Abstract
Actin-depolymerizing factors (ADFs) are the members of actin-binding proteins, which play pivotal roles in various cellular processes in plant cells. In this study, we isolated an ADF gene ZmADF3 from the kernels of a popcorn inbred N04. This gene had a 417 bp open reading frame (ORF) which encoded for 139 amino acids. Phylogenetic analysis showed that ZmADF3 had a high sequence identity with TaADF3 and OsADF4. Quantitative real-time PCR and western blotting showed that ZmADF3 expressed variously according to tissues and developmental stages during kernel development both in RNA and in protein levels. A 1738 bp promoter for ZmADF3 was obtained and the cis-acting regulatory elements were predicted. Furthermore, yeast two-hybrid screening revealed that ZmADF3 interacted with glyceradehyde-3-phosphate dehydrogenase (GAPDH). Heterologous overexpression of ZmADF3 showed an increase in seed size in transgenic Arabidopsis plants mainly through the increase in cell size. In addition, the positive regulation of seed size genes SHB1 and IKU1 were up-regulated while the negative regulators AP2 and ARF2 were down-regulated in the transgenic lines. Our results showed that ZmADF3 might play important roles in kernel development in maize. However, further research should be done to thoroughly explore the mechanism of ZmADF3 in playing these functions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The intracellular cytoskeleton consists of microtubules, actin filaments and intermediate filaments, which is involved in various cellular organizations and processes such as cell shape, cell division, differentiation, expansion, communication and metabolism (Ishikawa 1990; Kost et al. 1999; Volkmann and Baluska 1999; Staiger 2000; Smith 2003). Actin filaments play a decisive role in these cell functions (Tiezzi 1991; Kost et al. 2002). Globular actin (G-actin) and filamentous actin (F-actin) are the two forms of actin in eukaryotic cells, and G-actin as an actin monomer is the basic subunit of the double-stranded helical actin filament (Staiger 2000; Blanchoin et al. 2014). The activity of actin filaments are modulated by numerous actin binding proteins such as profilin, actin depolymerizing factor (ADF)/cofilin, cyclase associated protein (CAP), actin interacting protein (AIP), Rho of plants (ROP) GTPases, gelsolin, myosin, formins, fibrin, Arp2/3 complex and villin (Hussey et al. 2006; Ren and Xiang 2007; Ketelaar 2013). ADFs/cofilin, as the members of the actin-binding proteins, have a low molecular mass (15–20 kD) and ubiquitously distribute in eukaryotes (Bowman et al. 2000). They can bind to G-actin and F-actin and play a pivotal role in actin filament dynamics in cells (Carlier et al. 1997; Maciver and Hussey 2002; Zheng et al. 2013). As a functional node in cell biology, ADFs can be regulated by phosphorylation and ubiquitination, PIP2, pH, oxidation, and nuclear translocation (Bernstein and Bamburg 2010; Sun et al. 1995; Bowman et al. 2000).
There are much more ADFs in plants than in animals (Maciver and Hussey 2002). Quantities of ADFs had been isolated and characterized from plants, such as lily (Kim et al. 1993), Brassica napus (Kim et al. 1993), maize (Rozycka et al. 1995; Lopez et al. 1996), Petunia (Mun et al. 2000), Arabidopsis (Dong et al. 2001a; Bou et al. 2011), wheat (Ouellet et al. 2001), Nicotiana tabacum (Chen et al. 2002), rice (Feng et al. 2006) and cotton (Zhang et al. 2007; Li et al. 2010). In plants, ADF genes always exist in the form of large families (Mun et al. 2000; Feng et al. 2006). For example, there are 12 ADF genes have been isolated and characterized in Arabidopsis as well as in rice (Feng et al. 2006; Ruzicka et al. 2007). According to the tissue-specific expression and the subcellular localization, the ADF family in Arabidopsis was divided into four subclasses (Ruzicka et al. 2007).
Many relevant researches have been confirmed that ADFs were involved in plant growth and development (Huang et al. 2012). Overexpression of AtADF1 was associated with the disappearance of thick actin cables and caused abnormal cellular and tissue morphogenesis, while antisence expression of AtADF1 leaded to a delay in flowering, and promoted the cell expansion and organ growth (Dong et al. 2001b). Ectopic expression of ADF1 of Gossypium barbadense in tobacco resulted in shorter hypocotyls and fewer root hairs (Chi et al. 2013). The downregulation of GhADF1 increased fiber length and strength due to more abundant F-actin filaments in transgenic Gossypium hirsutum plants (Wang et al. 2009). Overexpression of NtADF1 resulted in the disruption of the actin cytoskeleton and the inhabitation of pollen tube growth in elongating pollen tubes (Chen et al. 2002). Silence expression a rice ADF gene resulted in the suppressed expression of α-amylase gene, which gave rise to the decrease of seed germination and depressed the immune responses (Cheng et al. 2013). In addition, further studies had also indicated that plant ADFs could respond to biotic and abiotic stimuli, such as light, drought, cold, salt and pathogen attack (Dong and Hong 2013; Burgos-Rivera et al. 2008; Huang et al. 2012; Porter et al. 2012; Fu et al. 2014; Henty-Ridilla et al. 2014).
In maize, three ADFs have been studied. ZmADF1 and ZmADF2 expressed specifically in pollen, while ZmADF3 expressed in all tissues except of pollen (Rozycka et al. 1995; Lopez et al. 1996). ZmADF3 was a key regulator of actin dynamics in plant cells, which could reset the cytoskeletal network during root hair initiation and elongation (Jiang et al. 1997a). ZmADF3 could be phosphorylated on serine Ser6 by a calmodulin-like domain protein kinase (CDPK), which was essential for the control of actin dynamics (Smertenko et al. 1998; Allwood et al. 2001). In our previous study, the protein profiles of endosperm and pericarp at three key developmental stages in popcorn were identified using iTRAQ labeling coupled with LC–MS/MS (Dong et al. 2015). As one of the differentially expressed protein and due to the important role of the ADF family in plant, we isolated the full-length cDNA sequence of ZmADF3 from a popcorn inbred and analyzed its expression patterns in the development of pericarp and endosperm. The promoter of ZmADF3 was also obtained from the genomic DNA of the inbred N04. Yeast two-hybrid library screening was carried out. Heterologous overexpression of ZmADF3 in Arabidopsis was used to investigate the possible functions of ZmADF3 in kernel development. These results were useful in further research to explore the biological functions of ZmADF3 in maize.
Materials and methods
Plant materials and growth conditions
Plant materials and sample collections had been described in our previous research (Liu et al. 2010). The popcorn inbred line N04 with small-sized grains was planted in the Scientific Research and Education Center of Henan Agricultural University in Zhengzhou, Henan, China in 2014. The rows were 4 m long with 0.67 m spacing between rows and standard cultivation management practices were performed. Each plant was self-pollinated by hand. Ears were harvested at 10, 20 and 33 days after pollination (DAP). Kernels were isolated from the middle part of the ears. At each time point, kernels were collected from at least three ears. Endosperm and pericarp were separated from the kernels, respectively. The collected samples were immediately frozen in liquid nitrogen and stored at −80 °C.
The Columbia ecotype of Arabidopsis thaliana was used for gene transformation. Arabidopsis seeds were sterilized by 6.25 % sodium hypochlorite solution with 0.01 % TritonX-100 for 15 min, washed three times with sterilized H2O and then grown on 0.8 % (w/v) agar plates containing 1/2 Murashige and Skoog medium (MS, Sigma) and 3 % sucrose. Seven-day-old seedlings were planted in square plates filled with soil mixture. The seedlings were cultured in a growth chamber under controlled conditions, 22 °C and 16 h light/8 h dark cycle.
RNA isolation and cDNA synthesis
Total RNAs were extracted from the endosperm and pericarp at different development stages in maize, and the different tissues of transgenic Arabidopsis plants using a Column Plant RNAout kit (Tiandz) according to the manufacturer’s instructions. DNA was digested using RNAse-free DNAse (TaKaRa). The quantity and quality of the total RNA were verified by 1 % agarose gel electrophoresis and spectrophotometric measurement (ND-1000 NanoDrop Technologies). The first-strand cDNA was synthesized from 2 µg of DNA-free RNA using a PrimeScript RT reagent Kit (TaKaRa).
Cloning and structural analysis of ZmADF3
We chose an important protein peptide (GRMZM2G060702_P02) from the isobaric tags for relative and absolute quantification (iTRAQ) protein libraries constructed in our laboratory as the objective protein in this study. The function of this protein was predicated as ADF3, and its expression tendency was greatly different between the two tissues and among the three developmental stages (Dong et al. 2015). Using TBLASTN in NCBI, the most highly homology sequences were assembled into an extended sequence by in silico cloning. The specific primers ADF3-F and ADF3-R (Table S1) were designed to amplify the assembled sequences. PCR was carried out using 1 µl of the obtained cDNA, 2.5 µl 10 × PCR buffer, 2.5 µl dNTPs mixture (2.5 mM each), 1 µl of each primer (10 µM), 0.125 µl TaKaRa La Taq, and ddH2O was added to make up the final volume of 25 µl. The PCR program was used as follows: 5 min at 95 °C, then 35 cycles of 30 s at 95 °C, 30 s at 58 °C and 90 s at 72 °C, and a final extension of 10 min at 72 °C. PCR products were cloned into the pMD18-T vector (TaKaRa) and sequenced. The deduced protein of ZmADF3 and other ADF proteins were aligned using ClustalW. A phylogenetic tree based on the result of ClustalW protein alignments was constructed using the Neighbor-Joining method in the software MEGA 6.0. The Poisson correction method was applied to compute the evolutionary distances.
Isolation of the promoter sequence of ZmADF3
The genomic DNA of inbred N04 was isolated from leaves using a Plant Genomic DNA kit (TianGen) according to the manufacturer’s instructions. To isolate the promoter sequence of ZmADF3 from inbred N04, a BLAST analysis with the mRNA sequence of ADF3 (X97726) was performed in MaizeGDB (www.maizegdb.org). The specific primers Pro-F and Pro-R (Table S1) were designed to amplify the promoter sequence of ZmADF3 by PCR amplification of the N04 genomic DNA. The cis-elements in the promoter sequence were analyzed using the PlantCARE (Lescot et al. 2002).
Quantitative real-time PCR
Quantitative real-time PCR (qPCR) was performed in ABI 7500 Real-time PCR System and using SYBR Premix ExTaq II (TaKaRa) and gene-specific primers (Table S1). The 25.0 μl reaction contained 12.5 µl 2 × SYBR mix, 1 µl PCR forward primer, 1 µl PCR reverse primer, 1 µl of a five times dilution of the cDNA as the template, and 9.5 µl sterilization of double distilled water. The amplification procedure was as follows: 95 °C for 3 min, then 40 cycles each of denaturation at 95 °C for 10 s, annealing at 57 °C for 20 s, and extension at 72 °C for 20 s. The 18S rRNA gene was used as the endogenous control, and each sample was repeated for three times. The relative transcript levels of synthesis were calculated using the 2−ΔΔCT method (Livak and Schmittgen 2001).
Western blot analysis
Total proteins were extracted by BPP method as described by Wang et al. (2007). Total proteins were electrophoresed in 12 % SDS-PAGE and the gels were transferred onto PVDF microporous membranes. After blocking for 2.5 h in TBST buffer (20 mM Tris-HCl, pH 7.6, 150 mM NaCl, and 0.05 % Tween 20) with 5 % nonfat dry milk at room temperature, membranes were incubated with special antibody of ZmADF3 at 1:1000 dilution for overnight at 4 °C. Following three times of washing with TBST, membranes were incubated with secondary antibodies (Goat anti-Rabbit IgG-HRP, Abmart) at 1:5000 dilution for 1.5 h at room temperature away from light. After three times of washing with TBST, signals were detected using an ECL Western Blotting Kit (Amersham) following the manufacturer’s instructions. Anti-ZmADF3 rabbit polyclonal antisera were generated against the peptide of ZmADF3 by the Abmart biomedical company (Shanghai China).
Yeast two-hybrid library screening
Yeast two-hybrid library screening was carried out using Matchmaker™Gold Yeast Two-Hybrid System (Clontech). The full-length ORF of ZmADF3 was cloned into the pGBKT7 vector and expressed as a BD fusion protein and then transformed into the yeast strain Y2HGold. The cDNA library cloned inframe with the GAL4 activation domain in the pGADT7 vector constructed previously in our laboratory from the kernels of inbred N04 (Wang 2015) was used to screen for the interaction clones with ZmADF3. Two-hybrid screening was performed as described in the Clontech’s Matchmaker™Gold Yeast Two-Hybrid user manual (Clontech). The positive clones were sequenced and characterized. For a further confirmation, the full-length ORFs of the positive clones were cloned into the pGADT7 vector, respectively. Then, as the described by Cai et al. (2014), they were co-transformed with the pGBKT7-ZmADF3 vector into the Y2HGold yeast strains. The mated culture was spread on DDO (SD/-Trp/-Leu), QDO/X (SD/-Trp/-Leu/-His/-Ade supplemented with X-α-gal) and QDO/X/A (SD/-Trp/-Leu/-His/-Ade supplemented with X-α-gal and Aureobasidin A) medium incubated at 30 °C for 5–10 d.
Vector construction and genetic transformation
The ORF of ZmADF3 with the termination codon was generated by PCR using the following primers: 5′ GCCCATGGATGGCAAACGCGAGATCGGGTG 3′ (NcoI site underlined) and 5′GCACTAGTCTAGCGAGCCCGATCCTTGATCT 3′ (SpeI site underlined). After verifying by sequencing, the PCR fragment was inserted into pCAMBIA1302 under the control of the CaMV 35S promoter. The fused plasmid (35S::ZmADF3) and the empty plasmid were introduced into the Agrobacterium tumefaciens strain EHA105 by freeze-thawing with liquid nitrogen, and then transferred into Arabidopsis plants (Col-0) using the floral dip method (Clough and Bent 1998), respectively. ZmADF3 transgenic Arabidopsis plants were selected on 1/2MS medium with hygromycin resistance and confirmed by PCR using specific primers (Table S1). Western blotting was used to confirm the expression of ZmADF3 in the T3 transgenic Arabidopsis.
Phenotypic analysis of seeds and mature embryos
The wild-type (WT) plants and three independent T3 homozygous progenies of transgenic lines were selected for morphological analysis. The average seed weight was determined by weighing the mature dry seeds in batches of 1000 using an electronic analytical balance (OHAUS DV215CD), and five sample batches were measured for each seeds lot. Seeds were observed and photographed under a stereoscopic microscope (Nikon SMZ1500), and the seed size was measured using the Image-Pro Plus6.0 software (http://www.mediacy.com).
The dried mature seeds were soaked in the water for 1 h and dissected under an SMZ-T2 microscope to isolate the mature embryos. The isolated embryos were treated as described by Ohto et al. (2005) and then photographed using a Leica differential interference contrast (DIC) microscope. About 30 transgenic and WT embryos were photographed to measure cell size and count the number of cells using Image-Pro Plus6.0 software.
Results
Cloning and characterization of ZmADF3
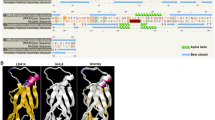
A 678 bp cDNA fragment was obtained via PCR from inbred N04, which contained an ORF of 417 bp encoded for 139 amino acids (Fig. 1). In addition, a 148 bp 5′ untranslated region (UTR) and a 113 bp 3′ UTR were predicted. The calculated molecular weight of the encoded protein was 15.91 kDa, with an isoelectric point (pI) of 5.47. The function domain for ZmADF3 was predicted by software CDD of NCBI on line (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi). The result showed that the protein belonged to the ADF/cofilin-like subtribe of the ADF-gelsolin family, which had two putative actin monomer (G-actin) binding sites (Ser6 and Gly7) and five putative actin filaments (F-actin) binding sites (Lys82, Arg84, Lys98, Asp125 and Glu128).
Multiple sequence alignment of ZmADF3 and other plant ADFs. Completely identical or partial conserved amino acids were shaded in colors. The putative actin monomers (G-actin) binding sites and actin filaments (F-actin) binding sites of ZmADF3 were marked by triangle and rhombus, respectively. The sequences were derived from the following accession numbers: AtADF1 (At3g46010), AtADF2 (At3g46000), OsADF1 (Os02g44470), OsADF2 (Os03g56790), OsADF3 (Os03g60580), OsADF4 (Os03g60590), TaADF3 (AIZ95472), TaADF4 (AGW22223), TaADF5 (AIT97056), TaADF6 (AIT97055), SiADF1 (XP_004953378), SiADF2 (XP_004957704), SiADF3 (XP_0049812564)
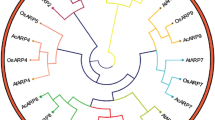
The multiple sequence alignment and phylogenetic analysis were performed to investigate the evolutionary relationships among ZmADF3 and other ADF proteins. Comparisons among the ADF protein sequences from Arabidopisis thaliana, Oryza sativa, Setaria italica and Triticum aestivum showed their high identity in plants. The similarity for their amino acid sequences were above 64 % (Fig. 1). As shown in Fig. 2, ZmADF3 had the highest homology with SiADF3, OsADF4 and TaADF3. They occupied an independent branch in the tree, while other four maize ADFs were classified into three different branches. This implied the specialty of ZmADF3 compared with other ADFs in maize.
Phylogenetic analysis of the ADF sequences from Arabidopisis thaliana, Oryza sativa, Setaria italica and Triticum aestivum using the Neighbor-Joining method. The percentages of replication trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) were shown above the branches. The Poisson correction method was applied to calculate the evolutionary distances and a ADF gene (AAB28361) of human was used as an outgroup. The sequences were derived from the following accession numbers: AtADF1 (At3g46010), AtADF2 (At3g46000), AtADF3 (At5g59880), AtADF4 (At5g59890), AtADF5 (At2g16700), AtADF6 (At2g31200), AtADF7 (At4g25590), AtADF8 (At4g00680), AtADF9 (At4g34970), AtADF10 (At1g01750), AtADF11 (At3q45990), AtADF12 (At5g52360), OsADF1 (Os02g44470), OsADF2 (Os03g56790), OsADF3 (Os03g60580), OsADF4 (Os03g60590), OsADF5 (Os03g13950), OsADF6 (Os04g46910), OsADF7 (Os05g02250), OsADF8 (Os07g20170), OsADF9 (Os07g30090), OsADF10 (Os10g37670), OsADF11 (Os12g43340), TaADF3 (AIZ95472), TaADF4 (AGW22223), TaADF5 (AIT97056), TaADF6 (AIT97055), TaADF7 (AFS49701), TaADF8 (AIZ95473), TaADF11 (AIT97057), ZmADF1 (NP_001105463), ZmADF2 (NP_001105590), ZmADF5 (NP_001167686), ZmADF6 (NP_001148357), SiADF1(XP_004953378), SiADF2(XP_004957704), SiADF3 (XP_004981256), SiADF5 (XP_004985039), SiADF7 (XP_004960504), SiADF10 (XP_004983892)
Isolation of the promoter region for ZmADF3
Based on the B73 genome sequence from 293162401 to 293166106 on chromosome 1, a 1738 bp promoter of ZmADF3 was obtained from the genomic DNA of inbred N04. Then the cis-acting regulatory elements were predicted using PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html). The results showed that the ZmADF3 promoter contained several basic regulatory elements, including 18 TATA boxes and 9 CAAT boxes. Some known cis-elements were also contained, including light responsive element ACE, AE-box, AT1, G-box, GATA, GT1, I-box and Sp1, abscisic acid responsive element ABRE, gibberellin responsive element GARE, MeJA responsive element CGTCA and TGACG, heat stress responsive element HSE, fungal elicitor responsive element Box-W1, defense and stress responsive element TC-rich repeats, wounding and pathogen responsive element W box, and meristem expression element CAT-box and CCGTCC-box (Table 1). In addition, endosperm expression element GCN4 and Skn-1, zein metabolism regulation element O2-site and MYB binding site element MBS and CCAAT-box were also identified (Table 1).
The expression profiles of ZmADF3 during kernel development in maize
The expression profiles of ZmADF3 in the kernels of inbred N04 were measured using qPCR. The results showed that ZmADF3 expressed differently in the two tissues and across three developmental stages. For the two tissues, the expression levels were much higher in pericarp than in endosperm at all stages. For the expression tendency among different stages, the overall trend was on the decline during the developmental periods (Fig. 3a). Comprehensively speaking, ZmADF3 expressed abundantly in pericarp at the early developmental stages.
The expression tendency of ZmADF3 for all samples by quantitative RT-PCR analysis (a) and western blot analysis (b). The total proteins were extracted from pericarp (Pe10, Pe20, Pe33) and endosperm (En10, En20, En33) at 10, 20 and 33DAP, respectively. 18S rRNA was used as the endogenous control for qRCR and the immunizing mice with Arabidopsis ACT11 full-length protein (Abmart China) as the endogenous control for western blot. The protein expression was estimated by integrated optical density (IOD). Data were represented as the mean ± SE (n = 3)
Western blot assay of ZmADF3 protein
Western blot was performed for the same two tissues at three developmental stages to explore the expression of ZmADF3 in the protein levels. The ZmADF3 proteins were detected for all samples (Fig. 3b). The result showed that the expression levels in pericarp increased at first and decreased subsequently and reached the highest at 20 DAP, while the expression tendency in endosperm were completely contrary with in pericarp and had the highest expression level at 33 DAP.
ZmADF3 interacted with GAPDH in yeast two-hybrid system
Protein–protein interaction assays were performed to investigate the interacting proteins with ZmADF3. The ORF of ZmADF3 was designed as the bait to screen the prey cDNA library prepared from maize kernels at different developmental stages of inbred N04 using yeast two-hybrid assay. There were 18 possible interacting proteins were detected with putative functions, which were involved in oxidoreductase, isomerase, nutrient reservoir, GTP binding, ATP binding, NADP binding and nucleotide binding activities (Table. S2). Three independent clones encoding a maize glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was identified. To confirm the interaction, the full-length ORF of GAPDH was cloned into the pGADT7 vector. The Y2HGold yeast strains co-transformed with pGADT7-GAPDH and pGBKT7-ZmADF3 vectors could grow on the DDO, QDO/X, and QDO/X/A medium incubated at 30 °C for 5-10 d (Fig. 4). This result showed that ZmADF3 could interact with GAPDH in yeast two-hybrid system.
Expression pattern of ZmADF3 in transgenic Arabidopsis plants
The heterologous overexpression transgenic plants in Arabidopsis were generated, and the hygromycin resistant T0 transformants were transferred into soil and grown in a growth chamber to obtain the segregating T1 progeny for genetic analysis. Among the transformants, six overexpression lines showed a 3:1 segregation pattern for hygromycin resistance. The relative expression levels of the exogenous ZmADF3 in the transgenic and WT Arabidopsis plants were analyzed by both qPCR and western blotting (Fig. 5). Three independent T3 homozygous progenies of transgenic lines with higher expression for ZmADF3 were selected for the phenotype assays. In addition, the expression tendency of ZmADF3 in root, stem, leaf, silique and inflorescence of the transgenic plants were determined by using both reverse transcription-polymerase chain reaction (RT-PCR) and qPCR. As shown in Fig. 6, the results showed that ZmADF3 expressed with no tissue specificity and the expression abundance in stem and inflorescence was significantly higher than that in root, leaf and silique.
Overexpression of ZmADF3 in Arabidopsis. a RT-PCR and qRT-PCR results for six homozygous T3 transgenic plants (3#, 7#, 8#, 14#, 31#, 33#) and wild-type Arabidopsis using the specific primers for ZmADF3. b Western blot analysis of ZmADF3 protein expression in the leaves of three homozygous T3 transgenic lines and wild-type plant. Data are represented as the mean ± SE
Expression pattern of ZmADF3 in transgenic plants. Total RNA was isolated from root, stem, leaf, silique and inflorescence of transgenic plants at 8DAF. a RT-PCR and b qRT-PCR results for different tissues of transgenic plants. Data were represented as the mean ± SE (n = 3). 18S rRNA was used as the endogenous control
Overexpression of ZmADF3 increased seed size in transgenic Arabidopsis plants
The morphological analysis of mature seeds was performed to investigate the possible function of ZmADF3 in seed development. Compared with the WT plant, the 1000-seed weights of the three independent transgenic lines (3#, 7# and 8#) increased 7.37, 29.97 and 21.16 %, respectively (Fig. 7a, b, c). Meanwhile, the seed area (+6.66, +23.16, +14.32 %) and length (+6.82 %, +20.27 %, +7.10) also increased significantly than the WT (Fig. 7d, e). Although the seed width of transgenic lines 3# and 8# had some increase (+0.44, +3.74 %), only line 7# reached the significant level (+5.42 %) compared with the WT plant (Fig. 7f).
Morphological analysis of the mature seeds in Arabidopsis of 35S::ZmADF3 and wild-type. Morphology of seed size (a) and mature embryo size (b). Statistics of seed weigth (c), seed area (d), seed length (e) and seed width (f). Seed weight was determined by weighing the mature dry seeds in batches of 1000. The weights of five sample batches were measured for each seed lot (n = 5). Seeds were observed and photographed under a stereoscopic microscope and 20 randomly selected seeds were used to measure seed area, seed length and seed width (n = 20). 3#, 7#, 8# represented three independent T3 progenies of transgenic lines. Data were represented as the mean ± SE. t test compared with the wild-type control: ** and *referred to significances at P < 0.01 and P < 0.05, respectively. Bars = 250 µm in (a) and (b)
We also isolated and visualized the mature embryos from mature seeds and found that the cotyledon areas of the three transgenic lines were 1.15, 1.26 and 1.23-fold larger than the WT plant (Fig. 8a, c). The cell area was also determined by measured at least 30 cells in the central region of cotyledon epidermis (Fig. 8b). The average sizes of the cotyledon cells for the three transgenic lines were 1.16, 1.28 and 1.22-fold than that the WT plant (Fig. 8d). However, there were no significant differences for the average cell numbers of cotyledon epidermis in the mature embryos between the three transgenic lines and the WT plant (Fig. 8e).
Phenotypic analysis of the mature embryos. The dissected mature embryos (a) and the epidermal cell layer from the central region of cotyledon (b). Statistics of cotyledon area (c), epidermal cell area (d) and average cell numbers (e). The isolated embryos after treated and then photographed using a Leica differential interference contrast (DIC) microscope (×300). The epidermal cell of the cotyledon were photographed using a DIC microscope (×700). 3#, 7#, 8# represented three independent T3 progenies of transgenic lines. Data were represented as the mean ± SE (n = 30). t test compared with the wild-type control: ** and * referred to significances at P < 0.01 and P < 0.05, respectively. Bars = 250 µm in (a) and 100 µm in (b)
Expression analysis of seed size-associated genes between transgenic lines and the WT plant in Arabidopsis
The expression patterns of seed size-associated genes SHORT HYPOCOTYL UNDER BLUE1 (SHB1), APETALA2 (AP2), AUXIN RESPONSE FACTOR2 (ARF2) and HAIKU1 (IKU1) of Arabidopsis were analyzed by qPCR between 35S::ZmADF3 and the WT plants to further investigate the underlying regulation mechanism of ZmADF3 in increasing seed size in transgenic Arabidopsis. The results showed that the expression levels of SHB1 and IKU1 were significantly increased in overexpression plants, whereas the expression of AP2 and ARF2 were observably higher in four-week-old WT plant leaves (Fig. 9a) and 7 DAP siliques (Fig. 9b).
Expression analysis of seed size-associated genes between 35S::ZmADF3 and wild-type plants. Total RNA was isolated from four-week-old leaves (a) and 7 DAP siliques (b). The primers for qRT-PCR analysis were referred to Jiang et al. (2013). 3#, 7#, 8# represented three independent T3 progenies of transgenic lines. Data were represented as the mean ± SE (n = 3). t test compared with the wild-type control: ** and * referred to significances at P < 0.01 and P < 0.05, respectively
Discussion
ADFs have been identified and cloned from a variety of plants. In Maize, three ADFs have been cloned and characterized (Rozycka et al. 1995; Lopez et al. 1996). ZmADF3 was firstly identified and cloned based on an EST collected from a leaf cDNA library by Lopez et al. (1996). Herein, we isolated ZmADF3 from a popcorn inbred N04 based on the protein sequence of GRMZM2G060702_P02 from the protein libraries for pericarp and endosperm at key developmental stages of the inbred constructed in our laboratory (Dong et al. 2015).
Histodifferentiation, reserve deposition and maturation drying are the three sequential stages during seed development (Wang et al. 2015). Cell division and expansion, as well as storage matter accumulation mainly occurred in the first two stages (Wang et al. 2015). In previous studies, ADFs showed high expression in the histodifferentiation stage and at the early stage of reserve deposition in the seeds of rice and Arabidopsis (Xu et al. 2008; Hajduch et al. 2010). Herein, ZmADF3 also had a similar expression tendency in these stages and the expression levels in pericarp were higher than that in endosperm, which implied that ZmADF3 might play a vital role in kernel development.
Obtaining mutations by overexpression or misexpression is an effective approach for exploring biological functions in plants (Prelich 2012; Liu et al. 2015; Su et al. 2015). ADFs regulated by light, drought, cold, salt and pathogen attack had been confirmed in the previous studies through this way (Dong et al.2013; Burgos-Rivera et al. 2008; Huang et al. 2012; Porter et al. 2012; Fu et al. 2014; Henty-Ridilla et al. 2014). In this study, the promoter region of ZmADF3 was obtained from the genomic DNA of the inbred N04, in which many cis-acting regulatory elements associated with light were found. Further study on the influence of light to the seedlings of 35S::ZmADF3 and WT plant showed that the length of hypocotyl of ectopic expression seedlings increased 55.8 % than that of the WT plant after 8 days darkness cultivation. Clearly, light had an important influence on the regulation of ZmADF3 in plant growth and development. Furthermore, the endosperm expression element GCN4 and Skn-1 (Takaiwa et al.1991; Wu et al. 1998), zein metabolism regulation element O2-site (Schmidt et al.1992) and MYB Binding Site element MBS and CCAAT box (Edwards et al.1998) identified in the promoter region of ZmADF3 implied that ZmADF3 might have special functions in endosperm, meristem and zein metabolism. These needed to be studied in further research.
The yeast two-hybrid system is a useful and powerful tool for studying protein–protein interactions (Finley and Jr 2007). In this study, 18 interacting proteins with ZmADF3 were detected by using yeast two-hybrid library screening method. Their molecular functions were predicted by Gene Ontology terms via the InterPro database (Mitchell et al. 2015). In addition, the interaction between ZmADF3 with GAPDH was confirmed also using the yeast two-hybrid system (Fig. 4). GAPDH was always used as internal control for transcription quantification experiments due to its relatively constant expression level (Lin et al. 2014). In previous studies, GAPDH had been shown to be involved in many cellular processes and played important roles in plant development and environmental stress response (Nicholls et al. 2012; Munoz-Bertomeu et al. 2010; Rius et al. 2008; Zhang et al. 2011; Cho et al. 2014). This implied that there might be a possible function contact between ZmADF3 and GAPDH. Due to the intrinsic defect of yeast-two hybrid screening system (Sprinzak et al. 2003), the verification for the interaction between them should be done using other methods.
Many recent studies confirmed that ADFs related to the plant growth and development particularly in the tip tissue (i.e. root hairs, pollen tube) growth, and majority of the observed phenotypes might be ascribed to altered cytoplasmic cytoskeletal activities affecting the meristem development (Jiang et al. 1997b; Chen et al. 2002; Daher and Geitmann 2012; Clement et al. 2009; Augustine et al. 2008; Burgos-Rivera et al. 2008; Allwood et al. 2002; Dong et al. 2013). Herein, our results showed that ZmADF3 had high expression in the inflorescence of transgenic plants in Arabidopsis (Fig. 6), which was in accordance with the previous results. In higher plants, seed size, as an important component of seed yield, is also a vital agronomic trait in crop domestication, and to improve seed yield is a key target for crop breeders (Xia et al. 2013). In this study, the possible functions of ZmADF3 in kernel development were studied by means of heterologous overexpression of ZmADF3 in Arabidopsis. The seed weight, seed area, seed length and seed width in transgenic lines all showed significant increase than in the WT plants. In dicotyledons, the endosperm is eventually replaced by the growing embryo, which then constitutes the majority of the mature seed (Sundaresan 2005). Accordingly, we isolated and visualized the mature embryos from the mature seeds. Our result showed that the cotyledon areas of transgenic lines were larger than that of the WT plant. The organ size was primarily determined by cell proliferation and cell expansion at the cellular level (Mizukami 2001). To reveal which was the determinant factor in this study, we measured the cells in the central region of cotyledon epidermis, and the average cell numbers of cotyledon epidermis. The result indicated that ZmADF3 enlarged seed size through the regulation of cell expansion in transgenic Arabidopsis.
In Arabidopsis, SHB1, IKU1, MINISEED3 (MINI3) and HAIKU2 (IKU2) can positively regulate seed size by promoting endosperm growth in the early stage of seed development, and both MINI3 and IKU2 are under the control of SHB1 and IKU1 in different pathway (Jiang et al. 2013; Luo et al. 2005; Zhou et al. 2009; Wang et al. 2010). IKU2 is regulated by MINI3 through the binding of W-box motif in the promoter region of IKU2 (Luo et al. 2005). In order to investigate the underlying regulation mechanism of ZmADF3 in increasing seed size in transgenic Arabidopsis, the expression patterns of seed size-associated genes SHB1, AP2, ARF2 and IKU1 in Arabidopsis were analyzed by qPCR between 35S::ZmADF3 and WT plants in our present study. SHB1 and IKU1 showed up-regulated expression in the transgenic lines at 7 DAP siliques, which implied that the increased mature seed size in the ZmADF3 heterologous overexpression lines might be also due to the endosperm proliferation in the early stage. In addition, a W-box motif (TTGACC) also was predicted in the promoter region of ZmADF3. Whether there is an internal relationship between the heterologous ZmADF3 and the SHB1-MINI3-IKU2 pathway on positive regulating seed size of Arabidopsis need to be proved in further research. In Arabidopsis, other pathways via maternal tissues to regulate seed size have been confirmed (Xia et al. 2013). AP2 and ARF2 are two representative genes in this pattern and both of them reduce seed size by restricting cell expansion and proliferation in the integuments of seeds (Ohto et al. 2005, 2009; Jofuku et al. 2005; Schruff et al. 2006). In this study, the ectopic expression of ZmADF3 in Arabidopsis promoted cell expansion in the cotyledon epidermis of mature embryo and the expression of AP2 and ARF2 were down-regulated in transgenic lines. Therefore, ZmADF3 could promote cell expansion through the influence of the expression of seed size-associated genes. However, further work still be required to uncover the underlying mechanism.
In conclusion, we isolated and characterized ZmADF3 from a popcorn inbred line N04. The promoter of ZmADF3 was obtained from the genomic DNA of N04 and then the cis-acting regulatory elements were predicted. 18 possible interacting proteins with ZmADF3 were screened using yeast two-hybrid system. Heterologous overexpression in Arabidopsis showed that ZmADF3 might increase seed size through promoting cell expansion and regulating the expression of seed size regulated genes. However, the underlying regulation mechanism of ZmADF3 in increasing seed size in transgenic Arabidopsis and the relationship between ZmADF3 and the seed size-associated genes was still unclear. Further research should be done to thoroughly explore the mechanism of ZmADF3 in playing these functions in maize.
References
Allwood EG, Smertenko AP, Hussey PJ (2001) Phosphorylation of plant actin-depolymerising factor by calmodulin-like domain protein kinase. Febs Lett 499:97–100
Allwood EG, Anthony RG, Smertenko AP, Reichelt S, Drobak BK, Doonan JH, Weeds AG, Hussey PJ (2002) Regulation of the pollen-specific actin-depolymerizing factor LlADF1. Plant Cell 14(11):2915–2927
Augustine RC, Vidali L, Kleinman KP, Bezanilla M (2008) Actin depolymerizing factor is essential for viability in plants, and its phosphoregulation is important for tip growth. Plant J 54(5):863–875
Bernstein BW, Bamburg JR (2010) ADF/cofilin: a functional node in cell biology. Trends Cell Biol 20(4):187–195
Blanchoin L, Boujemaa-Paterski R, Sykes C, Plastino J (2014) Actin dynamics, architecture, and mechanics in cell motility. Physiol Rev 94(1):235–263
Bou DF, van Oostende C, Geitmann A (2011) Spatial and temporal expression of actin depolymerizing factors ADF7 and ADF10 during male gametophyte development in Arabidopsis thaliana. Plant Cell Physiol 52(7):1177–1192
Bowman GD, Nodelman IM, Hong Y, Chua NH, Lindberg U, Schutt CE (2000) A comparative structural analysis of the ADF/cofilin family. Proteins 41(3):374–384
Burgos-Rivera B, Ruzicka DR, Deal RB, McKinney EC, King-Reid L, Meagher RB (2008) Actin depolymerizing factor 9 controls development and gene expression in Arabidopsis. Plant Mol Biol 68(6):619–632
Cai RH, Zhao Y, Wang YF, Lin YX, Peng XJ, Li Q, Chang YW, Jiang HY, Xiang Y, Cheng BJ (2014) Overexpression of a maize WRKY58 gene enhances drought and salt tolerance in transgenic rice. Plant Cell Tissue Organ Cult 119:565–577
Carlier MF, Laurent V, Santolini J, Melki R, Didry D, Xia GX, Hong Y, Chua NH, Pantaloni D (1997) Actin depolymerizing factor (ADF/cofilin) enhances the rate of filament turnover: implication in actin-based motility. J Cell Biol 136(6):1307–1322
Chen CY, Wong EI, Vidali L, Estavillo A, Hepler PK, Wu HM, Cheung AY (2002) The regulation of actin organization by actin-depolymerizing factor in elongating pollen tubes. Plant Cell 14(9):2175–2190
Cheng X, Wu Y, Guo J, Du B, Chen R, Zhu L, He G (2013) A rice lectin receptor-like kinase that is involved in innate immune responses also contributes to seed germination. Plant J 76(4):687–698
Chi J, Han Y, Wang X, Wu L, Zhang G, Ma Z (2013) Overexpression of the Gossypium barbadense actin-depolymerizing factor 1 gene mediates biological changes in transgenic tobacco. Plant Mol Biol Rep 31:833–839
Cho JI, Lim HM, Siddiqui ZS, Park SH, Kim AR, Kwon TR, Lee SK, Park SC, Jeong MJ, Lee GS (2014) Over-expression of PsGPD, a mushroom glyceraldehyde-3-phosphate dehydrogenase gene, enhances salt tolerance in rice plants. Biotechnol Lett 36(8):1641–1648
Clement M, Ketelaar T, Rodiuc N, Banora MY, Smertenko A, Engler G, Abad P, Hussey PJ, de Almeida EJ (2009) Actin-depolymerizing factor 2-mediated actin dynamics are essential for root-knot nematode infection of Arabidopsis. Plant Cell 21(9):2963–2979
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16(6):735–743
Daher FB, Geitmann A (2012) Actin depolymerizing factors ADF7 and ADF10 play distinct roles during pollen development and pollen tube growth. Plant Signal Behav 7(7):879–881
Dong CH, Hong Y (2013) Arabidopsis CDPK6 phosphorylates ADF1 at N-terminal serine 6 predominantly. Plant Cell Rep 32(11):1715–1728
Dong CH, Kost B, Xia G, Chua NH (2001a) Molecular identification and characterization of the Arabidopsis AtADF1, AtADF5 and AtADF6 genes. Plant Mol Biol 45(5):517–527
Dong CH, Xia GX, Hong Y, Ramachandran S, Kost B, Chua NH (2001b) ADF proteins are involved in the control of flowering and regulate F-actin organization, cell expansion, and organ growth in Arabidopsis. Plant Cell 13(6):1333–1346
Dong CH, Tang WP, Liu JY (2013) Arabidopsis AtADF1 is functionally affected by mutations on actin binding sites. J Integr Plant Biol 55(3):250–261
Dong Y, Wang Q, Zhang L, Du C, Xiong W, Chen X, Deng F, Ma Z, Qiao D, Hu C, Ren Y, Li Y (2015) Dynamic proteomic characteristics and network integration revealing key proteins for two kernel tissue developments in popcorn. PLoS One 10(11):e143181
Edwards D, Murray JA, Smith AG (1998) Multiple genes encoding the conserved CCAAT-box transcription factor complex are expressed in Arabidopsis. Plant Physiol 117(3):1015–1022
Feng Y, Liu Q, Xue Q (2006) Comparative study of rice and Arabidopsis actin-depolymerizing factors gene families. J Plant Physiol 163(1):69–79
Finley Jr RL (2007) A guide to yeast two-hybrid experiments. In: Zuk D (ed) Evaluating techniques in biochemical research (Cambridge, MA: Cell Press), http://www.cellpress.com/misc/page?page=ETBR
Fu Y, Duan X, Tang C, Li X, Voegele RT, Wang X, Wei G, Kang Z (2014) TaADF7, an actin-depolymerizing factor, contributes to wheat resistance against Puccinia striiformis f. sp. tritici. Plant J 78(1):16–30
Hajduch M, Hearne LB, Miernyk JA, Casteel JE, Joshi T, Agrawal GK, Song Z, Zhou M, Xu D, Thelen JJ (2010) Systems analysis of seed filling in Arabidopsis: using general linear modeling to assess concordance of transcript and protein expression. Plant Physiol 152:2078–2087
Henty-Ridilla JL, Li J, Day B, Staiger CJ (2014) Actin depolymerizing factor 4 regulates actin dynamics during innate immune signaling in Arabidopsis. Plant Cell 26(1):340–352
Huang YC, Huang WL, Hong CY, Lur HS, Chang MC (2012) Comprehensive analysis of differentially expressed rice actin depolymerizing factor gene family and heterologous overexpression of OsADF3 confers Arabidopsis thaliana drought tolerance. Rice (NY) 5(1):33
Hussey PJ, Ketelaar T, Deeks MJ (2006) Control of the actin cytoskeleton in plant cell growth. Annu Rev Plant Biol 57:109–125
Ishikawa H (1990) Functional significances of the cytoskeleton. Hum Cell 3(4):289–293
Jiang CJ, Weeds AG, Hussey PJ (1997a) The maize actin-depolymerizing factor, ZmADF3, redistributes to the growing tip of elongating root hairs and can be induced to translocate into the nucleus with actin. Plant J 12(5):1035–1043
Jiang CJ, Weeds AG, Khan S, Hussey PJ (1997b) F-actin and G-actin binding are uncoupled by mutation of conserved tyrosine residues in maize actin depolymerizing factor (ZmADF). Proc Natl Acad Sci USA 94(18):9973–9978
Jiang WB, Huang HY, Hu YW, Zhu SW, Wang ZY, Lin WH (2013) Brassinosteroid regulates seed size and shape in Arabidopsis. Plant Physiol 162(4):1965–1977
Jofuku KD, Omidyar PK, Gee Z, Okamuro JK (2005) Control of seed mass and seed yield by the floral homeotic gene APETALA2. Proc Natl Acad Sci USA 102(8):3117–3122
Ketelaar T (2013) The actin cytoskeleton in root hairs: all is fine at the tip. Curr Opin Plant Biol 16(6):749–756
Kim SR, Kim Y, An G (1993) Molecular cloning and characterization of anther-preferential cDNA encoding a putative actin-depolymerizing factor. Plant Mol Biol 21(1):39–45
Kost B, Mathur J, Chua NH (1999) Cytoskeleton in plant development. Curr Opin Plant Biol 2(6):462–470
Kost B, Bao YQ, Chua NH (2002) Cytoskeleton and plant organogenesis. Philos Trans R Soc Lond B Biol Sci 357(1422):777–789
Lescot M, Dehais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouze P, Rombauts S (2002) Plant care, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res 30(1):325–327
Li XB, Xu D, Wang XL, Huang GQ, Luo J, Li DD, Zhang ZT, Xu WL (2010) Three cotton genes preferentially expressed in flower tissues encode actin-depolymerizing factors which are involved in F-actin dynamics in cells. J Exp Bot 61(1):41–53
Lin F, Jiang L, Liu Y, Lv Y, Dai H, Zhao H (2014) Genome-wide identification of housekeeping genes in maize. Plant Mol Biol 86(4–5):543–554
Liu Y, Li J, Li Y, Wei M, Cui Q, Wang Q (2010) Molecular cloning, sequence and expression analysis of ZmArf2, a maize ADP-ribosylation factor. Mol Biol Rep 37(2):755–761
Liu YB, Qin LJ, Han LZ, Xiang Y, Zhao DG (2015) Overexpression of maize SDD1 (ZmSDD1) improves drought resistance in Zea mays L. by reducing stomatal density. Plant Cell Tissue Organ Cult 122:147–159
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4):402–408
Lopez I, Anthony RG, Maciver SK, Jiang CJ, Khan S, Weeds AG, Hussey PJ (1996) Pollen specific expression of maize genes encoding actin depolymerizing factor-like proteins. Proc Natl Acad Sci USA 93(14):7415–7420
Luo M, Dennis ES, Berger F, Peacock WJ, Chaudhury A (2005) MINISEED3 (MINI3), a WRKY family gene, and HAIKU2 (IKU2), a leucine-rich repeat (LRR) KINASE gene, are regulators of seed size in Arabidopsis. Proc Natl Acad Sci USA 102(48):17531–17536
Maciver SK, Hussey PJ (2002) The ADF/cofilin family: actin-remodeling proteins. Genome Biol 3(5):s3007
Mitchell A, Chang HY, Daugherty L, Fraser M, Hunter S, Lopez R, McAnulla C, McMenamin C, Nuka G, Pesseat S, Sangrador-Vegas A, Scheremetjew M, Rato C, Yong SY, Bateman A, Punta M, Attwood TK, Sigrist CJ, Redaschi N, Rivoire C, Xenarios I, Kahn D, Guyot D, Bork P, Letunic I, Gough J, Oates M, Haft D, Huang H, Natale DA, Wu CH, Orengo C, Sillitoe I, Mi H, Thomas PD, Finn RD (2015) The interpro protein families database: the classification resource after 15 years. Nucleic Acids Res 43:D213–D221
Mizukami Y (2001) A matter of size: developmental control of organ size in plants. Curr Opin Plant Biol 4(6):533–539
Mun JH, Yu HJ, Lee HS, Kwon YM, Lee JS, Lee I, Kim SG (2000) Two closely related cDNAs encoding actin-depolymerizing factors of petunia are mainly expressed in vegetative tissues. Gene 257(2):167–176
Munoz-Bertomeu J, Cascales-Minana B, Alaiz M, Segura J, Ros R (2010) A critical role of plastidial glycolytic glyceraldehyde-3-phosphate dehydrogenase in the control of plant metabolism and development. Plant Signal Behav 5(1):67–69
Nicholls C, Li H, Liu JP (2012) GAPDH: a common enzyme with uncommon functions. Clin Exp Pharmacol Physiol 39(8):674–679
Ohto MA, Fischer RL, Goldberg RB, Nakamura K, Harada JJ (2005) Control of seed mass by APETALA2. Proc Natl Acad Sci USA 102(8):3123–3128
Ohto MA, Floyd SK, Fischer RL, Goldberg RB, Harada JJ (2009) Effects of APETALA2 on embryo, endosperm, and seed coat development determine seed size in Arabidopsis. Sex Plant Reprod 22:277–289
Ouellet F, Carpentier E, Cope MJ, Monroy AF, Sarhan F (2001) Regulation of a wheat actin-depolymerizing factor during cold acclimation. Plant Physiol 125(1):360–368
Porter K, Shimono M, Tian M, Day B (2012) Arabidopsis actin-depolymerizing factor 4 links pathogen perception, defense activation and transcription to cytoskeletal dynamics. PLoS Pathog 8(11):e1003006
Prelich G (2012) Gene overexpression: uses, mechanisms, and interpretation. Genetics 190:841–854
Ren H, Xiang Y (2007) The function of actin-binding proteins in pollen tube growth. Protoplasma 230(3–4):171–182
Rius SP, Casati P, Iglesias AA, Gomez-Casati DF (2008) Characterization of Arabidopsis lines deficient in GAPC-1, a cytosolic NAD-dependent glyceraldehyde-3-phosphate dehydrogenase. Plant Physiol 148(3):1655–1667
Rozycka M, Khan S, Lopez I, Greenland AJ, Hussey PJ (1995) A Zea mays pollen cDNA encoding a putative actin-depolymerizing factor. Plant Physiol 107(3):1011–1012
Ruzicka DR, Kandasamy MK, McKinney EC, Burgos-Rivera B, Meagher RB (2007) The ancient subclasses of Arabidopsis Actin Depolymerizing Factor genes exhibit novel and differential expression. Plant J 52(3):460–472
Schmidt RJ, Ketudat M, Aukerman MJ, Hoschek G (1992) Opaque-2 is a transcriptional activator that recognizes a specific target site in 22-kD zein genes. Plant Cell 4(6):689–700
Schruff MC, Spielman M, Tiwari S, Adams S, Fenby N, Scott RJ (2006) The AUXIN RESPONSE FACTOR 2 gene of Arabidopsis links auxin signalling, cell division, and the size of seeds and other organs. Development 133:251–261
Smertenko AP, Jiang CJ, Simmons NJ, Weeds AG, Davies DR, Hussey PJ (1998) Ser6 in the maize actin-depolymerizing factor, ZmADF3, is phosphorylated by a calcium-stimulated protein kinase and is essential for the control of functional activity. Plant J 14(2):187–193
Smith LG (2003) Cytoskeletal control of plant cell shape: getting the fine points. Curr Opin Plant Biol 6(1):63–73
Sprinzak E, Sattath S, Margalit H (2003) How reliable are experimental protein-protein interaction data? J Mol Biol 327(5):919–923
Staiger CJ (2000) Signaling to the actin cytoskeleton in plants. Annu Rev Plant Physiol Plant Mol Biol 51:257–288
Su XH, Zhou P, Wang R, Luo ZP, Xia ZL (2015) Overexpression of the maize psbA gene enhances sulfur dioxide tolerance in transgenic tobacco. Plant Cell Tissue Organ Cult 120:303–311
Sun HQ, Kwiatkowska K, Yin HL (1995) Actin monomer binding proteins. Curr Opin Cell Biol 7(1):102–110
Sundaresan V (2005) Control of seed size in plants. Proc Natl Acad Sci USA 102(50):17887–17888
Takaiwa F, Oono K, Wing D, Kato A (1991) Sequence of three members and expression of a new major subfamily of glutelin genes from rice. Plant Mol Biol 17(4):875–885
Tiezzi A (1991) The pollen tube cytoskeleton. Electron Microsc Rev 4(2):205–219
Volkmann D, Baluska F (1999) Actin cytoskeleton in plants: from transport networks to signaling networks. Microsc Res Tech 47(2):135–154
Wang QL (2015) Functional research of ZmREP2 and ZmArf2 and characterization of dynamic quantitative proteomics of pericarp and endoperm in two maize inbred lines. Doctoral dissertation, Henan Agricultural University
Wang X, Li X, Deng X, Han H, Shi W, Li Y (2007) A protein extraction method compatible with proteomic analysis for the euhalophyte Salicornia europaea. Electrophoresis 28(21):3976–3987
Wang HY, Wang J, Gao P, Jiao GL, Zhao PM, Li Y, Wang GL, Xia GX (2009) Down-regulation of GhADF1 gene expression affects cotton fibre properties. Plant Biotechnol J 7(1):13–23
Wang A, Garcia D, Zhang H, Feng K, Chaudhury A, Berger F, Peacock WJ, Dennis ES, Luo M (2010) The VQ motif protein IKU1 regulates endosperm growth and seed size in Arabidopsis. Plant J 63(4):670–679
Wang WQ, Liu SJ, Song SQ, Moller IM (2015) Proteomics of seed development, desiccation tolerance, germination and vigor. Plant Physiol Biochem 86:1–15
Wu CY, Suzuki A, Washida H, Takaiwa F (1998) The GCN4 motif in a rice glutelin gene is essential for endosperm-specific gene expression and is activated by opaque-2 in transgenic rice plants. Plant J 14(6):673–683
Xia T, Li N, Dumenil J, Li J, Kamenski A, Bevan MW, Gao F, Li Y (2013) The ubiquitin receptor DA1 interacts with the E3 ubiquitin ligase DA2 to regulate seed and organ size in Arabidopsis. Plant Cell 25(9):3347–3359
Xu SB, Li T, Deng ZY, Chong K, Xue Y, Wang T (2008) Dynamic proteomic analysis reveals a switch between central carbon metabolism and alcoholic fermentation in rice filling grains. Plant Physiol 148:908–925
Zhang C, Guo L, Wang X, Zhang H, Shi H, Xu W, Li X (2007) Molecular characterization of four ADF genes differentially expressed in cotton. J Genet Genomics 34(4):347–354
Zhang XH, Rao XL, Shi HT, Li RJ, Lu YT (2011) Overexpression of a cytosolic glyceraldehyde-3-phosphate dehydrogenase gene OsGAPC3 confers salt tolerance in rice. Plant Cell Tissue Organ Cult 10:1–11
Zheng Y, Xie Y, Jiang Y, Qu X, Huang S (2013) Arabidopsis actin-depolymerizing factor7 severs actin filaments and regulates actin cable turnover to promote normal pollen tube growth. Plant Cell 25(9):3405–3423
Zhou Y, Zhang X, Kang X, Zhao X, Zhang X, Ni M (2009) Short hypocotyl under blue 1 associates with MINISEED3 and HAIKU2 promoters in vivo to regulate Arabidopsis seed development. Plant Cell 21(1):106–117
Acknowledgments
This work was funded by the Plan for Fundamental and Forward Technology Research in Henan Province (121100910400), the Plan for Scientific Innovation Talent of Henan Province (124200510003) and the Earmarked Fund for Henan Agriculture Research System (S2010-02-G01).
Author contribution statement
D. Qiao performed most experiments, data analyses and manuscript preparation. Y. Dong contributed to some supervision of experiments. L. Zhang assisted in some data analysis and manuscript preparation. Q. Zhou, C. Hu and Y. Ren took part in the Arabidopsis planting and management. Y. Li was responsible for the whole study and the manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declared that they had no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Qiao, D., Dong, Y., Zhang, L. et al. Ectopic expression of the maize ZmADF3 gene in Arabidopsis revealing its functions in kernel development. Plant Cell Tiss Organ Cult 126, 239–253 (2016). https://doi.org/10.1007/s11240-016-0994-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-016-0994-5