dr G. Luna-Palencia for the advice in chromatographic analysis and C. Fontaine for technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zaragoza-Martínez, F., Lucho-Constantino, G.G., Ponce-Noyola, T. et al. Jasmonic acid stimulates the oxidative responses and triterpene production in Jatropha curcas cell suspension cultures through mevalonate as biosynthetic precursor. Plant Cell Tiss Organ Cult 127, 47–56 (2016). https://doi.org/10.1007/s11240-016-1028-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-016-1028-z