Abstract
The present study reports, for the first time, an efficient in vitro plant regeneration protocol for Digitalis ferruginea subsp. ferruginea L. (rusty foxglove). We have used different concentrations of gibberellic acid (GA3) on Murashige and Skoog (MS) medium to assess the germination frequency of seeds. High frequency of germination was achieved on MS medium with 1.0 mg l−1 GA3. 6-Benzylaminopurine (BAP) combined with α-naphtaleneacetic acid (NAA) or 2, 4-dichlorophenoxy acetic acid (2, 4-D) in the induction MS medium induced both somatic embryogensis and shoot organogenesis. The highest percentage of callus growth (85 %) was obtained when hypocotyl explants were cultured on MS medium containing 0.5 mg l−1 2, 4-D plus 1.0 mg l−1 BAP. The maximum mean number of somatic embryos (7.3 ± 1.3 embryos) or shoots (12.0 ± 1.1 shoots) per callus was obtained when medium contained 0.25 mg l−1 NAA plus 1.0 mg l−1 BAP or 0.5 mg l−1 NAA plus 2.0 mg l−1 BAP. The regenerated shoots easily rooted on MS medium. Higher amounts of lanatoside C [13.2 ± 0.5 mg 100 g−1 dry weight (dw)] and digoxin (2.93 ± 0.31 mg 100 g−1 dw) accumulation were obtained when shoots were obtained by indirect regeneration. We also investigated derivatives of cardenolides, i.e., digitoxigenin (730 ± 180 mg 100 g−1 dw), gitoxigenin (50 ± 20 mg 100 g−1 dw) and digoxigenin (490 ± 170 mg 100 g−1 dw) from natural samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Digitalis ferruginea subsp. ferruginea (D. ferruginea L.), commonly known as rusty foxglove, which belongs to the family Scrophulariaceae, is a medicinally and economically important species. D. ferruginea is composed of biennial or perennial herbs and occasionally of small shrubs. This species is native to the Balkans, Hungary, Italy, Lebanon, Romania, Transcaucasia and Turkey (Davis et al. 1988). D. ferruginea of the Samtskhe-Javakheti region are included in the list of rare species of Georgia. D. ferruginea L. (i.e., D. ferruginea subsp. ferruginea L., and D. ferruginea subsp. schischkini L.) species is the most widespread member of the nine Digitalis species grown in Turkey and mainly found in the woods, scrub, and rocky places (Davis et al. 1988).
Digitalis has been used for centuries to treat heart disease. The active ingredient in the drug is cardiac glycoside, which increases the force of systolic contraction and regulate heart rhythm. Treatment with these compounds is still the only safe inotropic drug for the improvement of hemodynamics in patients with compromised cardiac function. It is also used as diuretic and for reduction of oedema due to its ability to improve cardiovascular circulation. In India, Digitalis glycosides have been used in Ayurvedic formulations as an ointment to treat wounds and burns. Digitalis is widely used as an allopathic medicine in doses of 0.125–0.25 mg day−1. Cardiac glycosides of different Digitalis species, are also well-known for their antiproliferative effects on tumor cells (Sales et al. 2011).
Establishment of tissue culture protocols is very critical for biennial Digitalis species as, in long-term cultivation, plants in open-fields have failed to germinate at the end of the second vegetation period. Moreover, indiscriminate collection of the species possess a serious threat to its existence in wild populations, especially when the plants are harvested well before seed setting. Many in vitro culture protocols exist, where somatic embryogenesis and organogenesis have been achieved in D. davisiana (Gurel et al. 2011), D. lamarckii (Verma et al. 2011a, b), D. lanata (Kreis et al. 1990; Kuberski et al. 1984), D. obscura (Lapeña and Brisa 1995; Pérez-Bermúdez et al. 1987; Sales et al. 2011), D. purpurea (Hagimori et al. 1982), D. minor (Sales et al. 2003), D. thapsi (Cacho et al. 1991) and D. trojana (Verma et al. 2012). However, there is no report concerning in vitro regeneration of D. ferruginea. In this report, for the first time, we described a simple and very effective protocol for indirect somatic embryogenesis, organogenesis, plant regeneration and cardiac glycoside determination from both in vitro and natural samples of D. ferruginea.
Materials and methods
Plant material
Seeds of D. ferruginea, were collected from natural populations around Abant, Bolu, Turkey (at the altitude of 1,334 m, 40° 36.02N, 031°16.36E) in September 2008. Identification of species was done according to Davis et al. (1988), and specimens were deposited appropriately at the Abant Izzet Baysal University Herbarium, Bolu, Turkey.
Seed sterilization, germination and culture conditions
Prior to the surface sterilization process, seeds were stratified by using sand paper with very fine microgrits. Further, seeds were soaked in sterilized water for 24 h and were finally kept in different concentrations of gibberellic acid (GA3) (0.1, 0.5 and 1.0 mg l−1) at 4 °C for further use. Surface sterilization of seeds was done using 20 % commercial bleach (Domestos) with few drops of Tween-20 for 10 min in a sonicator and finally rinsed with sterile distilled water three times. An average of 25 seeds were aseptically cultured on 100 × 15 mm Petri dishes containing 30 ml of MS (Murashige and Skoog 1962)-based media MSO (MS medium without hormones) or containing different concentrations of GA3 (0.1, 0.5 or 1.0 mg l−1) and 3 % (w/v) sucrose. The medium was solidified with 0.8 % (w/v) agar and autoclaved at 121 °C temperature and 1.06 kg cm−2 pressure for 15 min after adjusting the pH to 5.8 with 0.1 N HCl or 0.1 N KOH. The culture was kept at 23 ± 1 °C for the first 2 days in dark, and then transferred to 16 h light:8 h dark photoperiod (provided by cool-white fluorescent light, irradiance 50 μmol m−2 s−1) at a relative humidity of 55–60 %. Hypocotyl segments (5–8 mm) from one-month-old seedlings were used as explants for culture initiation.
Callus induction, somatic embryogenesis and shoot regeneration
The hypocotyl segments (5–8 mm) excised from one-month-old in vitro-germinated seedlings were cultured in Petri dishes (90 × 15 mm) containing 30 ml solid MS medium containing different concentrations 0.25–0.5 mg l−1 of NAA (α-naphtaleneacetic acid) or 2, 4-dichlorophenoxy acetic acid (2, 4-D) and combined with 0.5–2.0 mg l−1 BAP (6-benzylaminopurine). Experiments were repeated three times, each using 20 replicates (i.e., a total of 60 explants per treatment). Both the frequency (%) of callus developing somatic embryos or shoots and mean numbers of somatic embryos or shoots per hypocotyl derived callus were recorded after 8 or 12 weeks of culture, respectively.
Histological studies
For histological studies, the embryogenic calli were fixed in formaldehyde/glacial acetic acid/ethanol (5:5:90, v/v/v) for 24 h, dehydrated through a graded tertiary butyl alcohol (TBA) series, each for 24 h and embedded in saturated paraffin wax. Embedded materials were sectioned to 5 μm thickness on a rotary microtome. Paraffin wax was removed by xylene prior to rehydration of the tissues in a graded ethanol series and staining of the tissues were performed using 1.0 % (w/v) safranin. Tissues were briefly washed in water to remove excess stain and then dehydrated in a graded ethanol series. The photographs were taken with an Olympus microscope.
Rooting of the shoots and hardening off
Twelve weeks old shoots (3–4 cm long) that developed from callus were transferred to Magenta vessels containing MS medium supplemented with 0.1, 0.5 and 1.0 mg l−1 IAA or IBA to observe root formation for 6 weeks. Plantlets with well-developed roots and shoots were removed from the cultured medium and washed gently under running tap water. The plantlets were then transferred to plastic pots containing an autoclaved mixture of peat moss, compost and soil (1:1:1). Pots were covered with transparent polyethylene bags having small pore to maintain humidity and kept in the culture room at 25 °C and 50 µmol m−2 −1S photon flux density provided by white fluorescent tubes. After 3 weeks, the plantlets were transferred to large pots containing peat moss, compost and garden soil (1:1:1) and finally transferred outdoors, under the sun.
Sample preparation, cardenolide extraction and acid hydrolysis
Whole shoots of in vitro germinated seedlings or in vitro regenerated plantlets of D. ferruginea were freeze-dried at −56 °C and later used for cardenolide extraction. Cardenolides were extracted as described by Wiegrebe and Wichtl (1993) with slight modifications. Briefly, 50 mg powdered dry tissue was transferred to centrifuge tube containing 1 ml of 70 % methanol. The extract obtained was further treated in ultrasonic bath for 30 min at 65–70 °C. The extract was finally cooled in ice bath for 3 min followed by centrifugation at 13,000 rpm for 10 min. The supernatant was thoroughly mixed with 0.25 ml of 15 % lead acetate solution (w/v) and centrifuged. After elucidating the lead acetate residue, 0.5 ml of 4 % monosodium phosphate was added and centrifuged. The supernatant was transferred to 2 ml centrifuge tubes, diluted to a final volume of 2 ml with water and again centrifuged at room temperature (RT) for 8 min at 13,000 rpm. The supernatant was mixed with 0.5 ml chloroform: isopropanol (3:2) and centrifuged for 5 min as before. The lower phase was transferred to 2 ml centrifuge tubes (the first extraction), the remaining methanolic solution was used (second extraction) by adding chloroform: isopropanol and centrifuged at RT for 5 min at 13,000 rpm. Both extractions were combined and evaporated under an air flow chamber for 3 h and finally dissolved in 500 µl methanol (HPLC grade). Thin layer chromatography (TLC) was performed on TLC plates (10 × 20 cm silica gel 60 W, Merck, Germany) after loading 50 µl of each sample.
The Acid hydrolysis of obtained extract was performed as mentioned (Eisenbeiß et al. 1999). Cardenolide extraction in centrifuge tubes was vacuum dried. It was dissolved in 240 µl of acetone: HCl (1:100) solution. After 1 min vortex, samples were ultrasonicated in water bath for 5 min at 40 °C. The cellular debris were pelleted at 13,000 rpm for 10 min. Samples were kept in dark at room temperature for about 12 h prior to the addition of 102 µl saturated sodium bicarbonate (NaHCO3) solution. After centrifugation, the supernatant was pipetted out and stored at 4 °C until further use. The residue was washed repeatedly with 1 ml acetone. Finally, the organic phase was evaporated and dried prior to the addition of 500 µl HPLC grade methanol.
Reference substances and determination of fingerprint TLC analysis
Chromatography was performed using 50 µl of each extract spotted on a precoated TLC plate. The plate was air dried and kept in a glass chamber containing mobile phase (chloroform: methanol: water; 81:18:1). After 10–25 min run plate was taken out from the chamber and sprayed with Jensen-Kny’s reagent to identify the cardenolide groups depending on coloration of the bands at 120 °C for 10 min. The spots appeared on the chromatogram at 366 nm UV light as yellow, green or blue bands refer to A, B or C type of cardenolides, respectively. For determination of genin groups on TLC plates, mixture of lanatoside A, B and C were treated with acid hydrolysis.
HLPC determination and reference substances
Cardenolide estimation using HPLC was performed as reported (Wiegrebe and Wichtl 1993). Briefly, chromatography was run at a flow rate of 1.2 ml min−1 with a binary pump solvent system. A dual λ absorbance detector operating at 220 and 350 nm, an autosampler (Waters Autosampler 717 Plus) injecting 20 µl of each sample (n:3 sample for each injection) was used in present work. Reprosil-Pur C18 AQ, 5 µm, 250 × 4 mm column for reverse phase (RP)-HPLC was used. Cardenolides were eluted with acetonitrile (A) and water (B) gradients as follows: 0–20 min 20 % (A), 80 % (B); 20–27 min 32 % (A), 68 % (B); 27–35 min 58 % (A), 42 % (B); 35–40 min 60 % (A), 40 % (B); 40–60 min 0 % (A), 100 % (B); 60–65 min 20 % (A), 80 % (B). HPLC calibration was done according to internal standard (IS, β-methyl-digitoxin).
Statistical analysis
Data were statistically analyzed using SPSS, Version 18.0 (SPSS Inc., Chicago,IL, USA). Analysis of variance was used to calculate statistical significance and mean ± SE (Standard error) were significantly determined using Duncan’s multiple range test at p < 0.05 level.
Results and discussion
The present study reports (1) an efficient in vitro plant regeneration protocol for D. ferruginea via shoot organogenesis and somatic embryogenesis from callus and (2) cardenolides detremination of the species from in vitro samples (Lanatoside C, Digoxin) and natural populations (genine group) employing various chromatographical techniques. To achieve these, two sets of experiments were carried out. First, the germination frequency of seeds on MS medium with different concentrations of GA3 was determined. Then, different concentrations and combinations of NAA or 2, 4-D with BAP were tested using MS medium for callus induction, somatic embryogenesis, shoot regeneration, plant regeneration and cardenolide estimation.
Germination
Following the GA3 pretreatment, 25 seeds were placed on MSO medium for germination. Pretreatment with GA3 significantly increased seed germination (Fig. 1a, b) and rate of germination was compared with control (MSO medium) (Table 1). Moreover, the seeds from control treatment did not start to germinate until 7 days after beginning of experiment, whereas seeds from all GA3 treatments started germinating after 48 h. Total germination in control treatment was not higher than 40 %, while seed germination was 80 % on MS medium supplemented with GA3 (1.0 mg l−1). Under in vitro conditions, germination rates of Digitalis endemic species were compared using above mentioned protocol and the order from highest to lowest is as follows : D. lamarckii > D. trojana > D. davisiana > D. ferruginea > D. cariensis (data not provided). It was earlier reported that GA3 have shown to break dormancy in various genera of seeds, especially in Pestemon Digitalis. In other plants of Scrophulariaceae family (D. ferruginea), the endosperm surrounds the embryo and occupies up to half of the seed (Atwater 1980). Thus, it is concluded from the results that if seeds have dormancy, it could be released by soaking in GA3.
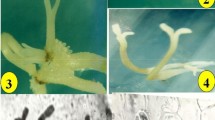
Plant regeneration through indirect organogenesis from hypocotyl segments of D. ferruginea L., (rusty foxglove). a Emergence of the radicle from the seeds, b Germinated seedlings on MS medium supplemented with 1.0 mg l−1 GA3, c–f Well-developed globular somatic embryos at different stages developing from hypocotyle explants after an 8 weeks of culture on MS medium supplemented with 0.25 mg l−1 NAA combined with 1.0 mg l−1 BAP and 0.25 mg l−1 2, 4D plus 0.5 mg l−1 BAP (g); h histological section of somatic embryos; globular embryo differentiating from embryogenic mass of calli (arrowheads) with a suspensor , i, j shoot regeneration from the hypocotyl derived calli after 12 weeks of cultures on MS medium fortified with 0.5 mg l−1 NAA plus 2.0 mg l−1 BAP, k Rooting of the regenerated shoots on medium containing 0.5 mg l−1 IAA , l Regenerated plants in potting soil
Callus induction and somatic embryogenesis
The hypocotyle explants of D. ferruginea were cultured on MS medium containing different concentrations and combinations of NAA or 2, 4-D with BAP. A small amount of friable callus developed on the cut surface of explants, and subsequently it covered their entire surface within one week. Callus induction and morphogeny data were collected after 4 weeks of culture initiation (Table 2). All concentrations and combinations, ranging from 0.25 to 0.5 mg l−1 2, 4-D plus 0.5 to 2.0 mg l−1 BAP, were effective for callus induction within the range of 76.6–85 % callusing rate (Table 2). These results are in good agreement with the earlier reported work on cotyledonary leaf culture of D. lamarckii (Verma et al. 2011a).
Only green friable calli were selected and transferred to the embryo regeneration medium contaning same hormone composition as the callus induction medium (Table 2). After 4 weeks, the embryogenic calli were observed and a part of callus was developed into embryos. Within 8 weeks of the culture initiation, numerous globular somatic embryos were visible all around the surface of the embryogenic callus. All of these globular embryos first appeared as protrusions on the tissue surface and then individual embryo enlarged into a distinct bipolar structure and passed through each of the typical stages of embryo development. The description of such stages (Fig. 1c–g) in D. ferruginea is similar to those previously reported for several Digitalis species including D. lamarckii (Verma et al. 2011a), and D. trojana (Verma et al. 2012). The highest frequency (33.3 %) of somatic embryogenesis and mean number of embryos (6.8 ± 1.4) was obtained when medium contained 0.25 mg l−1 NAA plus 1.0 mg l−1 BAP. There was no significant effect of the treatment on mean number of somatic embryos per hypocotyl derived callus (Table 2). Similar results were obtained with cotyledonary leaf explants of D. lamarckii (Verma et al. 2011a). The present findings suggest that the combination of auxin (2, 4-D) and cytokinin (BAP) is effective for somatic embryo development (Table 2; Fig. 1g). These findings were further supported by Kuberski et al. (1984) who reported that auxins (2, 4-D and NAA) triggered somatic embryogenesis and improved the later development of embryos in D. lanata. The plant growth regulators especially auxins are known to trigger the stimulation of pre-embryogenic determined cells to undergo cell division and then expression of embryogenesis in many plant species (Verma et al. 2011a).
An appearance of globular structure in somatic embryogenesis, which was coupled with the concomitant development of the protoderm. The outermost layer of a developing embryo with evident anticlinal divisions, and the presence of this layer was one of the unique features of the somatic embryo development. In the current study, the protoderm was clearly visible at the late globular stage (Fig. 1h), indicating that the emerging meristemoids were following the normal path of somatic embryogenesis. Importance of protoderm development in relation to somatic embryogenesis has been reported previously (Quiroz-Figueroa et al. 2002). A similar observation was also reported for somatic embryo of Hyoscyamus muticus L. (Verma and Chand 2009).
Shoot organogenesis
Shoot regeneration was achieved from organogenic callus that was already cultured for somatic embryos as described (Table 2). The organogenic callus was transferred to fresh medium (Table 2) for shoot regeneration and multiplication within 12 weeks. However, shoot development on the callus was mediated via organogenic-like shoot meristems. Therefore, it cannot be excluded that next to somatic embryogenesis, adventitious shoot formation was also involved. No shoot formation was achieved on 2, 4-D combination with BAP containing media (Table 2). Within the tested different auxins (2, 4-D and NAA) and cytokinin (BAP), BAP in combination with NAA induced highest shoot regeneration. Our results are consistent with the previous research on D. lanata and D. trojana (Çördük and Akı 2010; Schöner and Reinhard 1986) in which combinations of auxin and cytokinin positively influenced the growth in the cultures media. The 8 week-old hypocotyle derived friable organogenic callus was subcultured on fresh medium for shoot induction (Table 2; Fig. 1i, j). The percentage of culture showing shoot regeneration and number of shoots per culture was highest on MS medium fortified with 0.5 mg l−1 NAA plus 2.0 mg l−1 BAP. On this medium, maximum 80 % cultures showed shoot regeneration with 12.0 ± 1.1 shoots per culture, after 12 weeks callus culture initiation. There was no significant difference among the mean number of shoots per hypocotyl derived callus (Table 2). A green friable callus was directly capable of forming shoot primordia via organogenesis. The regenerated shoot did not show further elongation on regeneration medium in which a maximum shoot length was 4 cm. On the other hand, promoting effect of NAA or BAP on callus induction and/or shoot regeneration has been reported for many plant species, including some medicinal endemics to Turkey (Turker et al. 2010; Verma et al. 2011a).
Acclimatization and transfer of rooted plants
Regenerated shoots (3–4 cm long) were isolated from the callus and transferred to MS medium contained 0.1, 0.5 or 1.0 mg l−1 IAA or IBA (Table 3). The roots started to emerge from the cut ends of the shoots within 2 weeks in rooting medium. In general, IAA showed a better response than IBA in terms of both the percentages of shoots that rooted and the mean number of roots per rooted shoot. However, IAA (1.0 mg l−1) was found most effective by producing mean value of 6.3 ± 0.3 roots per shoot with a 90 % rooting frequency. Absence of roots were observed on medium containing no plant growth regulators. A significant difference was observed among mean number of roots per rooted shoots (Table 3). Plantlets with well-developed roots and shoots were removed from the cultured medium and transferred to a mixture of peat moss, compost and soil (1:1:1). The plantlets continued to grow well and get acclimatized in greenhouse conditions. Almost 90 % of the transplanted plantlets survived over the next 10 weeks.
Cardenolide estimation in in vitro samples and natural population
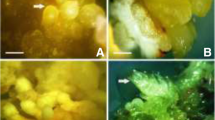
HPLC was used to quantify the presence of cardenolides in both in vitro and leaves of natural D. ferruginea populations. Lanatoside C and digoxin were estimated in callus, regenerated plantlets or seedlings (Table 4), while digitoxigenin, gitoxigenin and digoxigenin derivatives of cardenolides from leaves of natural samples were also estimated after acid hydrolysis of the genine groups (Fig. 2).
Germinated seedlings
The values of two isomers (lanatoside C and digoxin) of cardenolides from the germinated seedlings are presented in Table 4. It was observed that germinated seedlings had less cardenolides (3.04 ± 0.41 mg 100 g−1 lanatoside C and 0.27 ± 0.2 mg 100 g−1 digoxin; see Table 4) than in vitro regenerated shoots. There was a significant difference among the mean number of cardiotonic glycoside (Table 4). In case of D. ferruginea seedlings, lanatoside C and digoxin were higher than D. davisiana (Gurel et al. 2011). It is noticeable that, Digitalis cardenolides are mainly stored in vacuole of mesophyll cells (Hoelz et al. 1992; Kreis and May 1990) and the reduction in mesophyll cell size on the other hand implied the reduction in cardenolide content (Eisenbeiß et al. 1999). Whereas D. ferruginea has broad leaves as compared to Turkish Digitalis endemic species (D. davisiana Heywood, D. cariensis Boiss., D. lamarckii Ivanina, and D. trojana Ivanina), this could be a reason for the higher cardenolides content. It has been reported previously that there is a direct correlation between the leaf size and the productivity of cardenolides in several culture lines of Digitalis species (Gavidia and Pérez-Bermúdez 1997).
Green undifferentiated callus tissue
The present study observed that undifferentiated callus tissue (mainly green) produced trace amounts of lanatoside C (Table 4), compared to the steadily increasing amounts of digoxin detected upon callus re-differentiation into organized tissue. Hagimori et al. (1982) studied the involvement of chloroplast in cardenolides synthesis and reported that undifferentiated cells containing chloroplasts, could be a reason for cardenolides synthesis. Several groups have studied the presence or absence of cardenolides in undifferentiated callus of Digitalis species and trace amount of cardenolides in mitochondrial and chloroplast fractions (Gurel et al. 2011; Hirotani and Furuya 1977; Kreis et al. 1986; Lui and Staba 1979; Voigt et al. 1969).
Regenerated plantlets
Higher amount of lanatoside C and digoxin accumulation was obtained when shoots were regenerated on MS medium containing auxin (NAA) and cytokinin (BAP). Combinations of NAA and BAP produced 13.2 ± 0.50 and 2.93 ± 0.31 mg 100 g−1 dry weight (dw) of lanatoside C and digoxin, respectively (Table 4; Fig. 1i, j). We found that cytokinin alone produced twofold of lanatoside C and fivefold digoxin as compared with auxin alone. Moreover, cytokinin combined with auxin increased twofold lanatoside C and digoxin as compared to cytokinin alone (Table 4). Auxin and cytokinin combination was more effective than either auxin or cytokinin alone. When BAP was used alone or combined with NAA, there was no significant difference among the cardiotonic glycoside (Table 4). In addition, lanatoside C accumulation in in vitro regenerants was higher than that of callus and seedlings. Moreover, in this study the hormonal composition of the initial regeneration (shoot development) medium had a significant effect on the cardenolide production in in vitro grown materials. A similar finding was observed by Hagimori et al. (1982), where shoot forming callus accumulated considerable amounts of cardenolides in six Digitalis species. In the present investigation, the level of lanatoside C was higher than digoxin in in vitro samples, while on the contrary digoxin was higher than lanatoside C in D. davisiana (Gurel et al. 2011). It is well known that, lanatoside C is transformed into digoxin by deglucosylation using degilanidase present in the leaves and subsequent deacetylation (Gisvold and Wright 1957; Ikeda et al. 1992). Whereas in vitro culture from shoot tips of D. lanata on MS medium with increased thiamine content (up to 1 mg l−1) supplemented with 2–2.5 mg l−1 BAP and 0.17 mg l−1 IAA was found not to affect the lanatoside C content as reported by Kubalákoyá et al. (1987).
The majority of studies on in vitro cardenolides accumulation in Digitalis have been focused on two species, namely D. lanata and D. obscura. Rhenius et al. (1997) reported that, feeding deacetyl lanatoside C to senescent shoot cultures of D. lanata resulted in the formation of new product, which was isolated by semi-preparative HPLC. A 12β-hydroxlyase which catalyzes the hydroxylation of digitoxin and other cardenolides of the A-series to digoxin and the corresponding cardenolides of the C-series was isolated from suspension cultures of D.lanata (Petersen and Seitz 1985). Eisenbeiß et al. (1999) reported that, shoot culture of D. lanata accumulated up to 0.6 µmol cardenolides when cultivated under continuous white light. After its transfer to permanent dark, the cardenolide content of cultured shoot gradually decreased and reached no-detectable levels after 12 weeks. After transfer back to the light conditions, cardenolide started to accumulate and reached again the levels of the light-grown controls after 4 weeks. Vela et al. (1991) determined digoxigenin derivatives in all clonally propagated plants of D. obscura, but the amount of these glycosides were much higher in those obtained from axillary buds. In particular, the recent study have emphasized the relationship between the growth regulators and cardenolides production in D. ferruginea.
Natural populations
The lanes in different colors (i.e., pale blue, green, and blue), indicate digitoxigenin (A-series), gitoxigenin (B-series) and digoxigenin (C-series) derivatives of cardenolides (genine group), respectively (Fig. 2). TLC analysis of genine groups were also correlated with quantitative analysis by means of HPLC. In Fig. 2 is shown the gitoxigenin content of cardenolid, which was poor with a mean value of 50 ± 20 mg 100 g−1 dw. However the digitoxigenin (730 ± 180 mg 100 g−1 dw) and digoxigenin derivatives of cardenolides (490 ± 170 mg 100 g−1 dw) had prevalence in the natural population of D. ferruginea. This was also observed in 2 years old plant of D. purpurea (Usai et al. 2007) having ranges of 1.13–24.0 and 0.4–17.8 mg 100 g−1 dw for digitoxigenin and gitoxigenin, respectively. According to these data, it was evident that the predominant cardenolides in D. ferruginea plants were those of the series A, which was in an agreement with previous results reported for other Digitalis species including D. obscura (Gavidia and Pérez-Bermúdez 1997). Digitalis lanata is known to contain primary glycosides including lanatoside A, B and C series, on the other hand, Digitalis ambigua contains lanatoside A and B, but lanatoside C is not present (Wichtl et al. 1987).
In summary, we have successfully established an efficient regeneration protocol via indirect shoot organogenesis and indirect somatic embryogenesis of D. ferruginea. The developed method opens up a new approach towards somatic embryogenesis development and also to produce genetically modified Digitalis with the characteristics of commercial interest. Numerous pharmaceutical companies are dependent on materials produced from natural plant populations of several important medicinal species to a considerable extent, yet there is a lack of systematic effort for the cultivation of D. ferruginea. Therefore, the protocol described above can contribute to the establishment of a large scale tissue culture production with specific reference to germplasm conservation, commercial cultivation and genetic improvement studies, as well as cardenolide production. Today digoxin is one of the most important drugs and widely used medicine for the therapy of congestive heart failure and atrial fibrillation. We expect that the D. ferruginea will serve well for a large-scale production for digoxin and other related compounds.
Abbreviations
- BAP:
-
6-Benzylaminopurine
- GA3 :
-
Gibberellic acid
- HPLC:
-
High performance liquid chromatography
- IAA:
-
Indole-3-acetic acid
- IBA:
-
Indole-3-butyric acid
- NAA:
-
α-Naphthalene acetic acid
- PGR:
-
Plant growth regulator
- TDZ:
-
Thidiazuron
- 2, 4-D:
-
2, 4-Dichlorophenoxy acetic acid
- MS:
-
Murashige and Skoog
- MSO:
-
MS medium without hormones
- TLC:
-
Thin layer chromatography
References
Atwater BR (1980) Germination, dormancy and morphology of the seeds of herbaceous ornamental plants. Seed Sci Technol 8(4):523–573
Cacho M, Morán M, Herrera MT, Fernández-Tárrago J (1991) Morphogenesis in leaf, hypocotyl and root explants of Digitalis thapsi L. cultured in vitro. Plant Cell Tissue Organ Cult 25(2):117–123
Çördük N, Akı C (2010) Direct shoot organogenesis of Digitalis trojana Ivan., an endemic medicinal herb of Turkey. Afr J Biotechnol 9(11):1587–1591
Davis PH, Cullen J, Coode MJ (1988) Flora of Turkey: and the East Aegean Islands (supplement), vol 10. Edinburgh University Press, Edinburgh
Eisenbeiß M, Kreis W, Reinhard E (1999) Cardenolide biosynthesis in light-and dark-grown Digitalis lanata shoot cultures. Plant Physiol Biochem 37(1):13–23
Gavidia I, Pérez-Bermúdez P (1997) Cardenolides of Digitalis obscura: the effect of phosphate and manganese on growth and productivity of shoot-tip cultures. Phytochemistry 45(1):81–85
Gisvold O, Wright S (1957) Enzymatic decomposition of digitalis glycosides. J Am Pharm Assoc 46(9):535–538
Gurel E, Yucesan B, Aglic E, Gurel S, Verma SK, Sokmen M, Sokmen A (2011) Regeneration and cardiotonic glycoside production in Digitalis davisiana Heywood (Alanya Foxglove). Plant Cell Tissue Organ Cult (PCTOC) 104(2):217–225
Hagimori M, Matsumoto T, Obi Y (1982) Studies on the production of Digitalis cardenolides by plant tissue culture II. Effect of light and plant growth substances on digitoxin formation by Undifferentiated cells and shoot-forming cultures of Digitalis purpurea L. grown in liquid media. Plant Physiol 69(3):653–656
Hirotani M, Furuya T (1977) Restoration of cardenolide-synthesis in redifferentiated shoots from callus cultures of Digitalis purpurea. Phytochemistry 16(5):610–611
Hoelz H, Kreis W, Haug B, Reinhard E (1992) Storage of cardiac glycosides in vacuoles of Digitalis lanata mesophyll cells. Phytochemistry 31(4):1167–1171
Ikeda Y, Fujii Y, Yamazaki M (1992) Determination of lanatoside C and digoxin in Digitalis lanata by HPLC and its application to analysis of the fermented leaf powder. J Nat Prod 55(6):748–752
Kreis W, May U (1990) Cardenolide glucosyltransferases and glucohydrolases in leaves and cell cultures of three Digitalis (Scrophulariaceae) species. J Plant Physiol 136(2):247–252
Kreis W, May U, Reinhard E (1986) UDP-glucose: digitoxin 16′-O-glucosyltransferase from suspension-cultured Digitalis lanata cells. Plant Cell Rep 5(6):442–445
Kreis W, Hoelz H, May U, Reinhard E (1990) Storage of cardenolides in Digitalis lanata cells. Effect of dimethyl sulfoxide (DMSO) on cardenolide uptake and release. Plant Cell Tissue Organ Cult 20(3):191–199
Kubalákoyá M, Spltzova I, Novak F (1987) Stability of lanatoside C content in the in vitro propagated Digitalis lanata clones. Biol Plant 29(1):7–9
Kuberski C, Scheibner H, Steup C, Diettrich B, Luckner M (1984) Embryogenesis and cardenolide formation in tissue cultures of Digitalis lanata. Phytochemistry 23(7):1407–1412
Lapeña L, Brisa MC (1995) Influence of culture conditions on embryo formation and maturation in auxin-induced embryogenic cultures of Digitalis obscura. Plant Cell Rep 14(5):310–313
Lui JH, Staba EJ (1979) Effects of precursors on serially propagated Digitalis lanata leaf and root cultures. Phytochemistry 18(12):1913–1916
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15(3):473–497
Pérez-Bermúdez P, Falcó JM, Segura J (1987) Morphogenesis in root tip meristem cultures of Digitalis obscura L. J Plant Physiol 130(1):87–91
Petersen M, Seitz HU (1985) Cytochrome P-450-dependent digitoxin 12β-hydroxylase from cell cultures of Digitalis lanata. FEBS Lett 188(1):11–14
Quiroz-Figueroa F, Fuentes-Cerda C, Rojas-Herrera R, Loyola-Vargas V (2002) Histological studies on the developmental stages and differentiation of two different somatic embryogenesis systems of Coffea arabica. Plant Cell Rep 20(12):1141–1149
Rhenius M, Porzel A, Diettrich B, Luckner M (1997) 21′-di-dehydro-deacetyllanatoside C, a biotransformation product of deacetyllanatoside C from senescent shoot cultures of Digitalis lanata. Phytochemistry 44(6):1061–1064
Sales E, Segura J, Arrillaga I (2003) Agrobacterium tumefaciens-mediated genetic transformation of the cardenolide-producing plant Digitalis minor L. Planta Med 69(02):143–147
Sales E, Müller-Uri F, Nebauer SG, Segura J, Kreis W, Arrillaga I (2011) Digitalis wild crop relatives: genomic and breeding resources. Springer, Berlin, pp 73–112
Schöner S, Reinhard E (1986) Long-term cultivation of Digitalis lanata clones propagated in vitro: cardenolide content of the regenerated plants. Planta Med 52(06):478–481
Turker AU, Yucesan B, Gurel turk E (2010) Adventitious shoot regeneration from stem internode explants of Verbena officinalis L., a medicinal plant. J Biol 34:297–304
Usai M, Atzei AD, Marchetti M (2007) Cardenolides content in wild Sardinian Digitalis purpurea L. populations. Nat Prod Res 21(9):798–804
Vela S, Gavidia I, Pérez-Bermúdez P, Segura J (1991) Micropropagation of juvenile and adult Digitalis obscura and cardenolide content of clonally propagated plants. In Vitro Cell Dev Biol Plant 27(3):143–146
Verma S, Chand S (2009) Somatic embryogenesis and histological study in cotyledonary callus of Hyoscyamus muticus L. J Med Aroma Plant Sci 31(3):234–237
Verma SK, Yucesan BB, Gurel S, Gurel E (2011a) Indirect somatic embryogenesis and shoot organogenesis from cotyledonary leaf segments of Digitalis lamarckii Ivan., an endemic medicinal species. Turk J Biol 35:743–750
Verma SK, Yücesan BB, Şahin G, Gürel S, Gürel E (2011b) Direct shoot regeneration from leaf explants of Digitalis lamarckii, an endemic medicinal species. Turk J Bot 35:689–695
Verma SK, Sahin G, Yucesan B, Eker I, Sahbaz N, Gurel S, Gurel E (2012) Direct somatic embryogenesis from hypocotyl segments of Digitalis trojana Ivan and subsequent plant regeneration. Ind Crops Prod 40:76–80
Voigt W, Reissbrodt R, Baumgarten G (1969) On the question of binding of cardenolides to plant cell particles. Pharmazie 24(7):422
Wichtl M, Bühl G, Huesmann K (1987) Fingerhut. Digitalis L.—bekannte und weniger bekannte Vertreter einer wichtigen Arzneipflanzengattung. Deutsche Apotheker Ztg 127:2391–2400
Wiegrebe H, Wichtl M (1993) High-performance liquid chromatographic determination of cardenolides in Digitalis leaves after solid-phase extraction. J Chromatogr A 630(1):402–407
Acknowledgments
The authors thanks to TÜBİTAK for financial support (project no: TOVAG-106O470), a research grant for the international research fellowship programme (2214) to B.Y. and the Turkish Government for providing scholarship to S.K.Verma through the MHRD, India. Special thanks to Prof. Dr. W. Kreis (Friedrich-Alexander-Universität Erlangen-Nürnberg, Germany) for their technical support towards cardenolide analyses (genine group). The authors express their sincere thanks to Prof. Sergio J. Ochatt for his critical reading of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Verma, S.K., Yucesan, B., Sahin, G. et al. Embryogenesis, plant regeneration and cardiac glycoside determination in Digitalis ferruginea subsp. ferruginea L. Plant Cell Tiss Organ Cult 119, 625–634 (2014). https://doi.org/10.1007/s11240-014-0562-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-014-0562-9