Abstract
In vitro chromosome doubling techniques have been used to produce polyploids in several species, including table grapes, but limited research in this field has been performed using winegrapes. An efficient procedure was established to induce tetraploid grapevine plants by colchicine treatment of embryogenic cell aggregates (ECAs) grown in winegrape cultivar (cv. Mencía) suspension cultures. Colchicine treatment caused a significant decrease in the survival and embryogenic potential of the ECAs compared with the control. However, plantlets were regenerated and assessed for ploidy level by flow cytometry. Almost all plants not treated with colchicine maintained their ploidy level, whereas colchicine treatment resulted in a high variation in ploidy level. Colchicine at 0.2 % was the most effective concentration for obtaining tetraploid plantlets (25 % tetraploids). No chimeric or mixoploid plantlets were detected in this study. Based on the unique cell origin of grapevine somatic embryos, our system prevented obtaining chimeric plants. This protocol allows an increase in grapevine genetic diversity and could be a valuable tool for improving this crop. To our knowledge, this is the first report on in vitro chromosome doubling by colchicine treatment of embryogenic cell aggregates growing in grapevine suspension cultures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polyploid plant production is commonly used in traditional plant breeding, producing polyploid fruits and vegetables used as human and animal food for years. Recent progress reveals the great potential of in vitro culture for inducing polyploids. Because there is evidence that plant tissue culture may cause chromosome alterations and ploidy changes, the most probable origin of ploidy variation during micropropagation is somaclonal variation (Predieri 2001; Weber et al. 2008). In addition, polyploids can be obtained by treating plant material with the chemical agent colchicine, a toxic alkaloid extracted from the seeds and bulbs of Colchicum autumnale L., a member of the Liliaceae family. Colchicine has an affinity for tubulin, a microtubule-subunit protein, and inhibits spindle function, thereby preventing both cell and nuclear division during the replication of chromosomes and division to form sister chromosomes. Colchicine treatment of in vitro-cultured plant tissues has been successfully used to artificially produce polyploids in several plant species, such as tubers (Oumar et al. 2011), grasses (Glowacka et al. 2010), forage (Tulay and Unal 2010), medicinals (Omidbaigi et al. 2010; Xing et al. 2011), ornamentals (Zhang et al. 2008) and fruit crops (Blasco et al. 2015).
Tetraploids often generate variants that may contain favorable horticultural characteristics, such as a large fruit size, sturdiness, high productivity and tolerance to environmental stresses (Estilai and Shannon 1993). Moreover, in medicinal plants, polyploids can show increased content of bioactive compounds. For example, tetraploid lines of Catharanthus roseus increase the production of terpenoid indole alkaloids by modulating the expression of genes involved in their biosynthesis pathway (Xing et al. 2011).
Flow cytometry (FCM) has been described as one of the most reliable techniques for estimating DNA ploidy level and nuclear DNA content in plants. In comparison with other methods, such as Feulgen microdensitometry and chromosome counting, FCM provides unsurpassed ease, speed and accuracy (Doležel and Bartoš 2005). The application of FCM in plants has significantly increased in the last decade and has been successfully applied in the analysis of somaclonal variation in a variety of plant species, including cotton (Jin et al. 2008), grapevine (Acanda et al. 2013; Leal et al. 2006; Prado et al. 2010b) and Pinus pinaster (Marum et al. 2009).
Grapevine (Vitis sp.) is one of the most important fruit crops grown worldwide. In Galicia (North-western Spain), quality wine production has a long tradition, with up to five growing areas with a denomination of origin (DO, Rias Baixas, Ribeiro, Ribeira Sacra, Valdeorras and Monterrei) in which some distinguished cultivars, such as Albariño or Mencía, are grown. Polyploidization in grapevine could allow a greater fruit size and a loose berry cluster, characteristics that are very important for table grapes (Alleweldt and Possingham 1988). In fact, an increase in berry size and in the content of sensory and functional compounds in tetraploid table grape cultivars in comparison with their diploid counterparts has been reported (Shiraishi et al. 2008, 2010, 2012), although without a significant variation in soluble solids and total sugar content (Shiraishi et al. 2012). In addition, tolerance to environmental stress and the increased production of secondary metabolites could also be interesting in wine grapes. Tetraploids have been also used in grapevine breeding to obtain seedless triploid cultivars through embryo rescue (reviewed by Li et al. 2015).
Tetraploid, octoploid and mixoploid grapevine plants have been regenerated from anther-, ovary-, and stamen filament-derived somatic embryos (Acanda et al. 2013; Prado et al. 2010b), with percentages of polyploid occurrence ranging from 2.2 to 7.7 %, depending on the cultivar. Similar percentages of polyploid plants were obtained in the grapevine cv. Sinsaut by treating somatic embryos derived from immature zygotic embryos with colchicine (Yang et al. 2006). However, an undesirable effect of treating developed somatic embryos was a reduction in their germination efficiency. Based on that work, it appears that the use of colchicine did not have a significant effect on the production of polyploid plants versus somaclonal variation. More recently, Sinski et al. (2013) reported the induction of tetraploid plants in two seedless grapevine cultivars using cotyledonary somatic embryos treated with colchicine or oryzalin. These authors obtained higher percentages of tetraploid plants by using colchicine concentrations comparable to those used by Yang et al. (2006), although their polyploid detection system was based only on chromosome counts and stomatal size parameter determinations (Sinski et al. 2013).
In this study, we treated an embryogenic suspension culture of grapevine (cv. Mencía) with high concentrations of colchicine and evaluated the effects on the survival of the embryogenic aggregates and their embryogenic potential. The plantlets derived from colchicine-treated embryogenic cultures were analyzed by flow cytometry to determine possible alterations to the ploidy level. Considering the early embryogenic nature of the aggregates of which the suspension culture is composed (Acanda et al. 2013) and that the most probable origin of somatic embryos of grapevine are single cells (Faure et al. 1996), this method is expected to produce polyploid plants with a reduced or null probability of being mixoploid or chimeric.
Materials and methods
Initiation of embryogenic cultures and embryogenic suspension culture establishment
Adult field-grown plants of Vitis vinifera L. cv. Mencía were selected from the grapevine collection at the “Centro de Formación y Experimentación de Viticultura y Enología de Ribadumia” (Galicia, northwestern Spain). Inflorescences at stage H on the Baggiolini (1952) phenological scale (corresponding to separated clusters) were collected when the flowers were at the late binucleate microspore developmental stage (Prado et al. 2010a). This stage was identified microscopically after anther squashing in the presence of 4′,6-diamidino-2-phenylindole (DAPI) with the aid of an MZ10F fluorescence stereomicroscope (Leica Microsystems GmbH, Wetzlar, Germany). The collected flower clusters were washed twice for 5 min with 200 mL of distilled water containing a drop of detergent, chilled at 4 °C for 4 days and then sterilized as described by Kikkert et al. (2005). Embryogenic cultures were induced from stamen filaments in SEIM4 medium (Somatic Embryogenesis Induction Medium 4; Acanda et al. 2013) containing NN (Nitsch and Nitsch 1969) salts, MS (Murashige and Skoog 1962) vitamins, 1 µM 2,4-dichlorophenoxyacetic acid (2,4-D), 4.5 µM thidiazuron (TDZ), 6 % sucrose and 0.1 % casein hydrolysate. The pH of the medium was adjusted to 5.8 before autoclaving at 98 kPa and 121 °C, and the medium was solidified using 0.3 % gelrite (Duchefa Biochemie, Haarlem, The Netherlands). Twenty-five stamens were placed on 90 mm-diameter polystyrene Petri plates containing 25 mL of medium. The cultures were maintained in the same medium in continuous darkness at 23 ± 1 °C and subcultured onto fresh medium at 30 day intervals.
To initiate embryogenic suspension cultures (ESCs), embryo masses (400 mg, fresh weight) were transferred to 100 mL Erlenmeyer flasks containing 50 mL of liquid SEIM4 medium, as described by Acanda et al. (2013). The suspensions were incubated on an orbital rotary shaker (150 rpm) at 24 °C in continuous darkness and maintained by weekly replacement of 75 % of the medium with fresh SEIM4 medium. After 4 weeks, the suspensions were passed through a 850 µm sieve, and the retained masses were discarded, producing a homogeneous ESC composed of embryogenic cell aggregates (ECAs) smaller than 850 µm in diameter. This homogeneous ESC was subsequently used for the colchicine treatments described below.
Colchicine treatments
Colchicine was prepared by dissolving 500 mg in 2 mL of deionized water containing 1 % (v/v) dimethyl-sulfoxide (DMSO). This solution was filter sterilized (0.22 µm pore diameter) and then aseptically added to 15 mL conical polypropylene tubes containing 10 mL of grapevine ESC to result in final concentrations of 0, 0.1, 0.2 and 0.4 % (w/v) colchicine. The cultures were incubated on a tube rotator mixer (150 rpm) at room temperature for 24 h. Following the colchicine treatment, the ECAs were rinsed several times with sterilized SEIM4 liquid medium without colchicine, plated (1 mL) onto SEIM4 solid medium and incubated as described above to generate somatic embryo clusters. The mean number of somatic embryos produced per ECA (embryogenic potential) was determined after 30 days of incubation. To assess the effect of colchicine treatment on the survival of the grapevine ECAs, aliquots (1 mL) of the colchicine-treated ESCs were plated onto SEIM4 solid medium containing 0.1 % tetrazolium chloride (TZC) and incubated at 24 °C in darkness; the red (viable) ECAs were counted after 48 h of incubation.
Plant regeneration
Clusters of globular embryos regenerated from colchicine-treated ECAs were subcultured on 90 mm-diameter Petri plates containing 25 mL of DM1 differentiation medium (Differentiation Medium 1; Prado et al. 2010a) consisting of NN (Nitsch and Nitsch 1969) salts and MS (Murashige and Skoog 1962) vitamins, 6 % Sucrose, 0.25 % activated charcoal, pH 5.8 and 0.3 % gelrite. The cultures were maintained in continuous darkness for 4 weeks under the same conditions described for the embryogenic culture induction. For germination, opaque-white, well-developed embryos were isolated and cultured for 30 days on 90 mm-diameter Petri plates containing 25 mL of germination medium, which consisted of DM1 medium with 3 % sucrose, 0.3 % gelrite and lacking casein hydrolysate (16 embryos per plate). The cultures were maintained for 2 weeks in darkness at 20 ± 1 °C followed by another 2 weeks at 25 ± 1 °C (20 ± 1 °C night temperature) under a 16 h photoperiod with a photon flux density of 45 µmol m−2 s−1 provided by cool white light fluorescent tubes. For plant conversion, germinated embryos with hypocotyls and cotyledons and an apical root axis were transferred to test tubes (25 mm × 120 mm) containing 12.5 mL of MS medium with half-strength macronutrients, 0.15 % sucrose, pH 5.8 and 0.8 % plant agar (Duchefa). The cultures were maintained at 26 ± 1 °C (20 ± 1 °C night temperature) under a 16 h photoperiod with the same photon flux density of 45 µmol m−2 s−1 as above. The plants were propagated by culturing axillary buds on the same MS medium with half-strength macronutrients.
DAPI staining
Actively growing ECAs were collected by centrifuging aliquots of the ESC at 6000×g for 5 min. The supernatant was discarded, and the pellet was washed with phosphate-buffered saline (PBS; 137 mM NaCl, 3 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, pH 7.4) and centrifuged again. The ECAs were fixed overnight in 4 % (w/v) paraformaldehyde in PBS buffer at 4 °C, dehydrated in an acetone series and embedded in Technovit 8100 resin (Heraeus Kulzer GmbH, Wehrheim, Germany) at 4 °C. Semithin sections (2.5 μm) were obtained and stained with 1 μg/mL DAPI. After rinsing and drying, the preparations were mounted in Eukitt mounting medium (Kindler GmbH, Freiburg, Germany) and observed using an excitation wavelength of 405 nm and detection in the range of 415–490 nm with an E800 microscope (Nikon, Tokyo, Japan) equipped with a Nikon DS-U2 digital camera.
Ploidy determination
To determine plant ploidy levels, well-expanded leaves collected from twenty plants from each of the colchicine treatments were analyzed by flow cytometry. In brief, nuclei were isolated by chopping approximately 100 mg leaves with a sharp razor blade in 500 µL of a buffer containing 0.1 M Tris–HCl, 2 mM MgCl2·6H2O, 1 mM disodium EDTA, 43 mM NaCl, 5 mM Na2S2O5, 1 % PVP-10, and 0.5 % Triton-100, pH 7.5 (Acanda et al. 2013). The homogenate was then filtered through a 50 µm nylon mesh, and 50 µg/mL of propidium iodide and 50 µg/mL of RNase (Sigma, St. Louis, MO) were added to the samples. After a 10 min incubation period, the samples (at least 2000 nuclei each) were analyzed using an FC500 cytometer (Beckman Coulter Inc, Miami, FL).
Statistical analysis
The data were statistically analyzed using a Mann–Whitney U test (P ≥ 0.05) performed with the SPSS (Statistical Package for the Social Sciences) software (v18.0 for Windows, IBM Corp., Somers, NY). All of the experiments were repeated at least twice.
Results and discussion
The fact that somatic embryos can originate from single cells suggests that cell suspension culture is a very convenient micropropagation system to induce polyploids while avoiding the formation of unstable and undesirable mixoploids. Faure et al. (1996) found that grapevine somatic embryos initiate directly from anther connective cells or indirectly from connective-derived callus with starchy parenchymatous cells. In both cases, proembryos originate from single predetermined cells that do not show the cytological characteristics of meristematic cells, although they are highly differentiated cells with a large vacuole and numerous starch grains. We histologically identified proembryos as structured islands of cells with prominent nuclei on the surface of ECAs at an early developmental stage (Fig. 1) as previously described (Acanda et al. 2013). We previously found that the peripheral cells of ECAs are also starchy cells (Acanda et al. 2013). These results suggest that the somatic embryos derived from the ECAs in this study also originated from single cells, as described by Faure et al. (1996) for grapevine somatic embryos derived from anther connective tissue.
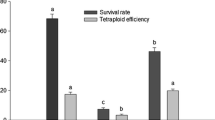
The stamen filament-derived embryogenic cultures were composed of somatic embryo masses (Fig. 2a) that were easily disaggregated in liquid medium to establish an ESC (Fig. 2b). The viability of the ECAs following treatment with increasing concentrations (0, 0.1, 0.2 and 0.4 %) of colchicine for 24 h was evaluated using TCZ (Fig. 2c). We found that all colchicine treatments caused a significant decrease in the survival of the ECAs compared with the control (Fig. 3a). Colchicine at 0.4 % seriously affected the survival of ECAs, which was reduced to 28.8 %. These results suggest that colchicine had a cytotoxic effect on the suspension culture. Other studies have indicated that colchicine is toxic to several plant materials from different plant species, especially at high concentrations (Adaniya and Shirai 2001; Glowacka et al. 2009, 2010; Omidbaigi et al. 2010). Yang et al. (2006) found that the survival and germination of grapevine somatic embryos after colchicine treatment were dependent on the colchicine concentration and treatment duration.
Embryogenic cultures of grapevine cv. Mencía. a Proliferation of somatic embryos on SEIM4 induction medium; b embryogenic suspension culture established in SEIM4 liquid medium in a 100 mL Erlenmeyer flask; c embryogenic culture treated with 0.2 % colchicine showing non-surviving (white) and surviving (red) embryogenic cell aggregates on SEIM4 medium containing 0.1 % TZC; d necrotic aggregates and globular embryos derived from embryogenic cell aggregates treated with 0.2 % colchicine after plating on SEIM4 solid medium and culturing for 4 weeks. Bars = 1 cm. (Color figure online)
After plating the colchicine-treated ESCs onto SEIM4 solid medium, a fraction of the ECAs became necrotic and did not differentiate into embryos (Fig. 2d), showing that the embryogenic potential of the aggregates was more affected by the colchicine treatment than was their survival (Fig. 3b). At 0.1 % colchicine, the embryogenic potential decreased to 38.3 %. In the ESC treated with 0.2 % colchicine, although survival decreased to 52.5 %, the embryogenic potential decreased to 13.8 %; at 0.4 % colchicine, the embryogenic potential was almost completely abolished (Fig. 3). The fact that the embryogenic potential was more affected than survival by the colchicine treatment may be due to the higher exposure received by the embryogenic cells located at the surface of the aggregates, causing them to die.
The somatic embryos derived from the colchicine-treated ESC aggregates plated onto SEIM4 solid medium were able to differentiate after 5 weeks of culture on DM1 differentiation medium. Somatic embryos developed asynchronously, with embryos at different developmental stages being easily distinguished (Fig. 4a). The asynchronous development of grapevine somatic embryos typically occurs when using different somatic embryogenesis protocols (Acanda et al. 2013; Prado et al. 2010a). The developed somatic embryos germinated normally (Fig. 4b) on germination medium and converted to plantlets (Fig. 4c) on a modified MS medium, as we previously reported (Acanda et al. 2013).
Plant regeneration from somatic embryos derived from 0.1 % colchicine-treated grapevine ECAs. a Embryogenic cluster showing embryos at different developmental stages after 5 weeks of culture on DM1 differentiation medium; b somatic embryos germinating after 4 weeks of culture on germination medium; c plantlet development after 4 weeks of culture on modified MS medium. Bars = 1 mm (a); 1 cm (b, c)
The effects of colchicine on shoot differentiation and embryo germination have been studied in several plant species. In grapevine, the number of germinated somatic embryos was found to decrease with increasing colchicine concentration (Yang et al. 2006), although it must be taken into consideration that these authors treated somatic embryos that were already developed with colchicine. In other species, colchicine causes a decrease in the survival of colchicine-treated explants, in the number of regenerated shoots and in the percentage of embryo germination (Ishfaq et al. 2012; Mahadevamma et al. 2012; Nilanthi et al. 2009; Pande and Khetmalas 2012).
To investigate the effect of colchicine treatment on the ploidy level of somatic embryo-derived plantlets, 20 plantlets derived from ESCs treated with each of the colchicine concentrations and 20 plantlets derived from non-treated ESCs were analyzed by FCM. Five tetraploid plants (25 % of the analyzed plantlets, Table 1) were identified from the 0.2 % colchicine-treated ESC-derived plantlets. Based on the presence of an intermediate G0/G1 peak, most of the polyploid plantlets obtained from the 0.1 and 0.4 % colchicine treatments probably were near-triploids (Fig. 5). Mixoploid plantlets were not detected in this study.
Histograms of the relative fluorescence intensity of propidium iodide (PI) obtained after the analysis of nuclei isolated from Vitis vinifera cv. Mencía leaves. a A diploid somatic embryo-derived plant; b a tetraploid somatic embryo-derived plant; c a near-triploid somatic embryo-derived plant showing an intermediate G0/G1 peak
Using colchicine at a range from 0.1 to 0.4 % w/v (equivalent to 2.5–10 mM), we obtained a high percentage of polyploids (25 % tetraploids using 0.2 % colchicine) as well as variants showing an intermediate cytotype (Table 1). Yang et al. (2006) achieved a lower percentage of polyploids (4 %) using 10–20 mg/L colchicine (equivalent to 50–100 µM), which is comparable to the percentage of polyploids achieved due to somaclonal variation in previously published grapevine somatic embryogenesis protocols (Acanda et al. 2013; Prado et al. 2010b). These results suggest that the effective concentration for colchicine-mediated polyploidization in grapevine should be on the order of 2.5 mM, as suggested by other studies of the in vitro application of colchicine in other plant species (Dhooghe et al. 2011). However, recent work (Sinski et al. 2013) in other grapevine cultivars (seedless cvs. Crimson Seedless and BRS Clara) demonstrated that it is possible to obtain high rates of polyploid plants (more than 30 %) using colchicine concentrations and target tissues (mature somatic embryos) similar to those used by Yang et al. (2006). In addition, Sinski et al. (2013) improved the induction of polyploids by using oryzalin instead of colchicine at the same concentrations. Based on this and the present research, it is evident that differences in the sensitivity of the target tissues as well as the grapevine genotype account for the differences observed. Hence, it can be concluded that, as frequently occurs in grapevine for every biotechnological method established (Couselo et al. 2006; Péros et al. 1998), the efficiency of the polyploidization method must be specifically tested for each grapevine cultivar in an empirical fashion.
In comparison to tetraploid plants, chimeric plants appear to be of much less agronomic value (Nilanthi et al. 2009) because the ratio of 4× cells is generally low and unstable, as 2× cells divide more rapidly (Notsuka et al. 2000). Chimeric plants are frequently observed in in vitro polyploidization experiments conducted through organogenesis, with many meristematic cells being involved in shoot formation in several species (Ishigaki et al. 2009; Omidbaigi et al. 2010; Sun et al. 2009; Zhang et al. 2008). In the present study, using a somatic embryogenesis system, the formation of chimeric plantlets was not produced, which may be due to the combination of three factors: the single-cell origin of the somatic embryos (Faure et al. 1996); the early developmental stage of the colchicine-treated embryogenic cultures (embryogenic cell aggregates producing proembryos); and the secondary embryogenesis process occurring on solid medium after plating the colchicine-treated embryogenic suspension.
References
Acanda Y, Prado MJ, González MV, Rey M (2013) Somatic embryogenesis from stamen filaments in grapevine (Vitis vinifera L. cv. Mencía): changes in ploidy level and nuclear DNA content. In Vitro Cell Dev Biol Plant 49:276–284. doi:10.1007/s11627-013-9499-7
Adaniya S, Shirai D (2001) In vitro induction of tetraploid ginger (Zingiber officinale Roscoe) and its pollen fertility and germinability. Sci Hortic 88:277–287. doi:10.1016/S0304-4238(00)00212-0
Alleweldt G, Possingham JV (1988) Progress in grapevine breeding. Theor Appl Genet 75:669–673. doi:10.1007/BF00265585
Baggiolini M (1952) Les stades repères dans le développement annuel de la vigne et leur utilisation pratique. Rev Romande Agric Vitic Arboric 8:4–6
Blasco M, Badenes ML, Naval MDM (2015) Colchicine-induced polyploidy in loquat (Eriobotrya japonica (Thunb.) Lindl.). Plant Cell Tissue Org Cult 120:453–461. doi:10.1007/s11240-014-0612-3
Couselo JL, Varela P, Rey M (2006) Effect of benzyladenine concentration and double-phase culture system on in vitro multiplication of adult Albariño plants. Am J Enol Vitic 57:109–112
Dhooghe E, Van Laere K, Eeckhaut T, Leus L, Van Huylenbroeck J (2011) Mitotic chromosome doubling of plant tissues in vitro. Plant Cell Tissue Org Cult 104:359–373. doi:10.1007/s11240-010-9786-5
Doležel J, Bartoš J (2005) Plant DNA flow cytometry and estimation of nuclear genome size. Ann Bot 95:99–110. doi:10.1093/aob/mci005
Estilai A, Shannon MC (1993) Salt tolerance in relation to ploidy level in guayule. In: Janick J, Simon JE (eds) New crops. Wiley, New York, pp 349–351
Faure O, Aarrouf J, Nougarède A (1996) Ontogenesis, differentiation and precocious germination in anther-derived somatic embryos of grapevine (Vitis vinifera L.): proembryogenesis. Ann Bot 78:23–28. doi:10.1006/anbo.1996.0090
Glowacka K, Jezowski S, Kaczmarek Z (2009) Polyploidization of Miscanthus sinensis and Miscanthus x giganteus by plant colchicine treatment. Ind Crops Prod 30:444–446. doi:10.1016/j.indcrop.2009.07.011
Glowacka K, Jezowski S, Kaczmarek Z (2010) In vitro induction of polyploidy by colchicine treatment of shoots and preliminary characterisation of induced polyploids in two Miscanthus species. Ind Crops Prod 32:88–96. doi:10.1016/j.indcrop.2010.03.009
Ishfaq M, Nasir IA, Mahmood N, Saleem M (2012) In vitro induction of mutation in tomato (Lycopersicon esculentum L.) cv. Roma by using chemical mutagens. Pak J Bot 44:311–314
Ishigaki G, Gondo T, Suenaga K, Akashi R (2009) Induction of tetraploid ruzigrass (Brachiaria ruziziensis) plants by colchicine treatment of in vitro multiple-shoot clumps and seedlings. Grassl Sci 55:164–170. doi:10.1111/j.1744-697X.2009.00153.x
Jin S, Mushke R, Zhu H, Tu L, Lin Z, Zhang Y, Zhang X (2008) Detection of somaclonal variation of cotton (Gossypium hirsutum) using cytogenetics, flow cytometry and molecular markers. Plant Cell Rep 27:1303–1316. doi:10.1007/s00299-008-0557-2
Kikkert JR, Striem MJ, Vidal JR, Wallace PG, Barnard J, Reisch BI (2005) Long-term study of somatic embryogenesis from anthers and ovaries of 12 grapevine (Vitis sp.) genotypes. In Vitro Cell Dev Biol Plant 41:232–239. doi:10.1079/IVP2004609
Leal F, Loureiro J, Rodriguez E, Pais MS, Santos C, Pinto-Carnide O (2006) Nuclear DNA content of Vitis vinifera cultivars and ploidy level analyses of somatic embryo-derived plants obtained from anther culture. Plant Cell Rep 25:978–985. doi:10.1007/s00299-006-0162-1
Li J, Wang X, Wang X, Wang Y (2015) Embryo rescue technique and its applications for seedless breeding in grape. Plant Cell Tissue Org Cult 120:861–880. doi:10.1007/s11240-014-0656-4
Mahadevamma M, Sahijram L, Kumari V, Shankarappa TH (2012) In vitro mutation studies in papaya (Carica papaya L.). CIBTech J Biotechnol 1:49–55
Marum L, Loureiro J, Rodriguez E, Santos C, Oliveira MM, Miguel C (2009) Flow cytometric and morphological analyses of Pinus pinaster somatic embryogenesis. J Biotechnol 143:288–295. doi:10.1016/j.jbiotec.2009.08.001
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
Nilanthi D, Chen X-L, Zhao F-C, Yang Y-S, Wu H (2009) Induction of tetraploids from petiole explants through colchicine treatement in Echinacea purpurea L. J Biomed Biotechnol 2009:343485. doi:10.1155/2009/343485
Nitsch JP, Nitsch C (1969) Haploid plants from pollen grains. Science 163:85–87. doi:10.1126/science.163.3862.85
Notsuka K, Tsuru T, Shiraishi M (2000) Induced polyploid grapes via in vitro chromosome doubling. J Jpn Soc Hortic Sci 69:543–551. doi:10.2503/jjshs.69.543
Omidbaigi R, Mirzaee M, Hassani ME, Moghadam MS (2010) Induction and identification of polyploidy in basil (Ocimum basilicum L.) medicinal plant by colchicine treatment. Int J Plant Prod 4:87–98
Oumar D, Sama AE, Adiobo A, Zok S (2011) Determination of ploidy level by flow cytometry and autopolyploid induction in cocoyam (Xanthosoma sagittifolium). Afr J Biotechnol 10:16491–16494. doi:10.5897/AJB11.2358
Pande S, Khetmalas S (2012) Biological efffect of sodium azide and colchicine on seed germination and callus induction in Stevia rebaudiana. Asian J Exp Biol Sci 3:93–98
Péros J-P, Torregrosa L, Berger G (1998) Variability among Vitis vinifera cultivars in micropropagation, organogenesis and antibiotic sensitivity. J Exp Bot 49:171–179. doi:10.1093/jxb/49.319.171
Prado MJ, Grueiro MP, González MV, Testillano PS, Domínguez C, López M, Rey M (2010a) Efficient plant regeneration through somatic embryogenesis from anthers and ovaries of six autocthonous grapevine cultivars from Galicia (Spain). Sci Hortic 125:342–352. doi:10.1016/j.scienta.2010.04.019
Prado MJ, Rodriguez E, Rey L, González MV, Santos C, Rey M (2010b) Detection of somaclonal variants in somatic embryogenesis-regenerated plants of Vitis vinifera by flow cytometry and microsatellite markers. Plant Cell Tissue Org Cult 103:49–59. doi:10.1007/s11240-010-9753-1
Predieri S (2001) Mutation induction and tissue culture in improving fruits. Plant Cell Tissue Org Cult 64:185–210. doi:10.1023/A:1010623203554
Shiraishi M, Fujishima H, Chijiwa H (2008) Tetraploid sucrose-accumulating grapevines. Vitis 47:191–192
Shiraishi M, Fujishima H, Chijiwa H (2010) Evaluation of table grape genetic resources for sugar, organic acid, and amino acid composition of berries. Euphytica 174:1–13. doi:10.1007/s10681-009-0084-4
Shiraishi M, Fujishima H, Chijiwa H, Muramoto K (2012) Estimates of genotypic and yearly variations on fruit quality and functional traits for tetraploid table grape breeding. Euphytica 185:243–251. doi:10.1007/s10681-011-0562-3
Sinski I, Bosco D, Pierozzi NI, Maia JDG, Ritschel PS, Quecini V (2013) Improving in vitro induction of autopolyploidy in grapevine seedless cultivars. Euphytica 196:299–311. doi:10.1007/s10681-013-1034-8
Sun Q, Sun H, Li L, Bell RL (2009) In vitro colchicine-induced polyploid plantlet production and regeneration from explants of the diploid pear (Pyrus communis L.) cultivar, “Fertility”. J Hortic Sci Biotechnol 84:548–552
Tulay E, Unal M (2010) Production of colchicine induced tetraploids in Vicia villosa Roth. Caryologia 63:292–303. doi:10.1080/00087114.2010.10589739
Weber J, Georgiev V, Pavlov A, Bley T (2008) Flow cytometric investigations of diploid and tetraploid plants and in vitro cultures of Datura stramonium and Hyoscyamus niger. Cytom Part A 73A:931–939. doi:10.1002/cyto.a.20628
Xing S-H, Guo X-B, Wang Q, Pan Q-F, Tian Y-S, Liu P, Zhao J-Y, Wang G-F, Sun X-F, Tang K-X (2011) Induction and flow cytometry identification of tetraploids from seed-derived explants through colchicine treatments in Catharanthus roseus (L.) G. Don. J Biomed Biotechnol 2011:793198. doi:10.1155/2011/793198
Yang XM, Cao ZY, An LZ, Wang YM, Fang XW (2006) In vitro tetraploid induction via colchicine treatment from diploid somatic embryos in grapevine (Vitis vinifera L.). Euphytica 152:217–224. doi:10.1007/s10681-006-9203-7
Zhang Z, Dai H, Xiao M, Liu X (2008) In vitro induction of tetraploids in Phlox subulata L. Euphytica 159:59–65. doi:10.1007/s10681-007-9457-8
Acknowledgments
This research was partially funded by the Spanish Ministry of Economy and Competitivity (Grant AGL2009-07488). This report is a contribution of the Interuniversity Research Group in Biotechnology and Reproductive Biology of Woody Plants (group code 08IDI1705). Yosvanis Acanda thanks the Spanish Ministry of Foreign Affairs and Cooperation for its support through a MAEC-AECID scholarship. The authors thank María José Graña and Julián Benéitez for their invaluable help during plant material collection at the Centro de Formación y Experimentación de Viticultura y Enología de Ribadumia (Pontevedra, Spain), a viticultural facility owned by the regional government of Galicia.
Authors contribution
Y.A. and M.R conceived the study, designed the experiments and drafted the initial manuscript. Y.A. and O.M. performed the experiments and analyzed the data. M.V.G. and M.J.P. revised the manuscript and provided helpful discussions. M.V.G. and M.R. developed the final manuscript and coordinated its revision. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Acanda, Y., Martínez, Ó., González, M.V. et al. Highly efficient in vitro tetraploid plant production via colchicine treatment using embryogenic suspension cultures in grapevine (Vitis vinifera cv. Mencía). Plant Cell Tiss Organ Cult 123, 547–555 (2015). https://doi.org/10.1007/s11240-015-0859-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-015-0859-3