Abstract
Somatic hybridization is a biotechnological tool, which allows the transfer of multiple resistance genes from sexually incompatible Solanum species into cultivated potato. Here we report the effect of the recipient commercial tetraploid potato cultivar on the genetic make-up of the somatic hybrids (SHs) with an accession of the incongruent diploid species Solanum bulbocastanum Dun. The SHs were produced by mesophyll protoplast electrofusion. The analysis of ploidy by flow cytometry was first used to select hexaploid putative SH shoots but SSR and AFLP markers, DAPI staining and later flow cytometry evaluation of ploidy reveals symmetric and asymmetric SH plants regeneration in proportions that depend on the potato cultivar. The growth and fertility of the SHs support the effect of recipient cultivar. Out of five different fusion combinations, the highest number of SHs and tuberosum morphology was recorded for the combinations: blb41 (+) ‘Delikat’ 235 SHs (104 symmetric and 131 asymmetric) and blb41 (+) ‘Rasant’ 33 SH plants (22 symmetric, 11 asymmetric). There were fertile SHs of these combinations and BC1 and BC2 progenies were obtained. Less successful were the combinations: blb41 (+) ‘Quarta’ 64 SHs (57 symmetric and 7 asymmetric), blb41 (+) ‘Baltica’ 25 SHs (16 symmetric and 9 asymmetric), which were infertile and blb41 (+) ‘Agave’ with only one highly asymmetric non-viable SH plant. The production of a large number of SHs with diverse commercial cultivars is a prerequisite for further selection of useful pre-breeding material. The causes of nuclear constitution asymmetry and somatic incompatibility of the two species are also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Potato is one of the world’s most productive and nutritious vegetable currently ranking fourth in terms of worldwide crop production (Vreugdenhil 2007; Haverkort et al. 2009). This crop produces more carbohydrate but also higher quality protein than any other vegetable except soybeans. Worldwide, potatoes are also an important source of animal feed, starch and ethanol (e.g. biofuel) and more recently human health promoting compounds, located in the skin of tubers (Reddivari et al. 2010).
The sexual incompatibility of potato with most of the wild Solanum species, which are highly resistant to major diseases and pests, makes it extremely difficult to improve potato by sexual hybridization (Hawkes 1990). The incompatibility of diploid, 1 endosperm balance number (EBN) species with tetraploid potato cultivars, even if chromosome numbers are doubled is a particular problem for potato breeding (Johnston et al. 1980).
S. bulbocastanum Dun. (blb) a wild diploid Mexican potato species (2n = 2x = 24), is highly resistant to all known races of Phytophthora infestans, even under high pressure of infection. Blb is a typical example of 1 EBN species and hence cannot be crossed directly with cultivated potato. Limited success has been obtained by interspecific bridge crossings. The derived clones ABPT (quadruple hybrids involving S. acaule and S. phureja as bridging species) were used to release the first potato cultivars in Europe incorporating resistance from S. bulbocastanum, i.e. ‘Biogold’, ‘Bionica’ and ‘Toluca’ (Hermsen and Ramanna 1973; Bradshaw et al. 2006; Haverkort et al. 2009). Nevertheless, classical breeding by bridge species is a very time consuming approach taking as long as 50 years of continuous crossings.
Somatic hybridization through protoplast fusion is a faster alternative method to exploit valuable genes of wild species of Solanum. It overcomes both pre- and post-zygotic sexual incompatibility barriers (Orczyk et al. 2003; Thieme et al. 2008, 2010). There are blb accessions that are resistant to late blight, early blight, Verticillium and the nematode Meloidogyne chitwoodi. Somatic hybrids (SHs) resistant to M. chitwoodi (Austin et al. 1993) or late blight (Helgeson et al. 1998; Thieme et al. 1997) were reported and characterized in the past but a limited number of potato cultivars or breeding lines were used.
The goal of our research was to produce and analyze a large number of SHs between a well-characterized, late blight resistant accession of Solanum bulbocastanum (blb41) and five commercial potato cultivars. The effect of potato recipient genotype on the genetic make-up of the SHs is outlined analyzing nuclear genetic constitution with cytogenetic and molecular methods (SSR, AFLP).
Materials and methods
Plant material
S. bulbocastanum GLKS-31741 [blb41; Gross Lüsewitz Potato Collections (GLKS) of the IPK Gene bank, Leibniz—Institute of Plant Genetics and Crop Plant Research, Germany] was germinated in vitro and only one plant was cloned by repeated subculture of apices on MS medium (Murashige and Skoog 1962) enriched with 1.15 g L−1 NH4NO3 (total content 2.80 g L−1). This clone is resistant (almost immune) to foliage late blight and gene specific marker analysis indicated the presence of two genes: Rpi-blb1 and Rpi-blb3 (data under publication). The potato commercial cultivars were cultured and maintained in vitro on MS medium, as previously described (Thieme et al. 2008). The following cultivars were used: ‘Agave’, ‘Delikat’, ‘Rasant’ (Nordring-Kartoffelzucht-und Vermehrungs-GmbH Gross Lüsewitz, Germany), ‘Baltica’ (Solana GmbH & Co K6, Germany) and ‘Quarta’ (Böhm-Nordkartoffel-Agrarproduktion, Germany). Those cultivars were selected because of their tuber production qualities and sensitivity to foliage blight.
Protoplast isolation, electrofusion and culture of fusion products
The isolation and culture of protoplast was done following the protocol described by Thieme et al. (1997), with some modifications of the enzyme concentrations: for potato cultivars 0.8 % Cellulase Onozuka R-10 and 0.2 % Macerozyme R-10 (Duchefa); for blb41 the cellulase concentration was raised to 1.2 %. The ratio and density of mesophyll protoplasts in the mixture transferred to the electrofusion chamber were 1:1 and 1 × 106 protoplasts mL−1, respectively. Electrofusion was carried out using the equipment produced by Krüss Company (Germany). Electrical parameters applied for electrofusion and fusion product culture conditions are as described by Thieme et al. (2008). Only a single shoot from each regenerating callus was selected for further cultivation and flow cytometry analysis. This selection was based on the assumption of hybrid vigorous growth. Only hexaploid putative SH shoots were maintained for further culture, the mixoploid or tetraploid shoots were discarded at this stage of selection.
Flow cytometry, AFLP and SSR analysis
The ploidy level of putative SH shoots was determined by flow cytometry using the Cell Lab Quanta SC, Beckman Coulter GmbH Krefeld, Germany equipped for UV excitation. For measurement of the relative DNA content samples of plants were prepared and histograms analyzed for ploidy determination (Thieme et al. 1997). From each regenerated callus only the hexaploid shoots were retained when analyzed by flow cytometry. The SHs maintained in vitro for years (7–10) as microtubers or plantlets and their derived BC1 clones were again investigated for ploidy by using the protocol described by Doležel et al. (2007), DNA being stained with propidium iodide.
DNA samples were prepared from leaf tissue of in vitro potato plants. About 50 mg of leaf material was vigorously homogenized in a mixer-mill MM300 (Retsch GmbH). A modified small-scale CTAB based DNA extraction was carried out as described by Saghai-Maroof et al. (1984). The PCR reaction was as previously described for the primers P1-P12 (Antonova et al. 2001, 2003) or for the other primers and AFLP markers as described by Thieme et al. (2008, 2010). SSR markers used to determine the hybridity of the plants were: P1, P2, P5 (Kawchuk et al. 1996), P3, P4, P7, P12 (Veilleux et al. 1995), ST13ST, STIIKA, STPRINPSG (as indicated by Provan et al. 1996), STM2022, STM1049 (Milbourne et al. 1998) and Stl057 (Feingold et al. 2005). The motifs of the AFLP markers are given in Table 1. At least two molecular markers were used for each SH clone but only the ones indicating asymmetry (missing bands of blb41) are listed in Table 2. The complete set of markers used for different SHs and their location on chromosomes are given in Table 1, and the combinations used to characterize sets of SHs in Supplementary Table S1.
Somatic metaphase chromosome preparation, DAPI staining and GISH
Roots were harvested from nodal segments cultured in vitro on root inducing medium: MS basal plus 20 g L−1 sucrose and 0.05 mg L−1 NAA. Roots 1–2 cm long were pretreated in 0.001 % α-bromonaphtalene for 4 h at 4 °C and fixed in 3:1 solution of ethanol–acetic acid for 2 h at room temperature. The protocol described by Khrustaleva and Kik (2000) was used to obtain chromosome spreads, which were stained with 0.1 % 4, 6-diamidino-2-phenylindole (DAPI) in 2xSSC for 5 min and mounted in Vectashield antifade solution. GISH was performed in order to visualize simultaneously the chromosomes of S. bulbocastanum and Solanum tuberosum. Total DNA from Solanum tuberosum was labelled with biotin-16-dUTP or digoxigenin-11-dUTP (Roche Diagnostics, Mannheim, Germany) using the random primed labelling protocol. Hybridization was performed at 42 °C overnight. Streptavidin-FITC (Roche) was used in the detection phase. The last step was counterstaining with 2 μg mL−1 DAPI, in antifade solution (Vectashield, Vector Laboratories). The slides were screened using a Zeiss Axioskop-2 fluorescence microscope. Images were captured with a Spot CCD camera (Diagnostic Instruments) and processed with Image Pro Plus software (Media Cybernetics). At least two good spreads for each plant were used for counting the number of chromosomes in selected SHs. The symmetric or asymmetric constitution of SHs was evaluated based on molecular markers, flow cytometry and chromosome counts. The asymmetry of SHs is considered in relation with nucleus genetic constitution because it is common that DNA from mitochondria and chloroplasts is not retained from both species.
Analysis of the morphology and fertility of the SHs and producing offspring by backcrossing
Qualitative analysis of growth, morphology, male and female fertility was carried out in a greenhouse as described previously for other potato SHs (Thieme et al. 2008, 2010). The selected SHs, based on morphology and normal growth in vitro, for each of the four combinations were transferred to a greenhouse. Flowers of greenhouse grown plants were emasculated at the bud stage and pollinated with cultivars ‘Rasant’, ‘Delikat’, ‘Romantze’ and ‘Quarta’, respectively (as detailed in Table 4) and regeneration of BC clones was further investigated. Berries were harvested and seeds cultivated in vitro using immature seeds or embryo rescue technique (Thieme 1991). The SHs were always used as female parents when backcrossing them with cultivars. Different cultivars from the ones originally involved in fusion were used for backcrossing to avoid inbreeding. For successful crossing the same SHs were tested in three different years.
Statistical data analysis
The data were analyzed for significance using R statistical software (R Development Core team 2010), ANOVA and pair wise comparison via Tukey’s test.
Results
Identification and characterization of the somatic hybrids
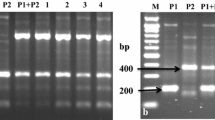
The protocol previously described for somatic hybridization of other fusion combinations proved also very efficient for blb41 (+) potato cultivars (Thieme et al. 1997, 2008, 2010). Out of five different fusion combinations, the highest number of SHs was produced for the combination blb41 (+) ‘Delikat’, with a total of 235 SHs (Table 2). The molecular markers (at least two, up to 13 SSR and 5 AFLP—see Table 1 and Supplementary Tables S1) reveal that the shoots were SHs (Fig. 1; Table 2). Of the SHs, 104 were symmetric, in having additive bands from both parental clones and 131 asymmetric i.e. in which the band for the wild parent was missing (Table 2; Fig. 1). Additionally, the plants presenting only typical bands of the cultivar were considered as not being SHs (Supplementary Table S1). For the combination blb41 (+) ‘Quarta’, 64 SHs were regenerated, 57 symmetric and 7 asymmetric. The majority of the 40 clones that were transferred to a greenhouse developed into weak morphologically abnormal plants. Moreover, those SHs were not fertile and could not be further used to produce BC progenies. Some of the asymmetric hybrids missed the band of P4 marker known to be located on chromosome 8 where the Rpi-blb1 (RB) gene is also mapped, but in an unlinked position (Tables 1, 2). In total, 33 SHs of the fusion combination blb41 (+) ‘Rasant’ were produced, 22 symmetric and 11 asymmetric, in some of which one specific marker for chromosome 8, namely P4 is also missing (Tables 1, 2 and Supplementary Table S1). After transfer to a greenhouse most of them developed into weak plants that did not flower. Nevertheless, ten hybrids growing well and flowering were selected for further investigation. For blb41 (+) ‘Baltica’ from 25 SHs, 16 symmetric and nine asymmetric were identified. The asymmetric hybrids also lacked the two SSR markers located on chromosome 8 (P4 and P7, Tables 1, 2 and Supplementary Table S1). Because, the SHs blb41 (+) ‘Baltica’ and ‘Quarta’ were growing slowly, showed a morphology less similar to potato tetraploid cultivar, developed infertile flowers or even missed flower development they could not be used for breeding purposes.
Evidence of the hybrid nature of plants 82/4 and 95/1, using different SSR markers: a–c StI057, STM1049 and STM2022 indicate symmetric somatic hybrids (SH) by additive DNA fragments from the parents, S. bulbocastanum and ‘Delikat’. c Missing peak from blb41 parent (arrow) indicates asymmetry of the somatic hybrid 82/4
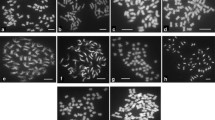
Only one asymmetric SH was obtained using the ‘Agave’, which proved non-viable in vitro (Table 2). The symmetric or asymmetric genetic constitution was proven using SSR and AFLP molecular markers (Tables 1, 2 and Supplementary Table S1), which revealed that bands of the wild parent are missing and consequently some chromosomes or chromosomal fragments are most probably missing, like for instance fragments of chromosome 8 (see Tables 1, 2 and Supplementary Table S1). Flow cytometry screening when the first shoots developed on each hybrid callus identified hexaploids or near-hexaploids and also mixoploid regenerants. Only hexaploid clones were maintained in in vitro culture as shoots. Moreover the SHs were induced to form microtubers for long-term storage at 4 °C. The evaluation of ploidy at a later stage revealed that the majority of the SHs were pentaploid or near hexaploid (Table 3; Fig. 2). The assessment of chromosome number using DAPI staining in selected SHs (the most interesting for producing pre-breeding material, but also some with interesting features) showed both symmetric (2n = 6x = 72) but mainly asymmetric hybrids in all fusion combinations (Table 3). The chromosome numbers of different root meristematic cells of the highly asymmetric and non-viable hybrid clone with ‘Agave’ also varied, proving its chimeric genetic structure. For the majority of analyzed SHs there were variations in chromosome numbers from 48 up to 72 (Table 3; Fig. 3). For the combination potato ‘Delikat’ (+) blb the ploidy was also assessed in some BC progenies, proving that most of BC1 and BC2 plants are losing chromosomes, mainly from blb species (Table 3). As illustrated by the first GISH results some of BC1 clones retain a reduced number of both parental chromosomes (ten out of twelve blb and 44 out of 48 potato chromosomes (Supplementary Fig. S1).
Assessment of somatic hybrids (SH) between Solanum bulbocastanum (GLKS 33741, blb41) and different potato commercial cultivars in terms of chromosome number revealed by DAPI staining: a Blb41-24 chromosomes; b the asymmetric SH blb41 (+) ‘Rasant’ 33/5, 66 chromosomes; c the asymmetric SH blb41 (+) ‘Quarta’ 40/1, 62 chromosomes; d the highly asymmetric SH with ‘Agave’ 93/1, 44–50 chromosomes, the insert shows the chromosomes in late prophase; e the symmetric SH 38/7 blb41 (+) ‘Baltica’, 72 chromosomes; f chromosomes of ‘Delikat’ 4x = 48; bar 2 µm
Fertility of somatic hybrids and production of BC progenies
The fertility of the SHs and regeneration of BC1 and BC2 plants are presented in Table 4 and morphological traits in Fig. 4a–c. The morphology of the SHs was always intermediate between the two parents, but only those clones which had a morphology very similar to potato parent were also setting flowers and part of them were fertile and could be used to produce BC1 and BC2 progenies. The greatest number of BC1 clones was obtained from the fusion combination of blb41 with ‘Delikat’ and blb41 with ‘Rasant’. In 3 years seven BC1 clones were produced from symmetric hybrids and 187 from asymmetric hybrids of blb41 (+) ‘Delikat’, respectively. Subsequently, another 86 BC2 clones were obtained from the symmetric and 118 from the asymmetric hybrids (Table 4). In 2012 from thirteen SHs of this combination 114 berries were harvested for a new BC1 generation. For the SH blb41 with ‘Rasant’ only one BC1 clone was obtained but this gave rise to 70 BC2 clones. No berries were harvested from the symmetric hybrids of this combination (Table 4).
Morphology of Solanum bulbocastanum (GLKS 33741, blb41) (+) ‘Delikat’ somatic hybrids, BC1 clones and crossing success. a Greenhouse-grown plants of blb41, the somatic hybrids (83/5, 85/1, 83/9), and ‘Delikat’ (from left to right). b Leaves and flowers of blb41, the hybrid 83/9 and ‘Delikat’. c Development of berries after crossing the somatic hybrids with cultivars
Discussion
Using mesophyll protoplast electrofusion it was possible to produce a large number of SHs between four out of five different potato commercial cultivars and cloned S. bulbocastanum accession blb41. This enabled the selection of SHs that are morphologically more similar to cultivars and fertile. Since the final goal of our research is to transfer late blight resistance genes, identified in blb41, into potato commercial cultivars, the selection of studied hybrids was also based on their capacity to set flowers and generate back-cross progenies. Shortening the way to breeding is also very important and this is why tetraploid cultivars instead of diploid lines were used. Both symmetric and asymmetric hybrids were identified in different ratios depending on the used cultivar. This is an important factor to consider in pre-breeding programs. Of the five fusion combinations, the most efficient for hybrid production was blb41 (+) ‘Delikat’ (235 SHs). This cultivar was also giving the highest number of SHs when combined with other wild species as S. tarnii or S. cardiophyllum (Thieme et al. 2008, 2010) and S. chacoense (data not published). For the combinations: blb41 (+) ‘Quarta’ 64 SHs, blb41 (+) ‘Rasant’ 33 SHs and blb41 (+) ‘Baltica’ 25 SHs were successfully produced but the morphology, the growth of the plants in in vitro or in a greenhouse and lack of flower fertility made them unsuitable for further use in pre-breeding, excepting few SHs with ‘Rasant’. For the combination blb41 (+) ‘Agave’ only one asymmetric, unviable, hybrid plant was regenerated. These results sustain the genotype effect not only for organ, tissues or cell culture (Henry et al. 1994) but also for genetic modification through protoplast fusion, as it was also demonstrated for asymmetric somatic hybridization in potato (Oberwalder et al. 1998). Nevertheless, it was also reported that potato genotype plays an important role in efficient gene transfer (Rakosy-Tican et al. 2007). Oberwalder et al. (1998) have discussed in detail the complex interaction of parental genotypes i.e. donor and recipient in two asymmetric SHs combinations of potato with the wild species blb and S. circaeifolium. These authors demonstrated after wild species X-ray irradiation a great genetic instability of the regenerated shoots. Although molecular data (AFLP) indicate the single-cell origin of each callus, the ploidy of the shoots regenerated from one callus varied. This was one reason for selecting only first hexaploid shoot from each callus in our fusion hybrids. Moreover, in the above mentioned report the variation in DNA content was higher when the recipient potato genotype was tetraploid in comparison with the diploid ones. This study of Oberwalder et al. (1998) has raised many questions on the interrelationship between donor and recipient genome, questions still unsolved even for the symmetric wild-cultivated potato fusion hybrids, where asymmetrization at nuclear genetic constitution level is probably caused by somatic incompatibility (Harms 1983). This author reviewed all possible nuclear and cytoplasmic interactions when two species are artificially force together by protoplast fusion. Although, in our experiments the protoplasts of the two partners were isolated from the same tissue, the mesophyll, differences between cell cycle are to be expected. Such differences might cause chromosomes of blb to be sorted out during SHs regeneration. More detailed analysis of genome composition of our SHs using GISH is under way. As for cytoplasmic composition, the first results on 22 SHs between S. tuberosum (tbr) ‘Rasant’ and blb41 by PCR amplification of specific regions of organelle DNA (cpDNA markers of the genes: trnD/trnT and atpE and mtADN markers of the genes: rpS14/cob and cob/rpS10), revealed a random distribution of cpDNA whereas mtDNA was of tbr type in 21 out of the 22 SHs analysed (Antonova—personal communication). In their experiments Iovene et al. (2007), using the cpDNA markers pucJ and Alc1/3 and mtDNA markers Alm4/5 and PumD, reported a nonrandom transmission of cpDNA and only tbr type mtDNA in a small number of SHs between dihaplod lines of potato and blb1C and blb2C. Moreover, nuclear and cytoplasmic genetic constitution play an important role in somatic incompatibility of different species and chromosome elimination, interrelation that may explain why potato ‘Delikat’ is suitable for somatic hybridization with other 1EBN wild species (Thieme et al. 2005). ‘Delikat’ and ‘Rasant’ both belong to a group of cultivars characterized as having W chloroplast DNA and α mitochondrial DNA haplotypes (Gavrilenko et al. 2007), while blb species has W chloroplasts but δ mt DNA (Lössl et al. 1999). Partial cytoplasm genetic compatibility might explain the fertility of our SHs with these two cultivars.The other varieties used in our experiments: ‘Agave’, ‘Baltica’ and ‘Quarta’ all belong to T cpDNA and β mtDNA haplotypes (Gavrilenko et al. 2007).
The regeneration of a large number of SHs for most combinations used in our experiments allows the selection of the best plants in terms of morphology, growth, fertility and resistance (data not shown). Moreover, fusing diploid wild species with tetraploid cultivars gives better results in producing a large number of hybrid plants with breeding value than blb (+) dihaploid potato breeding clones, as reported by Szczerbakowa et al. (2003), Greplová et al. (2008), Iovene et al. (2012) and Oberwalder et al. (1998). Szczerbakowa et al. (2003) reported only eight aneuploid and poorly growing SHs, which were not resistant to late blight. On the contrary, when tetraploid potato was fused to diploid blb more fertile SHs were recovered (Helgeson et al. 1998; Thieme et al. 2008, 2010). But, in this last reports only a limited number of potato cultivars or breeding lines were used. The genome composition AAAABB should induce a faster sorting out of wild genome and integration of resistance genes, particularly when the genes can be tackled by gene specific markers (data not shown).
In our experiments, the first shoots arising on each callus were screened for ploidy and only hexaploids retained. Nevertheless, further analyses by flow cytometry, molecular markers (SSR, AFLP) or DAPI staining reveal that, a high number of the SHs are asymmetric. The ratio between symmetric and asymmetric SHs depended also on potato cultivar. The regeneration of asymmetric hybrids after electrofusion of mesophyll protoplasts indicates that elimination of chromosomes or genes occurs at different stages in the regeneration of shoots and might be caused by: (1) Genome ratio (4x : 2x); (2) Protoclonal variation; (3) Somatic incompatibility or (4) epigenetic effects. Changes in DNA content after one year in culture was also demonstrated by flow cytometry in the asymmetric hybrids (Oberwalder et al. 1998). The asymmetry of the four hybrids regenerated after fusion of blb with a dihaploid clone of potato (H-8105), reported by Bałtowicz et al. (2005) suggest more than the effect of genome dosage. Chromosome-specific RAPD markers for blb were used, by above mentioned authors, to determine the fate of the wild species chromosomes in SHs. All four clones lacked linkage group II, which indicated they lost this chromosome. Such a variation at the cytogenetic level might be induced either by protoclonal variation or somatic incompatibility (Harms 1983). Our first results on GISH of a selected BC1 clone shows that one blb and two potato chromosome pairs are eliminated. There are other reports of SH clones with blb lacking one or more wild chromosomes or molecular markers (Bałtowicz et al. 2005; Masuelli et al. 1995; Iovene et al. 2012), which may be a result of protoclonal variation occurring during in vitro regeneration, similar to reports on cytoplasm DNA variation in potato protoclones (Kemble and Shepard 1984). It is likely however that somatic incompatibility, with its very complex cellular and genomic interactions occurring in each individual fusion product plays an important role (Harms 1983). S. bulbocastanum is sexually incompatible with potato that may explain somatic incompatibility reactions. Recent data also indicate chromosome rearrangements or deletions after fusion in the SHs between blb and a dihaploid potato that lack specific ISSR bands of one parent (Iovene et al. 2012). Masuelli et al. (1995) demonstrated clamping, lagging, dispersal and irregular pairing of chromosomes, accounting for the genetic instability of the BC progenies. A similar nuclear asymmetry also occurs during protoplast regeneration of the sexually incompatible Nicotiana × sanderae and N. debneyi along with efficient transfer of resistance to fungal disease caused by Peronospora tabacina (Patel et al. 2011).
Our results suggest that blb and cultivated potato are somatically incompatible although they can generate fertile hybrids with some chloroplast compatible constitutions. Moreover, the recipient potato cultivar influences the success of plant regeneration and their fertility as an expression of genetic, cytoplasmic and probably epigenetic compatibility which have to be further investigated. A better understanding of the mechanisms responsible for SHs asymmetry after mesophyll protoplast electrofusion will allow us to design schemes for the selection of those SHs that are genetically stable and show introgression of the desired multiple durable resistance genes.
The fertility of our SHs wasn’t only related to symmetric genetic makeup of the hybrids and only few clones resulting from the ‘Delikat’ and ‘Rasant’ produced BC1 and BC2 progeny. These clones are a useful pre-breeding material for further introgression of foliage late blight resistance into commercial potato cultivars (to be published).
References
Antonova OY, Kostina LI, Gavrilenko T, Schüler K, Thieme R (2001) Proof of long-term stored potato germplasm by use of molecular markers http://www.genres.de/infos/igrreihe.htm. Accessed 2 Dec 2014
Antonova OY, Kostina LI, Gavrilenko T, Schüler K, Thieme R (2003) Proof of long-term stored potato germplasm by use of molecular markers. In: Knüpffer H, Ochsmann J (eds.) Rudolf Mansfeld and plant genetic resources. Schriften zu Genetischen Ressourcen IGR-ZADI, Bonn 22:192–197
Austin S, Pohlman JD, Brown CR, Mojtahed H, Santo GS, Douches DS, Helgeson JP (1993) Interspecific somatic hybridization between Solanum tuberosum L. and S. bulbocastanum Dun. as a means of transferring nematode resistance. Am Potato J 70:485–495
Bałtowicz D, Szczerbakowa A, Wielgat B (2005) RAPD analysis of the interspecific somatic hybrids Solanum bulbocastanum (+) S. tuberosum. Cell Mol Biol Lett 10:151–162
Bradshaw JE, Bryan GJ, Ramsay G (2006) Genetic resources (including wild and cultivated Solanum species) and progress in their utilisation in potato breeding. Potato Res 49:49–65
Doležel J, Greilhuber J, Suda J (2007) Estimation of nuclear DNA content in plants using flow cytometry. Nat Protoc 2(9):2233–2244
Feingold S, Lloyd J, Norero J, Bonierbale N, Lorenzen M (2005) Mapping and characterization of new EST-derived microsatellites for potato (Solanum tuberosum L.). Theor Appl Genet 111:456–466
Gavrilenko TA, Antonova OY, Kostina LI (2007) Study of genetic diversity in potato cultivars using PCR analysis of organelle DNA. Russ J Genet 43(11):1301–1305 © Pleiades Publishing Inc
Greplová M, Polzerová H, Vlastníková H (2008) Electrofusion of protoplasts from Solanum tuberosum, S. bulbocastanum and S. pinnatisectum. Acta Physiol Plant 30:787–796
Harms CT (1983) Somatic incompatibility in the development of higher plant somatic hybrids. Quart Rev Biol 58:325–353
Haverkort AJ, Struik PC, Visser RGF, Jacobsen E (2009) Applied biotechnology to combat late blight in potato caused by Phytophthora infestans. Potato Res 52:249–264
Hawkes JG (1990) The potato: evolution, biodiversity and genetic resources. Smithsonian Institution Press, Washington, DC, pp 1–259
Helgeson JP, Pohlman JD, Austin S, Haberlach GT, Wielgus SM, Ronis D, Zambolim L, Tooley P, McGrath JM, James RV, Stevenson WR (1998) Somatic hybrids between Solanum bulbocastanum and potato: a new source of resistance to late blight. Theor Appl Genet 96:738–742
Henry Y, Vain IP, De Buyser J (1994) Genetic analysis of in vitro plant tissue culture responses and regeneration capacities. Euphytica 79:45–58
Hermsen JGT, Ramanna MS (1973) Double-bridge hybrids of Solanum bulbocastanum and cultivars of Solanum tuberosum. Euphytica 22:457–466
http://wheat.pw.usda.gov/ggpages/keygeneAFLPs.html Accessed Mar 2015
Iovene M, Savarese S, Cardi T, Frusciante L, Scotti N, Simon PW, Carputo D (2007) Nuclear and cytoplasmatic 425 genome composition of Solanum bulbocastanum (+) S. tuberosum somatic hybrids. Genome 50:443–450
Iovene M, Aversano R, Savarese S, Caruso I, DiMatteo A, Cardi T, Frusciante L, Carputo D (2012) Interspecific somatic hybrids between Solanum bulbocastanum and S. tuberosum and their haploidization for potato breeding. Biol Plant 56(1):1–8
Johnston SA, den Nijs TPM, Peloquin SJ, Hanneman RE Jr (1980) The significance of genic balance to endosperm development in interspecific crosses. Theor Appl Genet 57:5–9
Kawchuk LM, Lynch DR, Thomas J, Penner B, Sillito D, Kulcsar F (1996) Characterization of Solanum tuberosum simple sequence repeats and application to potato cultivar identification. Am Potato J 73:325–335
Kemble RJ, Shepard JF (1984) Cytoplasmic DNA variation in a potato protoclonal population. Theor Appl Genet 69:211–216
Khrustaleva LI, Kik C (2000) Introgression of Allium fistulosum into A. cepa mediated by A. roylei. Theor Appl Genet 100:17–26
Lössl A, Adler N, Horn R, Frei U, Wenzel G (1999) Chondriome-type characterization of potato: mt α, β, γ, δ, ε and novel plastid-mitochondrial configurations in somatic hybrids. Theor Appl Genet 98:1–10
Masuelli RW, Tanimoto EY, Brown CR, Comai L (1995) Irregular meiosis in a somatic hybrid between S. bulbocastanum and S. tuberosum detected by species-specific PCR markers and cytological analysis. Theor Appl Genet 91:401–408
Milbourne D, Meyer RC, Collins AJ, Ramsay LD, Gebhardt C, Waugh R (1998) Isolation, characterization and mapping of simple sequence repeat loci in potato. Mol Gen Genet 259:233–245
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol Plant 15:473–497
Oberwalder B, Schilde-Rentschler L, Ruoss B, Wittemann S, Ninnemann H (1998) Asymmetric protoplast fusions between wild species and breeding lines of potato: effect of recipients and genome stability. Theor Appl Genet 97:1347–1354
Orczyk W, Przetakiewicz J, Nadolska-Orczyk A (2003) Somatic hybrids of Solanum tuberosum: application to genetics and breeding. Plant Cell Tiss Organ Cult 74:1–13
Patel D, Power JB, Anthony P, Badakshi F, Heslop-Harrison JSP, Davey MR (2011) Somatic hybrid plants of Nicotiana × sanderae (+) N. debneyi with fungal resistance to Perenospora tabacina. Ann Bot 108(5):809–819
Provan J, Powell W, Waugh R (1996) Microsatellite analysis of relationships within cultivated potato (Solanum tuberosum). Theor Appl Genet 92:1078–1084
R Development Core Team. Version 2.11.1 (2010)
Rakosy-Tican E, Aurori CM, Dijkstra C, Thieme R, Aurori A, Davey MR (2007) The usefulness of the gfp reporter gene for monitoring Agrobacterium-mediated transformation of potato dihaploid and tetraploid genotypes. Plant Cell Rep 26(5):661–671
Reddivari L, Vanamala J, Safe SH, Miller JC Jr (2010) The bioactive compounds α-chaconine and gallic acid in potato extracts decrease survival and induce apoptosis in LNCaP and PC3 prostate cancer cells. Nutr Cancer 62(5):601–610
Saghai-Maroof MA, Soliman KM, Jorgensen RA, Allard RW (1984) Ribosomal DNA spacer-length polymorphisms in barley: mendelian inheritance, chromosomal location and population dynamics. Proc Natl Acad Sci USA 81:8014–8018
Szczerbakowa A, Boltowicz D, Wielgat B (2003) Interspecific somatic hybrids Solanum bulbocastanum (+) S. tuberosum H-8105. Acta Physiol Plant 25:365–373
Thieme R (1991) Embryo- und Samenkultur bei der Kartoffel. Vortr Pflanzenzüchtg 21:125–129
Thieme R, Darsow U, Gavrilenko T, Dorokhov D, Tiemann H (1997) Production of somatic hybrids between S. tuberosum L. and late blight resistant Mexican wild potato species. Euphytica 97:189–200
Thieme R, Rakosy-Tican E, Gavrilenko T, Antonova O, Heimbach U, Schubert J, Nachtigall M, Thieme T (2005) Utilization of the resistance to pathogens and pests in wild species of Solanum for breeding potatoes. 16th triennial confernece of the EAPR, 17–22.7.2005, Bilbao, Spain, pp. 246–250
Thieme R, Rakosy-Tican E, Gavrilenko T, Antonova O, Schubert J, Nachtigall M, Heimbach U, Thieme T (2008) Novel somatic hybrids and their fertile BC1 progenies of potato (Solanum tuberosum L.) (+) S. tarnii, extremely resistant to potato virus Y and resistant to late blight. Theor Appl Genet 116:691–700
Thieme R, Rakosy-Tican R, Nachtigall M, Schubert J, Hammann T, Antonova O, Gavrilenko T, Heimbach U, Thieme T (2010) Characterization of multiple resistance traits of somatic hybrids between Solanum cardiophyllum Lindl. and two commercial potato cultivars. Plant Cell Rep 29:1187–1201
Veilleux RE, Shen LY, Paz MM (1995) Analysis of the genetic composition of anther-derived potato by randomly amplified polymorphic DNA and simple sequence repeats. Genome 38:1153–1162
Vreugdenhil D (ed) (2007) Potato Biology and Biotechnology. Elsevier, Amsterdam
www.lsw.uni-heidelberg.de/users/christlieb/teaching/…./R-refman.pdf. Accessed Sep 2013
Acknowledgments
E R-T acknowledges the financial support of a grant of the Romanian Authority for Scientific Research, CNCS-UEFISCDI project number PNII-ID-PCE-2011-3-0586. Márta Molnár-Láng and Éva Szakács are kindly acknowledged for their help with GISH and late flow cytometry analyses. The authors wish to thank technical staff for excellent assistance. We gratefully thank T. Dixon for his helpful revision of the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
fig. S1
GISH analysis of the BC1 potato ‘Delikat’ (+) S. bulbocastanum (blb41) 94/5/5 after hybridization with potato DNA: in green = FITC stained potato chromosomes (22 pairs), in blue = counterstained DAPI blb41 chromosomes (5 pairs), total chromosome number = 54; bar = 2 µm (PDF 103 kb)
Rights and permissions
About this article
Cite this article
Rakosy-Tican, E., Thieme, R., Nachtigall, M. et al. The recipient potato cultivar influences the genetic makeup of the somatic hybrids between five potato cultivars and one cloned accession of sexually incompatible species Solanum bulbocastanum Dun.. Plant Cell Tiss Organ Cult 122, 395–407 (2015). https://doi.org/10.1007/s11240-015-0777-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-015-0777-4