Abstract
Kohlrabi (Brassica oleracea var. gongylodes) cultivars Vienna Purple (VP) and Vienna White (VW) were tested for their ability of de novo organogenesis in vitro. Root, cotyledon, hypocotyl explants and intact seedlings were cultivated on Murashige and Skoog (MS) media supplemented with different cytokinins: benzyladenine (BA), thidiazuron (TDZ), trans- or cis-zeatin. All tested cytokinins, including cis-zeatin, induced shoot regeneration from hypocotyl explants and intact seedlings, with seedlings being most successful for regeneration efficiency and viability of regenerated shoots in both cultivars. The highest frequency of shoot regeneration was achieved on MS with BA (60 %) or TDZ (50 %) for VP; and with BA (50 %), TDZ (47.5 %) or transZ (37.5 %) for VW. Measurements of the endogenous cytokinin and indole-3-acetic acid (IAA) contents in both hypocotyl explants and seedlings with regenerated shoots (HRSs and SRSs) suggested that the observed differences in organogenic response between these two types of explants were related to their cytokinin and IAA contents. HRSs generally exhibited elevated amounts of total cytokinins, while SRSs displayed a higher IAA/bioactive cytokinins ratio. Shoots regenerated from seedlings were further successfully multiplicated on a medium supplemented with BA (0.5 mg L−1). The rooting potential of multiplicated shoots was tested on media supplemented with 2 or 4 mg L−1 indole-3-butyric acid (IBA), with the higher concentration of IBA leading to more efficient rooting. Rooted plantlets were successfully planted into soil and flow cytometric analysis did not reveal ploidy variations, indicating that the described protocol is fast and efficient for kohlrabi regeneration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brassica oleracea is an economically important species in the Brassicaceae family, which comprises a wide range of vegetable crops of different morphologies such as cabbage, cauliflower, kale, broccoli, Brussels sprouts and kohlrabi. Kohlrabi (B. oleracea var. gongylodes) is a biennial plant, grown for the round swollen stem at the base of the plant (Ćosić et al. 2013). It is used for both food and feed as a good source of vitamins, minerals, fiber, with low amounts of fat (National Food Composition Database, Fineli®, Finland 2011). In the past decades, the high demand for kohlrabi stems with leaves increased the production of this vegetable in Europe, especially in Nordic countries (Escalona et al. 2007).
However, together with other varieties of Brassica oleracea, kohlrabi demonstrates high susceptibility to various infestations by different pests and pathogens (Grosch et al. 2004). Also, stems and leaves tend to toughen during cold storage, decreasing the nutritional value of edible parts (Escalona et al. 2007). Apart from the widespread breeding techniques used to solve these issues, biotechnological approaches offer new potential by developing and favoring genotypes with improved agronomical traits. The employment of biotechnological means such as in vitro growing requires development of an efficient and reproducible regeneration system in a controlled environment. Such a system would also offer further prospects for studying mechanisms involved in the process of kohlrabi stem thickening and the hormonal interactions that trigger this process (Selman and Kulasegaram 1966).
Substantial research on in vitro plant regeneration and transformation has been conducted in a number of Brassica species (reviewed in Cardoza and Stewart 2004; Vinterhalter et al. 2007). The most studied species include: a number of B. oleracea varieties (Hansen et al. 1999; Sparrow et al. 2004; Sretenović-Rajičić et al. 2004, 2006; Chikkala et al. 2009; Wang et al. 2011), as well as B. juncea (Zhang et al. 2006), B. napus (Koh and Loh 2000; Moghaieb et al. 2006; Haddadi et al. 2008), and B. campestris (Paul and Sikdar 2005). However, kohlrabi appears to be the least studied of all Brassica vegetables, presumably due to the difficulties related to plant regeneration. There are only few previous reports on kohlrabi regeneration in vitro (Glendening and Sjolund 1988; Klíma et al. 2004), including our earlier published results describing an optimized protocol for somatic embryogenesis from immature zygotic embryos (Ćosić et al. 2013).
The most utilized regeneration pathway for many Brassica vegetables is de novo shoot organogenesis (DNSO), as reviewed by Cardoza and Stewart (2004). Protocols have been developed for regeneration from various explants such as hypocotyls (Khan et al. 2003; Ghnaya et al. 2008; Pavlović et al. 2010), leaves (Glendening and Sjolund 1988; Abbasi et al. 2011), roots (Lillo and Shanin 1986), cotyledons (Ono et al. 1994; Cogbill et al. 2010) and floral segments (Bhalla and de Weerd 1999).
De novo organogenesis, relying on somatic cell totipotency, enables plants to regenerate organs from wounded parts or excised explants in vitro (Motte et al. 2014). This process is commonly divided into several distinct events: callus induction, induced partition of cell identity within the callus, radial patterning within shoot progenitors and shoot apical meristem morphogenesis (Duclercq et al. 2011 and the references therein). During each of these phases, distinct changes occur in the expression of genes involved in the auxin and cytokinin (CK) signal transduction pathways, as well as related transcription factors (Che et al. 2002, 2006).
The effect of exogenous PGRs, especially CKs, on the DNSO can vary due to differences in uptake from the nutrition media as well as in capability of the given genotype or even explant type to metabolize these CKs (Auer et al. 1992; Klemš et al. 2011). Furthermore, exogenously applied PGRs can affect the levels of endogenous plant hormones, by influencing their biosynthesis and distribution (Kamínek et al. 1997), subsequently altering in vitro development (Valdés et al. 2001; Moncaleán et al. 2003; Klemš et al. 2011). Furthermore, it has been shown that endogenous phytohormone levels depend on plant species, genotypes, type of explant and developmental stage (Bouza et al. 1993; Baroja-Fernández et al. 2002; Cuesta et al. 2009, 2012).
The aim of this work was to develop a simple and efficient in vitro DNSO protocol for kohlrabi and to provide a rationale for the role of plant hormones in this process. One of the key questions was whether successful DNSO, occuring from various explant types, was accompanied by distinct alterations in the endogenous levels of cytokinins and IAA, as influenced by the cytokinin composition of nutrition media used for shoot regeneration. The presented regeneration protocol offers an efficient and practicable system for clonal propagation of two cultivars of kohlrabi, Vienna Purple (VP) and Vienna White (VW), without the use of exogenous auxin. Changes in levels of cytokinins and IAA in plant tissues confirmed the role of endogenous hormones in de novo organogenesis. Since kohlrabi represents one of the few varieties of the cabbage group for which Agrobacterium-mediated transformation was not reported, this study could provide a useful background for genetic transformation prospects.
Materials and methods
Plant material and growth conditions
Seeds of two kohlrabi cultivars [Vienna Purple (VP) and Vienna White (VW)] were surface sterilized by a 5 min submersion in ethanol 70 %, followed by 30 min in 30 % commercial bleach (4–6 % NaOCl) with a drop of detergent (Fairy; Procter and Gamble, London, England) and thoroughly rinsed in autoclaved distilled water. Sterilized seeds were aseptically transferred to a hormone-free basal medium (MS) containing Murashige and Skoog (1962) mineral salts, Linsmaier and Skoog (1965) vitamins, 3 % sucrose, 100 mg L−1 inositol and 0.6 % agar, for germination. All media used in the investigation were adjusted to pH 5.8 with 1 N NaOH prior to autoclaving at 115 °C and 0.9 kPa for 25 min. Cultures were maintained in a growth chamber at 25 ± 2 °C under cool white fluorescent light (16 h photoperiod; 47 µmol m−2 s−1 irradiance).
Plant regeneration
Four explant types were used for testing kohlrabi regeneration efficiency: hypocotyls, roots, cotyledons and intact seedlings. Hypocotyl, root and cotyledon fragments, 5–8 mm in length, were excised from 2-week-old in vitro grown seedlings and placed on 90 × 15-mm Petri dishes (40 explants per Petri dish with appropriate regeneration medium). Regeneration media consisted of MS supplemented with different CKs: cis-zeatin (cisZ; 1 mg L−1 or 2 mg L−1), trans-zeatin (transZ; 1 mg L−1 or 2 mg L−1), thidiazuron (TDZ; 2 mg L−1) or N 6-benzyladenine (BA; 5 mg L−1). The respective concentrations of applied growth regulators have been previously established through a pilot experiment using all the four kohlrabi explant types of both cultivars (data not shown). For investigation of the regeneration potential of intact seedlings, surface-sterilized seeds were directly placed in tubes with 20 mL of MS media containing various CKs as listed above (20 seeds per respective CK treatment).
All experiments included control cultures with respective explants grown on MS media free of exogenous PGRs. The experiments were repeated 3 times. Shoot regeneration frequency [(number of explants with shoots/total numbers of explants) × 100 %] as well as the mean number of shoots per explant (number of shoots/number of shoot-forming explants) were calculated for the explants that had been cultured for 6 weeks.
Histological analysis of seedlings with regenerated shoots
Basal stems with surrounding callus of kohlrabi seedlings grown on each CK treatment for four weeks were used for histological analysis. Samples were fixed in FAA (formaldehyde: acetic acid: ethanol = 10:5:85 v/v/v) for 72 h followed by 5 min of vacuum infiltration. Tissue was dehydrated through a graded ethanol series, embedded in Paraplast, and sectioned at 8 µm. Sections were stained with hematoxylin and photographed using a Leitz DMRB photomicroscope (Leica, Wetzlar, Germany).
Phytohormone analysis
The content of endogenous plant hormones (CKs and IAA) was measured in two types of plant tissue. Hypocotyl explants, grown for 6 weeks in vitro on CK-supplemented shoot regeneration media, were sampled together with their respective regenerated shoots. These samples were named “hypocotyls with regenerated shoots” (HRSs). Seedlings grown for 6 weeks in vitro on the same shoot regeneration media with their respective regenerated shoots gave rise to the other set of samples, tagged as “seedlings with regenerated shoots” (SRSs). Hypocotyl explants and seedlings grown for 6 weeks in vitro on PGR-free medium were used as a control.
Endogenous plant hormones were extracted from HRSs or SRSs and respective control (1 g FW) by methanol/formic acid/water (15/1/4; v/v/v), homogenized in liquid nitrogen, and purified using the dual-mode solid phase extraction method (Dobrev and Kamínek 2002).
Detection and quantification of CKs were performed using HPLC/MS with LCQ ion trap mass spectrometer (Finnigan Corp., San José, CA, USA) operated in the positive-MS-MS (MS2) mode as described by Lexa et al. (2003). The internal standards, [2H]labeled CKs, were used for multilevel calibration graphs. Detection limits of different CKs ranged from 0.5 to 1 pmol/sample. CK nucleotides were determined from corresponding ribosides after their dephosphorylation by alkaline phosphatase. Each sample was analyzed by two parallel injections.
Purification and determination of IAA was carried out using solid phase extraction and two-dimensional high performance liquid chromatography (2D-HPLC) as previously described by Dobrev et al. (2005). Fluorescence detector LC 240 (Perkin Elmer, Wellesley, MA, USA) was used for quantification.
For evaluation of endogenous hormones, three biological replicates were used for each of cisZ 2 mg L−1, transZ 2 mg L−1, TDZ 2 mg L−1, or BA 5 mg L−1 treated regenerants. Analyses were repeated twice with comparable results.
Multiplication of regenerated shoots, rooting and acclimation
Both hypocotyl- and seedling-derived shoots were used in experiments with multiplication, while only shoots derived from seedlings were used for rooting and acclimatization experiments. Shoots over 1 cm in size were detached from the primary explants, and at least 25 shoots from each regeneration medium (its CK composition being designated as “pretreatment” in the shoot multiplication experiment) were further placed on subculture media composed of MS with addition of BA (0.5 mg L−1) for shoot multiplication. The average number of newly formed shoots was calculated for both cultivars after 4 weeks of culture. Experiments were repeated three times.
For root initiation, single shoots with 2–3 leaves were transferred from multiplication medium to 250 mL jars containing 50 mL MS media supplemented with IBA (either 2 mg L−1 or 4 mg L−1). For each IBA concentration, 30 elongated shoots per each CK pretreatment were used for both cultivars. The percentage of plantlets forming roots, root number and length, as well as plantlet height, were recorded after 5 weeks. The viable well-rooted plantlets (30 plantlets for each cultivar) were then transplanted into pots with soil mixture (60 % peat, 20 % red worm compost and 20 % sand) and further grown in the greenhouse. Plantlets were gradually acclimatized over a period of 2–4 weeks.
Flow cytometry analysis of ploidy level
Ploidy level of the regenerated plants was determined by flow cytometry analysis using leaf material taken from 30-day-old acclimatized kohlrabi plants; 15 plants for each cultivar were tested. Leaves of non-tissue cultured plants were used as control. Flow cytometric measurement was performed according to Otto (1988). Briefly, nuclei of sample chopped with a razor blade were released in 0.1 M citric acid containing 0.5 % Tween 20. The suspension was filtered through a 30 μm nylon-mesh filter. Staining buffer containing 4 mg L−1 of 4′,6-diamidino-2-phenylindole (DAPI) in 0.4 M disodium hydrogen phosphate was added. Measurements were done on a Partec PAS flow cytometer (Partec, Münster, Germany) using a linear scale. For DAPI staining, the UV spectrum excited with a HBO lamp was used and emissions were measured through a GG 435 long-pass filter. Approximately seven thousand nuclei per sample were measured and at least four repetitions were made. FLOMAX software (Partec, Münster, Germany) was used for calculating the positions of G0/G1 peaks. Ploidy level was determined using a diploid species, Trifolium repens L. cv. Milo, as an external standard.
Statistical analysis
The data were analyzed using SAS software (SAS Institute, 2002. SAS/STAT, ver. 9.00. SAS Institute Inc., Cary, NC, USA). To analyze the results for shoot induction efficiency, mean number of formed shoots and endogenous hormones content, two-way ANOVA (analysis of variance) was used; the type of treatment and explant being the factors analyzed. Statistical correlation was evaluated between endogenous content of certain hormones (CKs, IAA, IAA/bioactive CKs) and regeneration parameters (shoot induction efficiency, mean number of formed shoots) and presented using the Pearson's correlation coefficient (r). Two-way ANOVA was also used for rooting with the type of pretreatment (that is, composition of previously applied shoot regeneration media) and concentration of IBA as factors, whereas for evaluating multiplication the single analyzed factor was the type of pretreatment. The differences found among means were determined using the Fisher’s least significant difference (LSD) test at the 5 % level of probability. All percentage data were subjected to angular transformation before analysis. For presentation, the data were inversely transformed.
Results
Regeneration from root and cotyledon explants
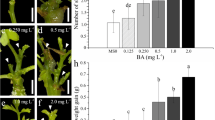
Using root or cotyledon explants as starting material for kohlrabi de novo shoot regeneration on CK-containing media was less successful than the use of hypocotyls and seedling explants, regardless of the genotype (Fig. 1).
The root explants grown in the presence of exogenous CKs were distinguished by elongation, branching and small friable yellowish calli forming at the branching points. Shoot buds appeared only on media with cisZ in VP (Fig. 2a) or transZ in VW (0.56 and 0.28 % respectively). Similarly, cotyledon explants cultivated on the CK-containing media thickened and overgrew and after 6 weeks of culture the only response was observed on MS media supplemented with TDZ with low percentage of shoot regeneration (data not shown). The non-responding explants, including control cultured on PGR-free medium, became necrotic. Because of the overall poor regeneration potential, cotyledon and root explants were excluded from further studies.
Indirect organogenesis from root and hypocotyl segments as well as from intact seedlings of kohlrabi cultivar Vienna Purple (VP) cultured on shoot induction media. a Shoot formation in VP root explant cultured for 6 weeks on regeneration medium supplemented with cis-zeatin (cisZ; 2 mg L−1); b VP hypocotyl segment grown on MS medium containing trans-zeatin (transZ; 2 mg L−1) for 6 weeks, forming a callus with a regenerated shoot; c Callus formation in the base of VP seedling stem after 2 weeks of culture on MS medium with N 6-benzyladenine (BA; 5 mg L−1); d Shoot regeneration in VP seedlings cultured on MS medium with N 6-benzyladenine (BA; 5 mg L−1); e Transection of basal stem of VP seedling grown from seed cultured on medium with BA 5 mg L−1 for 4 weeks: central cylinder (cc) with surrounding callus tissue (c), densely stained meristemoid formation (m) and shoot bud primordia (sb); f Shoot bud primordia were connected with callus tissue by a protodermal layer (pd) and provascular strands (pv); g Later stage of kohlrabi shoot formation showing shoot apical meristem (sam) encompassed with leaf primordia (lp) and young leaves (l); h Control VP plantlet grown on PGR-free medium for 6 weeks; i Effect of indole-3-butyric acid (IBA; 4 mg L−1) on root development in newly formed shoots of VP plantlets previously grown on primary MS medium with N 6-benzyladenine (BA; 5 mg L−1)
Regeneration from hypocotyl explants and seedlings
After 6 weeks of culture on different CKs all hypocotyl explants of both cultivars were characterized by thickening and appearance of friable light green-yellowish calli. A proportionate number of formed calli (up to 4 % for VP and up to 7 % for VW) gave rise to shoots, both at the cutting end and at the middle of ruptured hypocotyl fragments (Fig. 2b; Table 1). Organogenic response in control hypocotyl explants cultivated on PGR-free media was missing even after 6 weeks of culture and subsequently the explants became necrotic.
Seeds cultivated on media supplemented with different CKs germinated with 100 % efficiency and appearing seedlings were further tested as explants for shoot regeneration. Approximately 2 weeks after germination calli were formed at the base of the seedling stem (Fig. 2c) with 100 % efficiency on TDZ and BA treatments, and up to 95 % on cis- and trans-zeatin treatments. Seven days later, shoots started regenerating from these calli, reaching frequency of up to 50 % for VP and up to 60 % for VW after 6 weeks of culture.
Organogenesis did not occur in all seedling explants at the same time. Different stadiums of shoot development were observed among explants, as well as on the same explants (Fig. 2d). Histological analysis of basal stem with surrounding callus of kohlrabi seedlings grown for four weeks on each CK treatment confirmed that the origin of shoots was indirect de novo organogenesis (Fig. 2e–g). Dark colored meristemoid formations with densely packed meristematic cells were easily distinguished in callus tissue, as well as shoot buds in different developing phases (Fig. 2e). Bud primordia were connected with callus tissue by a protodermal layer and provascular tissue (Fig. 2f). Structural differentiation of the apical meristem and the foliar primordia (Fig. 2g) enabled formation of complete adventitious buds. All seeds grown on PGR-free medium germinated and gave rise to normal plantlets without callus formation. They were well rooted (Fig. 2h) in contrast to the treated ones, which lacked root formation.
Overall, shoots regenerated from seedling explants were vigorous and viable compared to those regenerated from hypocotyls, which were slow-growing and fragile.
A strong influence of explant type, regeneration medium, and explant type-regeneration medium interaction on both frequency of shoot organogenesis and mean number of shoots per explant was observed in both cultivars (Table 1). The frequency of regeneration from seedlings was significantly higher compared to hypocotyls on all CK treatments with the exception of cisZ applications in VP. Among all CKs tested the highest value of organogenic frequency in seedlings was obtained on media with BA (60 %) and TDZ (50 %) in VP (Table 1), while TDZ, BA and transZ exhibited the similar effect on DNSO in VW reaching frequency from 37 to 50 % (Table 1). For both VP and VW the percentage of seedlings with regenerated shoots on cisZ media was about 10 times lower than on media with other applied CKs.
Contrary to VP seedlings, cisZ and transZ induced the highest frequency of shoot regeneration in VP hypocotyl explants (4 %), while in VW hypocotyls there was no significant difference between the four tested CKs (4–7 %, Table 1). Concerning the effect of concentration of cisZ and transZ, the 2 mg L−1 treatment resulted in the higher organogenic response in both cultivars.
The mean number of regenerated shoots per explant was lower in seedlings than in hypocotyls and ranged from 2.00 (cisZ 2 mg L−1) to 5.42 (BA 5 mg L−1) in VP, and from 1.00 (cisZ 1 mg L−1) to 4.20 (BA 5 mg L−1) in VW. The highest average number of formed shoots in hypocotyl explants was 24.67 on transZ 2 mg L−1 in VP and 31.12 on TDZ 2 mg L−1 in VW (Table 1).
Effect of regeneration media on subsequent shoot multiplication
Shoots regenerated on different CK-containing media were detached from hypocotyls and seedling explants, and transferred to MS medium supplemented with BA 0.5 mg L−1 for further shoot multiplication. Hypocotyl-derived shoots of both cultivars showed poor viability on this medium and became necrotic within one week.
The shoots detached from seedlings of both cultivars were generally vigorous and viable, except for the shoots regenerated on media with cisZ, that were very small and necrotic so they were eliminated from the multiplication experiments.
After 4 weeks, a significant difference in mean numbers of newly formed shoots was recorded among different CK pretreatments in VP (Fig. 3a) but not in VW (Fig. 3b). This might indicate a shorter duration of the effects of applied CK pretreatments on the subseqeuent shoot multiplication in the latter cultivar. In VP, the shoots regenerated on BA pretreatment medium showed a significantly higher shoot multiplication rate than those regenerated on transZ (Fig. 3a).
Average number of newly formed kohlrabi shoots on shoot multiplication medium containing N 6-benzyladenine (BA; 0.5 mg L−1) in relation to the different cytokinin pretreatments in Vienna Purple (a) and Vienna White (b) cultivars. Data represent mean ± SE of three independent experiments with at least 25 replicates each. Means marked with the same letter were not significantly different according to Fisher’s least significant difference (LSD) test, P ≤ 0.05. transZ 1 = trans-zeatin 1 mg L−1, transZ 2 = trans-zeatin 2 mg L−1, TDZ 2 = thidiazuron 2 mg L−1, BA 5 = N 6-benzyladenine 5 mg L−1
Rooting
Due to unsuccessful multiplication of hypocotyl-derived shoots, the rooting ability was tested only in shoots regenerated from seedlings. After 5 weeks of cultivation, overall root formation efficiency in both cultivars was higher when IBA 4 mg L−1 was applied in the medium (Table 2; Fig. 2i). The rooting ability was significantly affected by pretreatment, genotype, as well as their interaction. The maximum proportion of rooted shoots in both VP (94.57 %) and VW (90.19 %) was found in shoots regenerated on transZ 2 mg L−1. Additionally, phenotypic characteristics of plantlets rooted on both IBA concentrations were determined. The mean number of roots differed significantly between the two IBA treatments only in VP shoots regenerated on transZ, whereas the mean length of the longest root did not depend on the applied concentration of IBA (Table 2). However, pretreatment alone significantly influenced the mean length of the plantlet’s longest root in VP, with the strongest effect achieved on BA (9.30 cm). Means for VW appeared in a similar range of values. Correspondingly, different hormonal pretreatments led to moderate variations in plant height only in VP cultivar.
Flow cytometric analysis of acclimatized plants
Well-rooted plantlets were further transferred to soil for one month of acclimatization under green-house conditions with a survival rate of 76.7 % for VP and 63.3 % for VW. All plants surviving the acclimatization exhibited regular morphology and developed a bulbous stem within 4 months (Fig. 4a). Plants flowered (Fig. 4b) 10 months after acclimatization, forming pods (Fig. 4c). 20–27 % of acclimatized plants, besides fertile pods, developed certain number of empty ones. Flow cytometry analysis conducted in acclimatized plants showed that 100 % of regenerated plants were diploid, indicating no ploidy variation.
Phytohormone analyses
Endogenous CKs and IAA contents were analyzed in hypocotyls with regenerated shoots (HRSs) as well as in seedlings with regenerated shoots (SRSs) cultivated on different CK-containing media and compared to control. Cotyledon and root explants were not taken into consideration due to their poor regeneration efficiency. Summary results for CKs are arranged and demonstrated based on the CK conjugation status as well as their physiological function (Figs. 5, 6) according to Dwivedi et al. (2010).
Cytokinin (CK) content (in pmol g−1 FW) in Vienna Purple hypocotyls with regenerated shoots (HRSs) and seedlings with regenerated shoots (SRSs) after 6 weeks of culture. Distribution of CKs is based on their conjugation status and biological activity: a total CK content, b bioactive forms, c storage forms, d irreversibly inactive forms and e CK phosphates. Different letters indicate significant differences between types of treatment and explant according to Fisher’s least significant difference (LSD) test, P ≤ 0.05 (n = 3). cisZ 2 = cis-zeatin 2 mg L−1, transZ = 2 trans-zeatin 2 mg L−1, TDZ 2 = thidiazuron 2 mg L−1, BA 5 = N 6-benzyladenine 5 mg L−1
Cytokinin (CK) content (in pmol g−1 FW) in Vienna White hypocotyls with regenerated shoots (HRSs) and seedlings with regenerated shoots (SRSs) after 6 weeks of culture. Distribution of CKs is based on their conjugation status and biological activity: a total CK content, b bioactive forms, c storage forms, d irreversibly inactive forms and e CK phosphates. Different letters indicate significant differences between types of treatment and explant according to Fisher’s least significant difference (LSD) test, P ≤ 0.05 (n = 3). cisZ 2 = cis-zeatin 2 mg L−1, transZ = 2 trans-zeatin 2 mg L−1, TDZ 2 = thidiazuron 2 mg L−1, BA 5 = N 6-benzyladenine 5 mg L−1)
The CK profile was similar in both cultivars, with CKs generally presenting higher levels in HRSs compared to SRSs. Levels of total CKs in both VP (Fig. 5a) and VW (Fig. 6a) depended on explant, regeneration medium, and explant-regeneration medium interaction according to two-way ANOVA. In VP, the amount of total CKs was significantly higher than control for cisZ and transZ treatments, as well as for HRSs on BA, while TDZ had no effect on total CK content in any type of regenerants (Fig. 5a). For VP HRSs, the highest levels of all classes of CKs were recorded on medium with transZ (Fig. 5a–d), the only exception being CK phosphates on cisZ-containing medium (Fig. 5e). For VP SRSs, the highest levels of total CKs were achieved on cisZ (as a result of extremely high levels of inactive CK forms, CK N-glucosides, Fig. 5d). On the other hand, TDZ caused the levels of CKs to be the lowest of all treatments (Fig. 5a–e).
Similarly, in both analyzed types of VW tissue, only cultivation on cis- and transZ led to a significant increase in levels of total CKs compared to control (Fig. 6a). Again, the transZ treatment led to the highest CK levels in HRSs, except for bioactive forms on cisZ treatment (Fig. 6b). In SRSs, the maximum levels were recorded either for cisZ or transZ treatments. Similarly to VP, the lowest levels of all classes of CKs appeared in both HRSs and SRSs cultivated on media with TDZ—except for CK storage forms in SRSs (Fig. 6a–e).
Bioactive CKs (transZ, cisZ, iP, DHZ and their corresponding ribosides) comprised only a small portion of total CKs in both HRSs and SRSs in VP as well as in VW. Statistical analysis showed that values recorded for VP HRSs cultured on all four CK-containing media significantly differed from control (higher on cisZ, transZ and BA; lower on TDZ), while for SRSs only the treatment on cisZ was shown to considerably increase the bioactive CK levels compared to control (Fig. 5b). In VW (Fig. 6b), HRSs displayed a similar distribution of bioactive CKs as in VP, whereas the cisZ, TDZ and BA treatments significantly decreased the level of bioactive CKs for SRSs.
Irreversibly inactive forms (CK N 7- and N 9-glucosides) represent the predominant CK forms in all tissues investigated (Figs. 5d, 6d) and are reflective of the distribution of total CKs (Figs. 5a, 6a). Furthermore, evident variations were found for CK storage forms (CK O-glucosides), depending on treatment, explant tissue as well as their interaction in both VP (Fig. 5c) and VW (Fig. 6c).
CK phosphates are a small fraction of total CK content, and a difference in CK profiles between the two types of explants was also observed for both genotypes. Figures 5e and 6e show significantly lower amounts of these CKs in SRSs compared to HRSs for all different treatments.
Additionally, the quantified endogenous CKs were also categorized based on their side chain structure into four types (Aremu et al. 2014a): cisZ type (cisZ, cisZR, cisZ7G, cisZ9G, cisZOG, cisZROG, cisZRMP); transZ type (transZ, transZR, transZ7G, transZ9G, transZOG, transZROG, transZRMP); DHZ type (DHZ, DHZR, DHZ7G, DHZ9G, DHZOG, DHZROG, DHZMP) and iP type (iP, iPR, iP7G, iP9G, iPRMP), the system of CK abbreviations being adopted and modified according to Kamínek et al. (2003). Statistical analysis showed that levels of all four types of CKs depended on tissue, treatment as well as on their interaction in both VP and VW (Table 3). For the most part values were higher for HRSs, as already shown for the CK distribution based on the cytokinin conjugation status and physiological function. As expected, cisZ type CKs were the most abundant on treatment with cisZ, while transZ and DHZ types showed their highest values on medium supplemented with transZ. This regarded HRSs as well as SRSs in both genotypes, although the effect of exogenously added CKs on the endogenous CK content was generally more pronounced in HRSs. Besides, in HRSs there was also a pronounced effect of exogenously added transZ on endogenous levels of cisZ-type CKs, as well as the effect of exogenously added cisZ on endogenous levels of transZ- and DHZ-type CKs. Endogenous levels of iP-type CKs were either decreased or increased by exogenous CK treatment in VP, while in VW they were consistently decreased (Table 3). The TDZ treatment mostly contributed to the decrease of all four CK types in VP and VW, while the effect of BA on different types of CKs was highly variable.
Endogenous levels of indole-3-acetic acid (IAA) were determined in both cultivars with comparable results (Table 4). In both VP and VW HRSs and SRSs, endogenous levels of IAA were significantly increased when grown on media supplemented with CKs in comparison to control media, except for VP HRSs grown on TDZ and VP SRSs grown on cisZ or BAP, where no significant difference from control was recorded. Generally, the highest level of IAA was recorded on media with transZ 2 mg L−1. The endogenous levels of IAA strongly positively correlated with the mean number of regenerated shoots in both HRSs (r = 0.92) and SRSs (r = 0.80) of VW, but for VP this correlation was much weaker (r = 0.37 for HRSs, 0.24 for SRSs).
The IAA/bioactive CKs ratios were analyzed and similar patterns were detected for both cultivars (Fig. 7). In SRSs, the IAA/bioactive CK ratio was mostly increased on regeneration media compared to control, while in HRSs there was no significant change in the IAA/bioactive CK ratio, except in VW explants with shoots grown on TDZ. On the other hand, values in VP (Fig. 7a) were generally lower than in VW (Fig. 7b). Ratios were mostly significantly lower for HRSs compared to SRSs. The highest value for SRSs was observed on treatment with TDZ both in VP and VW, in accordance with relatively low levels of CKs in explants cultivated on media with TDZ. The lowest IAA/bioactive CK ratios occurred either in cisZ (VP SRSs, VW HRSs) or control treatments (VP hypocotyls, VW seedlings). The IAA/bioactive CK ratio strongly correlated to the mean number of regenerated shoots in VW HRSs (r = 0.92) but this correlation was much lower in SRSs or in VP explants.
IAA/bioactive cytokinins ratios in Vienna Purple (a) and Vienna White (b) hypocotyls with regenerated shoots (HRSs) and seedlings with regenerated shoots (SRSs) after 6 weeks of culture. Mean ± SE with the same letter for each cultivar are not significantly different according to Fisher’s least significant difference (LSD) test, P ≤ 0.05 (n = 3). cisZ 2 = cis-zeatin 2 mg L−1, transZ = 2 trans-zeatin 2 mg L−1, TDZ 2 = thidiazuron 2 mg L−1, BA 5 = N 6-benzyladenine 5 mg L−1)
Discussion
Development of efficient protocols for in vitro regeneration of a plant species represents a prerequisite for a number of experimental approaches in modern plant physiology, biotechnology and genetical engineering. Here we present an efficient protocol for in vitro shoot organogenesis of two cultivars of kohlrabi—Vienna Purple (VP) and Vienna White (VW). Our study showed that the morphogenetic potential of kohlrabi is highly variable and dependent on explant tissue and applied growth regulators in both cultivars, as has been reported previously for other Brassicas (Ovesná et al. 1993; Sparrow et al. 2004; Akasaka-Kennedy et al. 2005; Ghnaya et al. 2008; Pavlović et al. 2010; Ravanfar et al. 2011). Each of the cytokinins tested (transZ, cisZ, TDZ, BA) was able alone to induce shoot regeneration from either hypocotyl explants or seedlings of both cultivars, with no need for addition of exogenous auxin. Regeneration protocols that do not include auxin are considered advantageous, as exogenous application of auxin in plant tissue culture may induce somaclonal variation (LoSchiavo et al. 1989; Neelakandan and Wang 2012). Composition of PGRs in the regeneration media affected the endogenous CK and IAA levels in both hypocotyls with regenerated shoots (HRSs) and in seedlings with regenerated shoots (SRSs). These changes in endogenous phytohormone levels have contributed to the shoot regeneration potential of these explants.
Effect of explant type on shoot regeneration efficiency
Although successful regeneration from both cotyledons (Teo et al. 1997; Mollika et al. 2011; Ravanfar et al. 2011, 2014) and roots (Wong and Loh 1988; Sharma and Thorpe 1989) has been reported in other Brassicas, in this study we showed that these explants were not suitable for in vitro regeneration of kohlrabi. As the regeneration media reported for other Brassica species contained auxin, it is possible that exogenous auxin is necessary when cotyledons or roots are used as primary explant. However, even on the media containing auxin, cotyledon explants proved to be less productive in Brassica oleracea var. tronchuda (Msikita and Skirvin 1989) and Brassica oleracea var. acephala (Dai et al. 2009), while root explants also showed poor regeneration potential in Brassica napus (Kamal et al. 2007).
Hypocotyl explants proved to be more suitable than root and cotyledon, but overall less productive than seedlings in kohlrabi shoot organogenesis, which is in contrast to previous observations that hypocotyls were the most suitable explants for both plant regeneration and transformation in most Brassica species (Cardoza and Stewart 2004). On the other hand, using seedlings as explant tissue was highly successful with nearly ten times higher regeneration efficiency. A successful early seedling morphogenesis has been also reported in Lotus corniculatus (Nikolić et al. 2006). Shoots regenerated from seedlings were vigorous and more viable than those regenerated from hypocotyl explants. However, the mean number of regenerated shoots was higher in hypocotyl explants than in seedlings. Shoot regeneration frequency could be affected by tissue age (Cardoza and Stewart 2004), by the levels of endogenous phytohormones and associated metabolites (Aremu et al. 2014b) and by exogenously applied phytohormones (Klemš et al. 2011).
Effect of exogenous CKs on shoot regeneration
The most frequently used CKs for inducing plant regeneration are BA and TDZ due to the highest response in many plant species, including the Brassica genus (Glendening and Sjolund 1988; Khan et al. 2003; Ghnaya et al. 2008; Abassi et al. 2011). A high response to these growth regulators has been obtained here for kohlrabi. However, we found that among the tested growth regulators, transZ was the most effective regarding the number of induced shoots in hypocotyls of the VP cultivar. It also contributed to high shoot regeneration efficiency, comparable to that of TDZ and BA, in hypocotyls of VW, as well as in seedlings of both cultivars. This is in contrast to previous work on different species where transZ was proved to be the least successful in inducing organogenesis (Bretagne et al. 1994; Pellegrineschi 1997; Ahmadabadi and Bock 2010; Verma et al. 2011). However, there are some reports of transZ being more effective in stimulating shoot regeneration than other CKs (Coleman and Ernst 1989; Souza et al. 2003) or having a similar effect as BA (Suri et al. 2005). In inducing the onset of regeneration in individual Lotus corniculatus seedlings, transZ was also the most effective CK, followed by BA and TDZ (Nikolić et al. 2006).
Kohlrabi regeneration also occurred on cisZ containing media, both from hypocotyls and intact seedlings. Interestingly, zeatin physiological activity has been attributed to transZ for years, while cisZ has been believed to be an inactive or weakly active CK form. These conclusions were due to the low biological activity of cisZ earlier reported in some CK bioassays (Schmitz et al. 1972; Kamínek et al. 1979). However, the topic of cis- versus transZ activity has been under discussion over the past years. Recent analyses demonstrated that cis isomers could be predominant CKs at particular stages during plant development (Emery et al. 1998; 2000; Gajdošová et al. 2011; Stirk et al. 2012). Furthermore, a gene encoding an enzyme O-glucosyltransferase with specifity to cisZ was identified in maize (Martin et al. 2001; Veach et al. 2003) and cisZ-type CKs were reported to be recognized by CK receptors (Spíchal et al. 2004; Yonekura-Sakakibara et al. 2004). These findings indicate that zeatin cis-isomers may have unique physiological functions in plant development. Here we demonstrated that cisZ is biologically active in the process of shoot regeneration from hypocotyl explants of kohlrabi, comparably to other tested CKs, but was much less effective than its trans-counterpart in shoot regeneration from kohlrabi seedlings.
Endogenous CK and IAA levels in explants with regenerated shoots grown on different regeneration media
In order to investigate the role of CKs in shoot regeneration, we measured the endogenous CK content of both hypocotyls with regenerated shoots (HRSs) and seedlings with regenerated shoots (SRSs). For the sake of interpretation of results, isoprenoid CKs can be divided based on their side chain structure into transZ-, cisZ-, DHZ- and iP-type cytokinins (Aremu et al. 2014a), or based on their conjugation status and physiological function, into bioactive cytokinins (free bases and ribosides), storage forms (O-glucosides), irreversibly inactive forms (N-glucosides) and CK phosphates/nucleotides (Dwivedi et al. 2010).
Addition of synthetic CKs (BA and TDZ) to the shoot regeneration media contributed to little or no increase in the endogenous CK levels of both HRSs and SRSs, compared to control. On the other hand, when regeneration media were supplied with the naturally occurring CKs trans- or cisZ, these contributed to remarkable increase in CK contents, in particular to the levels of the same type of CKs as that added to the nutrition medium (transZ- or cisZ-type, respectively) suggesting that the rise in CK levels is primarily a consequence of uptake from the media. Similarly, increases in endogenous levels of particular CKs upon their exogenous addition to the cultivation media have been reported earlier (Klemš et al. 2011; Montalbán et al. 2013; Aremu et al. 2014a). The more pronounced increase of endogenous CKs in HRSs cultivated on cisZ or transZ-supplemented media, compared to SRSs grown on the same media, suggests that the uptake of exogenous CKs might be more efficient for hypocotyl segments with regenerated shoots than for seedlings.
Addition of transZ also significantly contributed to the increase of endogenous levels of cisZ-type CKs and likewise exogenously added cisZ contributed to the increase of endogenous levels of transZ-type CKs; in both cases, levels of DHZ-type CKs were also significantly increased. These effects were again particularly pronounced in HRSs of both cultivars. It is not clear whether the increase in endogenous levels of cisZ upon cultivation on media supplemented with transZ and vice versa could be attributed to the uptake of one of the isomers from the media, which could then undergo conversion to the other one, possibly through cis-trans isomerisation. Interconversion between cis- and transZ by means of zeatin-cis-trans isomerase has been demonstrated in Phaseolus vulgaris (Bassil et al. 1993) and suggested also in Arabidopsis thaliana (Kasahara et al. 2004), although more recent research has rendered dubious the importance of this process in plants in vivo (Gajdošová et al. 2011). Increased endogenous levels of cisZ upon cultivation on media supplemented with transZ and vice versa has also been documented during shoot regeneration from vegetative buds of Pinus radiata (Montalbán et al. 2013).
In either HRSs or SRSs of both cultivars, exposure to exogenously added CKs in the nutrition media generally did not contribute to the increase of endogenous levels of iP-type CKs, which are believed to serve primarily as precursors for biosynthesis of zeatin-type CKs (Takei et al. 2004). Similar results have been published by Montalbán et al. (2013). In both HRSs and SRSs of VW exposed to exogenous CKs in the media, endogenous levels of iP-types even dropped in comparison to control, which might be an indicator of negative feedback-type regulation. It has been shown previously that endogenous CK levels in plants may be a subject to such kind of negative feedback control, either by suppression of biosynthesis (Miyawaki et al. 2004) or by enhanced catabolism (Motyka et al. 2003; Brugière et al. 2003).
Upon their apparent uptake from nutrition media, CKs seem to have been either kept as bioactive forms or conjugated in planta to form CK O- and N-glucosides, judging from the increase in levels of these classes of CKs when cultivated on transZ or cisZ in comparison to control, especially in HRSs of both cultivars. Accumulation of CK O- and N-glucosides has been documented in plant tissues exposed to enhanced uptake (Klemš et al. 2011; Montalbán et al. 2013) or altered hormonal homeostasis (Raspor et al. 2012) and is a consequence of metabolic glucosylation. The role of O-glucosylation consists in temporary inactivation, storage and protection from catabolic mechanisms (Veach et al. 2003; Stirk et al. 2012), while the biological role of N-glucosylation is still poorly understood (Bajguz and Piotrowska 2009).
Endogenous levels of CKs and IAA, as well as IAA/bioactive CKs ratio, revealed that there is a clear distinction between HRSs and SRSs with respect to the levels of endogenous CKs and IAA. In our experiments, HRSs contained generally higher amounts of CKs than SRSs, while SRSs included more IAA and even showed a higher IAA/bioactive CKs ratio. Stirk et al. (2008) reported that the developmental stage of the plant affected the levels of endogenous CKs and associated metabolites. In addition, various plant tissues generally differ in their phytohormone contents (Centeno et al. 1996; Valdés et al. 2001; Raspor et al. 2012; Aremu et al. 2014a, b). Exogenous addition of CKs to the regeneration media contributed to an increase in endogenous levels of IAA in both HRSs and SRSs of both cultivars, compared to explants grown on control media. This increase of endogenous IAA was pronounced enough to cause even an enhancement in the IAA/bioactive CK ratio in SRSs of both cultivars, grown on most media supplemented with CKs. CKs have been long known to affect endogenous auxin levels in plants. There are data concerning CKs as either positive (Jones et al. 2010) or negative regulators of auxin biosynthesis (Eklöf et al. 1997; Nordström et al. 2004; Liu et al. 2010). It is possible that the increase of endogenous IAA content in kohlrabi subjected to exogenous application of CKs was a consequence of CK-induced auxin biosynthesis. Increased rate of IAA biosynthesis has been observed in shoot apices, young leaves and roots of 10-day-old Arabidopsis seedlings grown in liquid media supplemented with BA, transZ or cisZ (Jones et al. 2010). Exogenous cytokinins also induced local auxin biosynthesis and polar transport during shoot regeneration of Arabidopsis (Cheng et al. 2013).
Endogenous CK and IAA content as a possible factor affecting regeneration efficiency
Endogenous phytohormone levels or their ratio may affect the ability of in vitro regeneration from different explants (Bouza et al. 1993; Centeno et al. 1996; Valdés et al. 2001; Moncaleán et al. 2003; Klemš et al. 2011; Cuesta et al. 2012; Montalbán et al. 2013; Aremu et al. 2014a, b). Furthermore, exogenous CKs applied to nutrition media can interact with endogenous hormones, altering the development of plants in vitro (Parker et al. 1986; Valdés et al. 2001; Moncaleán et al. 2003; Klemš et al. 2011).
According to our results, kohlrabi explants are able to form calli on media supplemented with only CKs, without any need for exogenous auxin. Contrary to that, standard in vitro regeneration protocols for most plant species include a series of two nutrition media, the first containing auxin for callus induction, whereas the second containing CK and intended for shoot regeneration (reviewed in Duclercq et al. 2011; Motte et al. 2014). Presumably, endogenous auxin levels, which were mostly elevated in kohlrabi hypocotyl and seedling explants grown on media supplemented with CKs, could have been sufficient to induce the first phase of organogenesis without any need for additional auxin in the nutrition media. It is assumed that phytohormone signaling in planta depends entirely on the concentration of hormones endogenuosly present in plant tissues, which does not necessarily reflect the composition of PGRs in the regeneration media (Gordon et al. 2007).
The best results for shoot regeneration frequency of both kohlrabi cultivars in our study were obtained in seedlings grown on media supplemented with BA, TDZ or transZ, where also the ratio of endogenous IAA/bioactive CKs happened to achieve the highest values. The mean number of shoots regenerated from seedlings strongly correlated to the endogenous levels of IAA in the SRSs of VW, although for VP this correlation was much weaker. Furthermore, the mean number of shoots regenerated from hypocotyls showed a strong statistical correlation to both endogenous IAA levels and IAA/bioactive CK ratio in HRSs of VW. The availability of endogenous auxin might thus represent an important factor for the beginning phases of shoot organogenesis in kohlrabi, rather than a high CK content, which is necessary for the subsequent formation of shoot primordia.
Even though with low regeneration frequency, the hypocotyl explants exhibited higher mean number of regenerated shoots compared to seedling explants, as well as a considerably lower IAA/bioactive CK ratio (due to both higher CK and lower IAA contents). Okubo et al. (1991) demonstrated that the lowermost section of the snapdragon hypocotyl was more capable of shoot regeneration than any other portion of the seedling when grown on medium with BA. Chemical analysis showed that this section also contained the highest levels of endogenous cytokinins and lowest amount of IAA.
Local changes in phytohormone concentration, including the establishment of local gradients, trigger the developmental events related to organogenesis (Benková et al. 2003; Pernisová et al. 2009; Cheng et al. 2013). Furthermore, as local variations in auxin content depend on CK and vice versa related to organogenesis (Pernisová et al. 2009; Cheng et al. 2013), they might be dependent on the phytohormonal signaling occurring within the intact plant. This could be the reason why intact kohlrabi seedlings undergo more efficient shoot regeneration than explants provided from isolated tissues, such as hypocotyl, cotyledon or root, where the phytochemical interaction with other plant parts is lacking. Thus, kohlrabi shoots regenerated from hypocotyls did not prove viable during the step of shoot multiplication and they eventually became necrotic. Conversely, shoots regenerated from seedlings successfully multiplicated and gave rise to plantlets which subsequently underwent successful rooting.
Conclusion
The results presented in this report have clearly shown a successful plant regeneration method in two kohlrabi cultivars via de novo shoot organogenesis from seedlings. Shoot regeneration was obtained on nutrition media supplemented with only cytokinins, either cis-zeatin, trans-zeatin, thidiazuron or benzyladenine. Despite being widely believed to be biologically inactive, cisZ was able to induce shoot regeneration from hypocotyl explants of kohlrabi similarly to the other tested CKs, but was much less effective than transZ in inducing regeneration from seedling explants. In addition, we showed that there is a specific profile of endogenous hormones in hypocotyl and seedling tissues both bearing regenerated shoots. This may be important for further investigation on regeneration control by application of exogenous plant growth regulators. Described protocols allow us to further investigate and enhance shoot production from young seedling explants and subsequent plant regeneration in order to create an efficient system for kohlrabi multiplication, as well as for potential genetic manipulation.
Abbreviations
- BA:
-
N 6-benzyladenine
- cisZ:
-
cis-Zeatin
- cisZR:
-
cis-Zeatin-9-riboside
- cisZ7G:
-
cis-Zeatin-7-glucoside
- cisZ9G:
-
cis-Zeatin-9-glucoside
- cisZOG:
-
cis-Zeatin-O-glucoside
- cisZROG:
-
cis-Zeatin-9-riboside-O-glucoside
- cisZRMP:
-
cis-Zeatin-9-riboside-5′-monophosphate
- CK:
-
Cytokinin
- DNSO:
-
De novo shoot organogenesis
- DHZ:
-
Dihydrozeatin
- DHZR:
-
Dihydrozeatin-9-riboside
- DHZ7G:
-
Dihydrozeatin-7-glucoside
- DHZ9G:
-
Dihydrozeatin-9-glucoside
- DHZOG:
-
Dihydrozeatin-O-glucoside
- DHZROG:
-
Dihydrozeatin-9-riboside-O-glucoside
- DHZRMP:
-
Dihydrozeatin-9-riboside-5′-monophosphate
- HRS:
-
Hypocotyl with regenerated shoots
- IAA:
-
Indole-3-acetic acid
- IBA:
-
Indole-3-butyric acid
- iP:
-
N 6-(∆2-isopentenyl)adenine
- iPR:
-
N 6-(∆2-isopentenyl)adenosine
- iP7G:
-
N 6-(∆2-isopentenyl)adenine-7-glucoside
- iP9G:
-
N 6-(∆2-isopentenyl)adenine-9-glucoside
- iPRMP:
-
N 6-(∆2-isopentenyl)adenosine -5′-monophosphate
- PGR:
-
Plant growth regulator
- SRS:
-
Seedling with regenerated shoots
- TDZ:
-
Thidiazuron
- transZ:
-
trans-Zeatin
- transZR:
-
trans-Zeatin-9-riboside
- transZ7G:
-
trans-Zeatin-7-glucoside
- transZ9G:
-
trans-Zeatin-9-glucoside
- transZOG:
-
trans-Zeatin-O-glucoside
- transZROG:
-
trans-Zeatin-9-riboside-O-glucoside
- transZRMP:
-
trans-Zeatin-9-riboside-5′-monophosphate
- VP:
-
Vienna Purple cultivar
- VW:
-
Vienna White cultivar
References
Abbasi BH, Khan M, Guo B, Bokhari SA, Khan MA (2011) Efficient regeneration and antioxidative enzyme activities in Brassica rapa var. turnip. Plant Cell Tissue Organ Cult 105:337–344
Ahmadabadi M, Bock R (2010) Development of a highly responsive leaf-based regeneration system for Peperomia species. Turk J Bot 34:329–334
Akasaka-Kennedy Y, Yoshida H, Takahata Y (2005) Efficient plant regeneration from leaves of rape seed (Brassica napus L.): influence of AgNO3 and genotype. Plant Cell Rep 24:649–654
Aremu AO, Plačková L, Bairu MW, Novák O, Plíhalová L, Doležal K, Finnie JF, Van Staden J (2014a) How does exogenously applied cytokinin type affect growth and endogenous cytokinins in micropropagated Merwilla plumbea? Plant Cell Tissue Organ Cult 118:245–256
Aremu AO, Plačková L, Bairu MW, Novák O, Szüčová L, Doležal K, Finnie JF, Van Staden J (2014b) Endogenous cytokinin profiles of tissue-cultured and acclimatized ‘Williams’ bananas subjected to different aromatic cytokinin treatments. Plant Sci 214:88–98
Auer CA, Cohen JD, Laloue M, Cooke TJ (1992) Comparison of benzyl adenine metabolism in two Petunia hybrida lines differing in shoot organogenesis. Plant Physiol 98:1035–1041
Bajguz A, Piotrowska A (2009) Conjugates of auxin and cytokinin. Phytochemistry 70:957–969
Baroja-Fernández E, Aguirreolea J, Martínková H, Hanuš J, Strnad M (2002) Aromatic cytokinins in micropropagated potato plants. Plant Physiol Biochem 40:217–224
Bassil NV, Mok DWS, Mok MC (1993) Partial purification of a cis-trans-isomerase of zeatin from immature seed of Phaseolus vulgaris L. Plant Physiol 102:867–872
Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115:591–602
Bhalla PL, de Weerd N (1999) In vitro propagation of cauliflower, Brassica oleracea var. botrytis for hybrid seed production. Plant Cell Tissue Organ Cult 56:89–95
Bouza L, Jacques M, Sotta B, Miginiac E (1993) The differential effect of N6-benzyl-adenine and N6-(Δ2-isopentenyl)-adenine on in vitro propagation of Paeonia suffruticosa Andr. is correlated with different hormone contents. Plant Cell Rep 12:593–596
Bretagne B, Chupeau MC, Chupeau Y, Fouilloux G (1994) Improved flax regeneration from hypocotyls using thidiazuron as a cytokinin source. Plant Cell Rep 14:120–124
Brugière N, Jiao S, Hantke S, Zinselmeier C, Roessler JA, Niu X, Jones RJ, Habben JE (2003) Cytokinin oxidase gene expression in maize is localized to the vasculature, and is induced by cytokinins, abscisic acid, and abiotic stress. Plant Physiol 132:1228–1240
Cardoza V, Stewart CN (2004) Brassica biotechnology: progress in cellular and molecular biology. In Vitro Cell Dev Biol Plant 40:542–551
Centeno ML, Rodríguez A, Feito I, Fernández B (1996) Relationship between endogenous auxin and cytokinin levels and morphogenic responses in Actinia deliciosa tissue cultures. Plant Cell Rep 16:58–62
Che P, Gingerich DJ, Lall S, Howell SH (2002) Global and hormone-induced gene expression changes during shoot development in Arabidopsis. Plant Cell 14:2771–2785
Che P, Lall S, Nettleton D, Howell SH (2006) Gene expression programs during shoot, root, and callus development in Arabidopsis tissue culture. Plant Physiol 141:620–637
Cheng ZJ, Wang L, Sun W, Zhang Y, Zhou C, Su YH, Li W, Sun TT, Zhao XY, Li XG, Cheng Y, Zhao Y, Xie Q, Zhang XS (2013) Pattern of auxin and cytokinin responses for shoot meristem induction results from the regulation of cytokinin biosynthesis by AUXIN RESPONSE FACTOR3. Plant Physiol 161:240–251
Chikkala VRN, Nugent G, Dix P, Stevenson T (2009) Regeneration from leaf explants and protoplasts of Brassica oleracea var. botrytis (cauliflower). Sci Hortic 119:330–334
Cogbill S, Faulcon T, Jones G, McDaniel M, Harmon G, Blackmon R, Young M (2010) Adventitious shoot regeneration from cotyledonary explants of rapid-cycling fast plants of Brassica rapa L. Plant Cell Tissue Organ Cult 101:127–133
Coleman GD, Ernst SG (1989) In vitro shoot regeneration of Populus deltoids: effect of cytokikin and genotype. Plant Cell Rep 8:459–462
Ćosić T, Vinterhalter B, Vinterhalter D, Mitić N, Cingel A, Savić J, Bohanec B, Ninković S (2013) In vitro plant regeneration from immature zygotic embryos and repetitive somatic embryogenesis in kohlrabi (Brassica oleracea var. gongylodes). In Vitro Cell Dev Biol Plant 49:294–303
Cuesta C, Rodriguez A, Centeno ML, Ordas RJ, Fernandez B (2009) Caulogenic induction in cotyledons of tone pine (Pinus pinea): relationship between organogenic response and benzyladenine trends in elected families. J Plant Physiol 166:1162–1171
Cuesta C, Novák O, Ordás RJ, Fernández B, Strnad M, Doležal K, Rodríguez A (2012) Endogenous cytokinin profiles and their relationships to between-family differences during adventitious caulogenesis in Pinus pinea cotyledons. J Plant Physiol 169:1830–1837
Dai XG, Shi XP, Ye YM, Fu Q, Bao MZ (2009) High frequency plant regeneration from cotyledon and hypocotyl explants of ornamental kale. Biol Plant 53:769–773
Dobrev P, Kamínek M (2002) Fast and efficient separation of cytokinins from auxin and abscisic acid and their purification using mixed-mode solid-phase extraction. J Chromatogr A 950:21–29
Dobrev P, Havlíček L, Vágner M, Malbeck J, Kamínek M (2005) Purification and determination of plant hormones auxin and abscisic acid using solid phase extraction and two-dimensional high performance liquid chromatograophy. J Chromatogr A 1075:159–166
Duclercq J, Sangwan-Norreel B, Catterou M, Sangwan RS (2011) De novo shoot organogenesis: from art to science. Trends Plant Sci 16:597–606
Dwivedi S, Vaňková R, Motyka V, Herrera C, Žižková E, Auer C (2010) Characterization of Arabidopsis thaliana mutant ror-1 (roscovitine-resistant) and its utilization in understanding of the role of cytokinin N-glucosylation pathway in plants. Plant Growth Regul 61:231–242
Eklöf S, Åstot C, Blackwell J, Moritz T, Olsson O, Sandberg G (1997) Auxin-cytokinin interactions in wild-type and transgenic tobacco. Plant Cell Physiol 38:225–235
Emery RJN, Leport L, Barton JE, Turner NC, Atkins CA (1998) cis-Isomers of cytokinins predominate in chickpea seeds throughout their development. Plant Physiol 117:1515–1523
Emery RJN, Ma Q, Atkins CA (2000) The forms and sources of cytokinins in developing white lupine seeds and fruits. Plant Physiol 123:1593–1604
Escalona VH, Aguayo E, Artés E (2007) Extending the shelf life of kohlrabi stems by modified atmosphere packaging. J Food Sci 72:308–313
Gajdošová S, Spíchal L, Kamínek M, Hoyerová K, Novák O, Dobrev PI, Galuszka P, Klíma P, Gaudinová A, Žižková E, Hanuš J, Dančák M, Trávníček B, Pešek B, Krupička M, Vaňková R, Strnad M, Motyka V (2011) Distribution, biological activities, metabolism, and the conceivable function of cis-zeatin-type cytokinins in plants. J Exp Bot 62:2827–2840
Ghnaya AB, Charles G, Branchard M (2008) Rapid shoot regeneration from thin cell layer explants excised from petioles and hypocotyls in four cultivars of Brassica napus L. Plant Cell Tissue Organ Cult 92:25–30
Glendening TM, Sjolund R (1988) In vitro propagation of kohlrabi from leaf explants. HortScience 23:772
Gordon SP, Heisler MG, Reddy GV, Ohno C, Das P, Meyerowitz EM (2007) Pattern formation during de novo assembly of the Arabidopsis shoot meristem. Development 134:3539–3548
Grosch R, Schneider JHM, Kofoet A (2004) Characterisation of Rhizoctonia solani anastomosis groups causing bottom rot in field-grown lettuce in Germany. Eur J Plant Pathol 110:53–62
Haddadi P, Moieni A, Karimzadeh G, Abdollahi MR (2008) Effects of gibberellin, abscisic acid and embryo desiccation on normal plantlet regeneration, secondary embryogenesis and callogenesis in microspore culture of Brassica napus L. cv. PF704. Int J Plant Prod 2:153–162
Hansen LN, Ortiz R, Andersen SB (1999) Genetic analysis of protoplast regeneration ability in Brassica oleracea. Plant Cell Tissue Organ Cult 58:127–132
Jones B, Andersson Gunnerås S, Petersson SV, Tarkowski P, Graham N, May S, Dolezal K, Sandberg G, Ljung K (2010) Cytokinin regulation of auxin synthesis in Arabidopsis involves a homeostatic feedback loop regulated via auxin and cytokinin signal transduction. Plant Cell 22:2956–2969
Kamal GB, Illich KG, Asadollah A (2007) Effect of genotype, explant type and nutrient medium components on canola (Brassics napus L.) shoot in vitro organogenesis. Afr J Biotechnol 6:861–867
Kamínek M, Pačes V, Corse J, Challice JS (1979) Effect of stereospecific hydroxylation of N6-(∆2-isopentenyl)adenosine on cytokinin activity. Planta 145:239–243
Kamínek M, Motyka V, Vaňková R (1997) Regulation of cytokinin content in plant cells. Physiol Plant 101:689–700
Kamínek M, Březinová A, Gaudinová A, Motyka V, Vaňková R, Zažímalová E (2003) Purine cytokinins: a proposal of abbreviations. Plant Growth Regul 32:253–256
Kasahara H, Takei K, Ueda N, Hishiyama S, Yamaya T, Kamiya Y, Yamaguchi S, Sakakibara H (2004) Distinct isoprenoid origins of cis- and trans-zeatin biosyntheses in Arabidopsis. J Biol Chem 279:14049–14054
Khan MR, Rashid H, Ansar M, Chaudry Z (2003) High frequency shoot regeneration and Agrobacterium-mediated DNA transfer in Canola (Brassica napus). Plant Cell Tissue Organ Cult 75:223–231
Klemš M, Slámová Z, Motyka V, Malbeck J, Trávníčková A, Macháčková I, Holík J, Procházka S (2011) Changes in cytokinin levels and metabolism in tobacco (Nicotiana tabacum L.) explants during in vitro shoot organogenesis induced by trans-zeatin and dihydrozeatin. Plant Growth Regul 65:427–437
Klíma M, Vyvadilová M, Kučera V (2004) Production and utilization of doubled haploids in Brassica oleracea vegetables. Hortic Sci Prague 31:119–123
Koh WL, Loh CS (2000) Direct somatic embryogenesis, plant regeneration and in vitro flowering in rapid-cycling Brassica napus. Plant Cell Rep 19:1177–1183
Lexa M, Genkov T, Malbeck J, Macháčková I, Brzobohatý B (2003) Dynamics of endogenous cytokinin pools in tobacco seedlings: a modelling approach. Ann Bot 91:585–597
Lillo C, Shanin EA (1986) Rapid regeneration of plants from hypocotyl protoplasts and root segments of cabbage. HortScience 21:315–317
Linsmaier EM, Skoog F (1965) Organic growth factor requirements of tobacco tissue cultures. Physiol Plant 18:100–127
Liu J, Mehdi S, Topping J, Tarkowski P, Lindsey K (2010) Modelling and experimental analysis of hormonal crosstalk in Arabidopsis. Mol Syst Biol 6: Article number 373
LoSchiavo F, Pitto L, Giuliano G, Torti G, Nuti-Ronchi V, Marazziti D, Vergara R, Orselli S, Terzi M (1989) DNA methylation of embryogenic carrot cell cultures and its variations as caused by mutation, differentiation, hormones and hypomethylating drugs. Theor Appl Genet 77:325–331
Martin RC, Mok MC, Habben JE, Mok DWS (2001) A cytokinin gene from maize encoding an O-glucosyltransferase specific to cis-zeatin. Proc Natl Acad Sci USA 98:5922–5926
Miyawaki K, Matsumoto-Kitano M, Kakimoto T (2004) Expression of cytokinin biosynthetic isopentenyltransferase genes in Arabidopsis: tissue specificity and regulation by auxin, cytokinin, and nitrate. Plant J 37:128–138
Moghaieb R, El-Awady M, El Mergawy R, Youssef S, El-Sharkawy A (2006) A reproducible protocol for regeneration and transformation in canola (Brassica napus L.). Afr J Biotechnol 5:143–148
Mollika SR, Sarker RH, Hoque MI (2011) In vitro plant regeneration in Brassica spp. Plant Tissue Cult Biotechnol 21:127–134
Moncaleán P, Rodríguez A, Fernández B (2003) Effect of different benzyladenine time pulses on the endogenous levels of cytokinins, indole-3-acetic acid and abscisic acid in micropropagated explants of Actinidia deliciosa. Plant Physiol Biochem 41:149–155
Montalbán IA, Novák O, Rolčik J, Strnad M, Moncaleán P (2013) Endogenous cytokinin and auxin profiles during in vitro organogenesis from vegetative buds of Pinus radiata adult trees. Physiol Plant 148:214–231
Motte H, Vereecke D, Geelen D, Werbrouck S (2014) The molecular path to in vitro shoot regeneration. Biotechnol Adv 32:107–121
Motyka V, Vaňková R, Čapková V, Petrášek J, Kamínek M, Schmülling T (2003) Cytokinin-induced upregulation of cytokinin oxidase activity in tobacco includes changes in enzyme glycosylation and secretion. Physiol Plant 117:11–21
Msikita W, Skirvin RM (1989) In vitro regeneration from hypocotyl and seedling cotyledons of tronchuda (Brassica oleracea var. tronchuda Bailey). Plant Cell Tissue Organ Cult 19:159–165
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15:473–497
National Institute for Health and Welfare, Nutrition Unit. Fineli (2011) Finnish Food Composition Database Release 14, Helsinki 2011. http://www.fineli.fi/food.php?foodid=329&lang=en. Accessed 8 June 2012
Neelakandan AK, Wang K (2012) Recent progress in the understanding of tissue culture-induced genome level changes in plants and potential applications. Plant Cell Rep 31:597–620
Nikolić R, Mitić N, Miletić R, Nešković M (2006) Effects of cytokinins on in vitro seed germination and early seedling morphogenesis in Lotus corniculatus L. J Plant Growth Regul 25:187–194
Nordström A, Tarkowski P, Tarkowska D, Norbaek R, Åstot C, Dolezal K, Sandberg G (2004) Auxin regulation of cytokinin biosynthesis in Arabidopsis thaliana: a factor of potential importance for auxin-cytokinin-regulated development. Proc Natl Acad Sci USA 101:8039–8044
Okubo H, Wada K, Uemoto S (1991) In vitro morphogenetic response and distribution of endogenous plant hormones in hypocotyl segment of snapdragon (Antirrhinum majus L.). Plant Cell Rep 10:501–504
Ono Y, Takahata Y, Kaizuma N (1994) Effect of genotype on shoot regeneration from cotyledonary explants of rapeseed (Brassica napus L.). Plant Cell Rep 14:13–17
Otto F (1988) High resolution DNA—flow cytometry using DAPI, Protocol 1, Partec Arlesheim, Münster
Ovesná J, Ptacek L, Opatrny Z (1993) Factors influencing the regeneration capacity of oilseed rape and cauliflower in transformation experiments. Biol Plant 35:107–112
Parker CW, Entsch B, Letham DS (1986) Inhibitors of two enzymes which metabolize cytokinins. Phytochemistry 25:303–310
Paul S, Sikdar SR (2005) Regeneration of plants from root explant of two Indian cultivars of Brassica campestris L. through somatic embryogenesis. Curr Sci 89:1323–1326
Pavlović S, Vinterhalter B, Mitić N, Adžić S, Pavlović N, Zdravković M, Vinterhalter D (2010) In vitro shoot regeneration from seedling explants in Brassica vegetables: red cabbage, broccoli, Savoy cabbage and cauliflower. Arch Biol Sci 62:337–345
Pellegrineschi A (1997) In vitro plant regeneration via organogenesis of cowpea [Vigna unguiculata (L.) Walp.]. Plant Cell Rep 17:89–95
Pernisová M, Klíma P, Horák J, Válková M, Malbeck J, Souček P, Reichman P, Hoyerová K, Dubová J, Friml J, Zažímalová E, Hejátko J (2009) Cytokinins modulate auxin-induced organogenesis in plants via regulation of the auxin efflux. Proc Natl Acad Sci USA 106:3609–3614
Raspor M, Motyka V, Žižková E, Dobrev PI, Trávníčková A, Zdravković-Korać S, Simonović A, Ninković S, Dragićević IC (2012) Cytokinin profiles of AtCKX2-overexpressing potato plants and the impact of altered cytokinin homeostasis on tuberization in vitro. J Plant Growth Regul 31:460–470
Ravanfar SA, Aziz MA, Kadir MA, Rashid AA, Haddadi F (2011) In vitro adventitious shoot regeneration and acclimatisation of Brassica oleracea subsp. italica cv Green Marvel. Afr J Biotechnol 10:5614–5619
Ravanfar SA, Aziz MA, Rashid AA, Salim S (2014) In vitro adventitious shoot regeneration from cotyledon explant of Brassica oleracea subsp. italica and Brassica oleracea subsp. capitata using TDZ and NAA. Pak J Bot 46:329–335
Schmitz RY, Skoog F, Playtis AJ, Leonard NJ (1972) Cytokinins: synthesis and biological activity of geometric and position isomers of zeatin. Plant Physiol 50:702–705
Selman LW, Kulasegaram S (1966) Development of the stem tuber in kohlrabi. J Exp Bot 18:471–490
Sharma KK, Thorpe TA (1989) In vitro regeneration of shoot buds and plantlets from seedling root segments of Brassica napus L. Plant Cell Tissue Organ Cult 18:129–141
Souza BM, Kraus JE, Enders L, Mercier H (2003) Relationships between endogenous hormonal levels and axillary bud development of Ananas comosus nodal segments. Plant Physiol Biochem 41:733–739
Sparrow PAC, Townsend TM, Morgan CL, Dale PJ, Arthur AE, Irwin JA (2004) Genetic analysis of in vitro shoot regeneration from cotyledonary petioles of Brassica oleracea. Theor Appl Genet 108:1249–1255
Spíchal L, Rakova NY, Riefler M, Mizuno T, Romanov GA, Strnad M, Schmülling T (2004) Two cytokinin receptors of Arabidopsis thaliana, CRE1/AHK4 and AHK3, differ in their ligand specificity in a bacterial assay. Plant Cell Physiol 45:1299–1305
Sretenović-Rajičić T, Ninković S, Vinterhalter B, Miljuš-Đukić J, Vinterhalter D (2004) Introduction of resistance to herbicide Basta® in Savoy cabagge. Biol Plant 48:431–436
Sretenović-Rajičić T, Ninković S, Miljuš-Đukić J, Vinterhalter B, Vinterhalter D (2006) Agrobacterium rhizogenes-mediated transformation of Brassica oleracea var. sabauda and B. oleracea var. capitata. Biol Plant 50:525–530
Stirk WA, Novák O, Václavíková K, Tarkowski P, Strnad M, van Staden J (2008) Spatial and temporal changes in endogenous cytokinins in developing pea roots. Planta 227:1279–1289
Stirk WA, Václavíková K, Novák O, Gajdošová S, Kotland O, Motyka V, Strnad M, van Staden J (2012) Involvement of cis-zeatin, dihydrozeatin, and aromatic cytokinins in germination and seedling establishment of maize, oats, and lucerne. J Plant Growth Regul 31:392–405
Suri SS, Saini ARK, Ramawat KG (2005) High frequency regeneration and Agrobacterium tumefaciens-mediated transformation of broccoli (Brassica oleracea var. italica). Eur J Hortic Sci 70:71–78
Takei K, Yamaya T, Sakakibara H (2004) Arabidopsis CYP735A1 and CYP735A2 encode cytokinin hydroxylases that catalyze the biosynthesis of trans-zeatin. J Biol Chem 279:41866–41872
Teo W, Lakshmanan P, Kumar P, Goh CJ, Swarup S (1997) Direct shoot formation and plant regeneration from cotyledon explants of rapid-cycling Brassica rapa. In Vitro Cell Dev Biol Plant 33:288–292
Valdés AE, Ordas RJ, Fernandez B, Centeno ML (2001) Relationships between hormonal contents and the organogenic response in Pinus pinea cotyledons. Plant Physiol Biochem 39:377–384
Veach YK, Martin RC, Mok DWS, Malbeck J, Vaňková R, Mok MC (2003) O-Glucosylation of cis-zeatin in maize. Characterization of genes, enzymes, and endogenous cytokinins. Plant Physiol 131:1374–1380
Verma SK, Yücesan BB, Şahin G, Gürel S, Gürel E (2011) Direct shoot regeneration from leaf explants of Digitalis lamarckii, an endemic medicinal species. Turk J Bot 35:689–695
Vinterhalter D, Sretenović-Rajičić T, Vinterhalter B, Ninković S (2007) Genetic transformation of Brassica oleracea vegetables. Transgenic Plant J 1:340–355
Wang Y, Tong Y, Li Y, Zhang Y, Zhang J, Feng J, Feng H (2011) High frequency plant regeneration from microspore-derived embryos of ornamental kale (Brassica oleracea L. var. acephala). Sci Hortic 130:296–302
Wong KW, Loh CS (1988) In vitro regeneration of plantlets from root segments of Brassica alboglabra Bailey. Sci Hortic Amst 35:7–14
Yonekura-Sakakibara K, Kojima M, Yamaya T, Sakakibara H (2004) Molecular characterization of cytokinin-responsive histidine kinases in maize. Differential ligand preferences and response to cis-zeatin. Plant Physiol 134:1654–1661
Zhang Y, Xu J, Han L, Wei W, Guan Z, Cong L, Chai T (2006) Efficient regeneration and Agrobacterium-mediated transformation of Brassica juncea. Plant Mol Biol Rep 24:255a–255i
Acknowledgments
This work was supported by the Ministry of Education, Science and Technological Development of Serbia (No. 173015) and the Czech Science Foundation (P506/11/0774).
Author information
Authors and Affiliations
Corresponding author
Additional information
Tatjana Ċosiċ and Václav Motyka have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ċosiċ, T., Motyka, V., Raspor, M. et al. In vitro shoot organogenesis and comparative analysis of endogenous phytohormones in kohlrabi (Brassica oleracea var. gongylodes): effects of genotype, explant type and applied cytokinins. Plant Cell Tiss Organ Cult 121, 741–760 (2015). https://doi.org/10.1007/s11240-015-0742-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-015-0742-2